Abstract
In previous studies, we generated knock-in mice with a cocaine-insensitive dopamine transporter (DAT-CI mice) and found cocaine does not stimulate locomotion or produce reward in these mice, indicating DAT inhibition is necessary for cocaine stimulation and reward. However, DAT uptake is reduced in DAT-CI mice and thus the lack of cocaine responses could be due to adaptive changes. To test this, we used adeno-associated virus (AAV) to reintroduce the cocaine-sensitive wild type DAT (AAV-DATwt) back into adult DAT-CI mice, which restores cocaine inhibition of DAT in affected brain regions but does not reverse the adaptive changes. In an earlier study we showed that AAV-DATwt injections in regions covering the lateral nucleus accumbens (NAc) and lateral caudate-putamen (CPu) restored cocaine stimulation but not cocaine reward. In the current study, we expanded the AAV-DATwt infected areas to cover the olfactory tubercle (Tu) and the ventral midbrain (vMB) containing the ventral tegmental area (VTA) and substantia nigra (SN) in addition to CPu and NAc with multiple injections. These mice displayed the restoration of both locomotor stimulation and cocaine reward. We further found that AAV-DATwt injection in the vMB alone was sufficient to restore both cocaine stimulation and reward in DAT-CI mice. AAV injected in the VTA and SN resulted in DATwt expression and distribution to the DA terminal regions. In summary, cocaine induced locomotion and reward can be restored in fully developed DAT-CI mice, and cocaine inhibition of DAT expressed in dopaminergic neurons originated from the ventral midbrain mediates cocaine reward and stimulation.
Keywords: Addiction, Cocaine, Dopamine transporter, Locomotion, Reward, adeno-associated virus
1. Introduction
Cocaine is a powerfully addictive stimulant and a popular drug of abuse. Cocaine inhibits the transporters for dopamine (DA), norepinephrine (NE), and serotonin (5-HT) at roughly similar concentrations in both brain preparations and in cultured cells expressing the cloned transporters (Ritz et al., 1987, Amara and Kuhar, 1993, Surratt et al., 1993, Gu et al., 1994, Han and Gu, 2006). Knockout mice with each of the transporters disrupted individually still exhibit the rewarding effect of cocaine in conditioned place preference (CPP) and/or cocaine self-administration experiments (Rocha et al., 1998, Sora et al., 1998, Xu et al., 2000), suggesting that none of the these transporters are required for cocaine reward. However, the knock-out mice showed very significant adaptive changes to compensate for the lack of an important transporter (Giros et al., 1996, Bengel et al., 1998, Rocha et al., 1998, Sora et al., 1998, Li et al., 1999, Rioux et al., 1999, Xu et al., 2000). We have made a knock-in mouse line carrying a functional but cocaine-insensitive dopamine transporter (DAT-CI mice) (Wu and Gu, 2003, Chen et al., 2006). In these mice, cocaine does not block DAT and does not produce reward or stimulate locomotion, indicating that cocaine blockade of DAT is required for the cocaine reward and stimulation in “normal” mice. In addition, cocaine actually produces conditioned place aversion (CPA) and locomotor suppression in these mice (Chen et al., 2006, O’Neill et al., 2013). However, DAT function in DAT-CI mice is also significantly reduced which might also cause some adaptive changes. It is possible that the lack of cocaine responses in these mice is due to adaptive changes. To test this possibility, we use viral vectors to reintroduce the wild type DAT (DATwt) back into the brains of fully developed adult DAT-CI mice, which partially restore cocaine inhibition of DAT but will not reverse any adaptive changes occurred during development.
In our prior study, we injected recombinant adeno-associated viral (AAV) vectors containing the wild-type mouse DAT (AAV-DATwt) into various brain regions of DAT-CI mice. We found that restoring DAT inhibition in the lateral striatum including the dorsal and ventral areas is sufficient for restoring cocaine’s stimulating effect but not for the rewarding effect (O’Neill et al., 2014). In the current study, we expanded the tested areas to identify the brain regions where cocaine inhibition of DAT leads to stimulation and reward.
2. Materials and methods
2.1 Animal Subjects
Homozygous C57-congenic DAT-CI mice (Chen et al., 2006, O’Neill and Gu, 2013) and wild-type littermates were produced from sibling pairings of heterozygous DAT-CI mice. Mice were kept in a 12-h light/dark cycle and group housed up to five per cage. Water and standard rodent chow were provided ad libitum. Nesting material and hiding/nesting devices were provided for enrichment purposes. Only male mice were used for experiments, and all mice were between 2-6 months of age at the time of testing. All animal related procedures were performed in accordance with the Ohio State University Institutional Animal Care and Use Committee (IACUC). The animals went through surgeries of AAV injections 4 weeks before behavioral tests followed by immunohistochemical analysis. Figure 1 illustrate the timeline of these procedures.
Figure 1. Timeline of AAV injection and behavior tests.
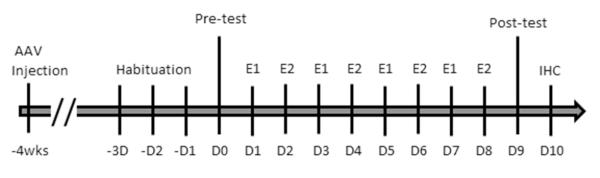
The stereotaxic surgery was performed to inject AAV vectors into selected brain regions 4 weeks before the behavior tests. The 4 weeks of recovery after the surgery is necessary for stable expression of virus carried genes. The mice were first habituated to being handled each day for 3 days (−D3 to −D1). Then the mice went through the CPP paradigm with the pre-test on day 0 (D0), followed by four treatment/environment pairings of two alternating environments (E1 and E2) which are saline or cocaine injection. Half of the mice had cocaine treatment first and half of the mice had saline treatment first. The post-conditioning test was on day 9 (D9). After the last behavioral test, the mice were sacrificed and processed for immunohistochemical (IHC) localization of the AAV injection site (D10) and HA-DAT expression.
2.2 Viral Vectors
Recombinant adeno-associated viral vectors (AAV) carrying the wild-type mouse dopamine transporter (AAV-DATwt) were used in this study. The virus carried DAT has a hemagglutinin (HA) tag at the N-terminal, allowing the detection of its expression. The vectors were prepared by the OSU viral vector core, where viruses were packaged and purified using a procedure similar to that described (Clark et al., 1999). Briefly, HEK293 cells were co-transfected with a capsidation plasmid (AAV1 serotype), a helper plasmid, and the recombinant genome plasmid containing HA tagged wild type mouse DAT. The AAV-DATwt viruses were then isolated from the cell culture by ultracentrifugation and chromatography purification. The final preparation was tittered by real-time PCR and the concentration was 2.6×1012 vg/ml. The virus stock was diluted to 2.6×10 11 vg/ml for microinjections.
2.3 Surgeries and Microinjection of Viral Vectors
The surgery and microinjection procedures are similar to those described in our earlier publication (O’Neill et al., 2014). Briefly, mice were anesthetized with a mixture of 100mg/kg ketamine and 20mg/kg xylazine (Sigma-Aldrich, St. Louis, MO). Mice were mounted on a stereotaxic frame and a small straight incision was made along the midline of the head to expose the underlying skull. The bregma coordinates were recorded and used as the reference point.
The stereotaxic injection setup consisted of a Hamilton syringe connected to a 33 gauge injector cannula (Plastics one, Roanoke VA). A volume of 2-10 μl of virus was injected per mouse, at a rate of 0.1-0.25 ul/min controlled by a syringe pump.
Microinjections of viral vectors were carried out to cover multiple regions. Injection 1 covered olfactory tubercle (Tu), nucleus accumbens shell (Acbsh) and core (AcbC), and the Caudate Putamen (CPu). Injection 2 covered the ventral midbrain (vMB) including the ventral tegmental area (VTA) and the substantia nigra (SN). In injection 1, four boli of 1 μL virus were infused along the injector’s path at 4 different dorsal/ventral coordinates (Table 1). The coordinates for each of the targeted regions are listed in Table 1.
Table 1.
Coordinates used for stereotaxic AAV-DATwt injection
| Brain Region | Injection site relative to Bregma (in mm) | |||
|---|---|---|---|---|
| Anterior/Posterior | Medial/Lateral | Dorsal/Ventral | ||
| CPu | +1.5 | ±1.3 | −3.5 | |
| AcbC | −4.6 | |||
| AcbSh | −5.0 | |||
| Tu | −5.5 | |||
| vMB | −2.9 | ±0.9 | −4.5 | |
The stereotaxic coordinates for targeted brain regions in mm relative to bregma. CPu: caudate putamen (dorsal striatum); AcbC and AcbSh: nucleus accumbens core and shell; Tu: olfactory tubercle; vMB: ventral MidBrain including ventral tegmental area (VTA) and substantia nigra (SN).
After infusion was finished, the injector was left in place for 2 minutes before being raised. The mice were sutured after the surgery and administered post-operative care for one week. After four weeks recovery, the wild-type DAT would be stably expressed in the injected region.
2.4 Drugs Administered
All drugs were dissolved in 0.9% saline and given in a volume of 10ul/g body weight. Cocaine HCL was provided by the NIDA drug supply program, and administered at 20mg/kg doses intraperitoneally (i.p.). Ketamine and xylazine were administered for anesthesia before the virus injection surgeries at doses of 100mg/kg and 20mg/kg respectively (i.p.).
2.5 Conditioned Place-Preference and Locomotion Test
The conditioned place-preference (CPP) scores and the locomotor activities were recorded during the same CPP procedure (O’Neill et al., 2014). The apparatus was a acrylic box divided into three interconnected compartments: two side compartments and a center compartment. The CPP procedure is outlined in Figure 1. Mice were first habituated to being handled for three days. On the pre-conditioning test day (D0), the three compartments had distinct visual and tactile cues, creating three different “environments”. Mice were placed into the center compartment and allowed to explore all three compartments for 30minutes. Mouse locations and movements were recorded by the AnyMaze video tracking system (Stoelting Co.). Time mice spent in each of the three compartments and the total distance mice traveled were calculated. Their preference was defined as the difference in time spent in one side compartment versus the other side. The pre-test (preexisting) preference was counterbalanced in each group of mice by designating individual mice to receive cocaine in either their initially preferred or initially non-preferred environment - such that the group bias was minimized.
During the conditioning phase (D1 to D8), all three compartment had the same environmental cue set. The mice were then administered the treatment (either cocaine or saline) corresponding with one of the two environments and immediately placed in the environment for 30 minutes. The mice were monitored and the distance travelled was recorded. On the following day, the opposite agent was administered and mice were placed in the alternate environment for 30 min. These alternations proceeded for 4 cycles (four pairings, D1 to D8). The distances traveled after the first saline injection and after the first cocaine injection were used to assess the basal locomotion and cocaine induced locomotor stimulation in these mice.
On the post-conditioning test day (D9), the apparatus was configured to have three-environments and the mice were tested exactly the same way as on the pre-conditioning test day. There were no treatments during the pre or post-conditioning tests. The “CPP score” is defined as the time spent in the cocaine-paired environment (conditioned stimulus, CS+) minus the time spent in the saline-paired environment (unconditioned stimulus, CS-) during the pre- and postconditioning tests. Differences in CPP score during the pre-conditioning test versus the post-conditioning test indicate an effect of the drug.
2.6 Immunohistochemistry
After the behavior experiments, mice were sacrificed and perfused with 4% paraformaldehyde and the mouse brains were harvested. Areas of HA tagged DATwt expression were determined by immunohistochemical staining on floating sections. A freezing microtome was used to make 60 μm coronal brain sections through the striatal region and through the midbrain. All incubations were carried out at room temperature in PBS containing 0.3% triton X-100. The sections were first incubated in a blocking buffer containing 5% goat serum for 60 minutes and then incubated in a 1:6000 dilution of the primary antibody, a mouse monoclonal anti-HA (Sigma). The sections were washed and incubated in a 1:1000 dilution of goat-anti mouse secondary antibody (sigma) followed by an incubation in a 1:1000 dilution of peroxidase-conjugated mouse anti-peroxidase (Jackson ImmunoResearch). The sections were reacted with a chromogen using a glucose oxidase-catalyzed, nickel-intensified Diaminobenzidene (DAB) procedure (Tian et al., 2008). The sections were mounted onto slides and visualized on a CarlZeiss axioscope. The immunofluorescence procedure was similar in early steps. The secondary antibody used was a goat anti mouse Cy3 secondary antibody (1:2000) (Jackson Immunoresearch, West Grove, PA). The images were visualized using a fluorescence microscope.
2.7 Statistical Analysis
Simple t-tests were performed to compare between the locomotor activities after saline and after cocaine injections by the same group of mice. No comparisons were made between groups and thus more sophisticated statistics (such as Two Way ANOVA) were not used. Similarly, t-tests were used to compare between the CPP score in the preconditioning test and the CPP score in the postconditioning test of the same group of mice.
3. Results
3.1 AAV-DATwt injection in Multiple Brain Regions
We have shown that AAV-DATwt injections into the dorsal and ventral striatum can restore cocaine locomotor stimulation but not cocaine reward (O’Neill et al., 2014). It is possible that cocaine actions in more brain regions are required for cocaine reward. We thus injected AAV-DATwt into the brains of DAT-CI mice through 4 injections. The two rostral injections (bilateral) each had 4 infusions at different dorsal/ventral coordinates (see Table 1) to target the olfactory tubercle (Tu), the nucleus accumbens shell (AsbS) and core (AsbC), and the caudate putamen (CPu). The two caudal injections targeted the ventral midbrain (vMB) covering the ventral tegmental area (VTA) and the substantia nigra (SN). The coordinates used to target these regions are listed in Table 1. Immunohistochemical staining was performed to confirm the expression of HA tagged wild type DAT in targeted regions using a primary antibody recognizing the HA tag. Figure 2A and 2B show representative micrographs of coronal brain slices at or near the needle paths of the rostral and caudal injections respectively. We examined multiple adjacent brain slices and observed approximate spherical symmetry of the virus spread around the injection sites.
Figure 2. DAB immunostaining of the HA tag to assess AAV mediated wild type DAT expression in DAT-CI mice.
Representative micrographs of coronal brain slices from DAT-CI mice with AAV-DATwt injections. The brain slices were stained with the DAB procedure using an antibody recognizing the HA tag on the AAV delivered wild type DAT. (A) A coronal slice near the striatum injection sites showing that the spread of the virus covers the olfactory tubercle (Tu), the nucleus accombens shell (AcbSh) and core (AcbC), lateral AcbSh (LAcbSh) and the caudate putamen (CPu). (B) A coronal slice near the ventral mid brain injection site showing that the spread of the virus covers the ventral tegmental area (VTA) and the substantia nigra reticular part (SNR) and compact part (SNC). The drawings on the right illustrate the locations of these brain regions.
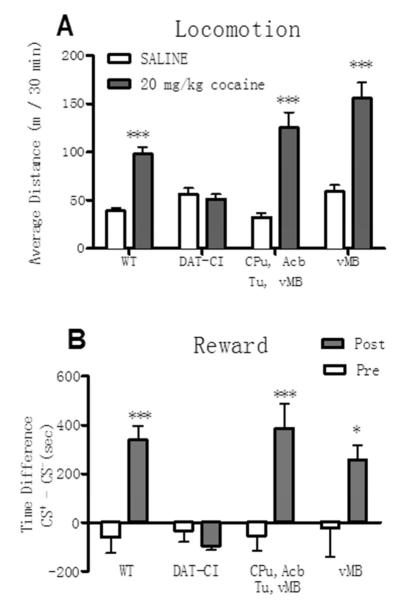
As expected, re-introduction of wild type DAT into multiple brain regions restored cocaine induced locomotor stimulation. Figure 3A shows that, compared to the saline administration, 20 mg/kg cocaine stimulated locomotor activities in the wild type mice and in the DAT-CI mice with AAV-DATwt injected in multiple regions, but not in the control DAT-CI mice without virus injection. Most importantly, DAT-CI mice with AAV-DATwt injected in multiple regions also displayed the restored cocaine conditioned place preference. Figure 3B shows that 20 mg/kg cocaine induced robust CPP in the wild type mice, whereas the uninjected DAT-CI mice clearly did not develop CPP to cocaine. In contrast, DAT-CI mice with AAV-DATwt injected into multiple brain regions displayed statistically significant CPP (P<0.0001, T test).
Figure 3. Cocaine-induced locomotor stimulation and conditioned place preference in AAV-injected DAT-CI mice.
Panel A shows the locomotor activities presented as horizontal distances traveled (meters) by mice in 30 min (mean± SEM) during the first saline or cocaine conditioning session of the CPP paradigm. Wild-type mice (WT) display significant locomotor stimulation induced by 20 mg/kg cocaine compared to that after saline treatment (n = 12, P< 0.0001), whereas DAT-CI mice do not (n = 8, p > 0.05). Cocaine administration significantly stimulated locomotor activities compared to saline (n = 25, p < 0.0001) in DAT-CI mice injected with AAV- DATwt to the ventral midbrain (vMB) and striatum regions including olfactory tubercle (Tu), the nucleus accombens (Acb), and the caudate putamen (CPu). The restored locomotor stimulation by cocaine was also observed in DAT-CI mice with AAV- DATwt injected into the ventral midbrain (vMB) only (n = 13, p < 0.0001). ***, P<0.0001 in t-test comparing cocaine and saline induced locomotor activities in the same groups of mice.
Panel B shows the reward activities represented by the cocaine conditioned place preference (CPP). The CPP scores are defined as the time (in seconds) spent in the cocaine-paired environment (CS+) minus the time spent in the saline-paired environment (CS-) during the preconditioning test (Pre) and postconditioning test (Post). The wild-type mice displayed significant conditioned place-preference to 20 mg/kg cocaine – the CPP score in the post-test is significantly higher than that in the pre-test (n = 12, P<0.0001), whereas the uninjected DAT-CI mice did not (P>0.05). Injections of AAV-DATwt into the vMB and combined striatum regions of DAT-CI mice displayed significant CPP to 20mg/kg cocaine (n = 25, p < 0.001). Injections of AAV-DATwt into the vMB only also restored cocaine CPP (n = 13, P < 0.05). *, P<0.05; **, P<0.001 ***, P<0.0001 in t-tests comparing post and preconditioning CPP scores in the same group of mice.
3.2 AAV-DATwt Injection In Ventral Midbrain (vMB) Alone
The ventral midbrain injection covers the VTA and SN (Figure 2B) which contain the cell bodies of the dopaminergic neurons projecting to NAc, CPu, Tu, and other important brain structures. AAV injection in the areas having DA neuron cell bodies would lead to the wild type DAT expression in the DA neurons. It is likely that the virus derived wild type DAT were transported from the injection site in the VTA and SN to the DA terminals in the projection areas, which may contribute to restoring cocaine responses. Therefore, we injected AAV-DATwt only in the ventral midbrain in another group of DAT-CI mice. Immunofluorescence staining shows that the midbrain virus injection resulted in the expression of the HA tagged wild type DAT in CPu, NAc, and Tu (Figure 4). Behavioral tests show that AAV-DATwt injection in the ventral midbrain (vMB) alone is sufficient to restore cocaine induced locomotor stimulation (Figure 3A) and cocaine reward measured by conditioned place preference (Figure 3B) in DAT-CI mice.
Figure 4. Fluorescence immunostaining of the AAV mediated wild type DAT expression in DA terminal brain regions of DAT-CI mice injected with AAV-DATwt in the ventral midbrain.
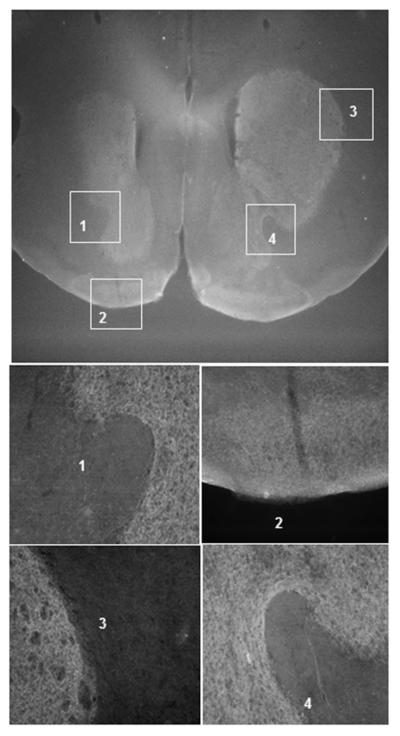
Coronal brain slices from DAT-CI mice with AAV vector injected in vMB. The brain slices were stained with anti HA tag primary antibody and goat anti mouse Cy3 secondary antibody. The rostral/caudal coordinate for the image is about +1 mm relative to Bregma. Images 1- 4 are higher magnification images of the boxed regions specified.
4. Discussion
Cocaine inhibits DAT, SERT and NET leading to complex effects. Each of the 3 monoamine transporters has been disrupted in knockout mouse models to dissect out the contributions of each transporter to the cocaine effects. Surprisingly, knocking out any of the transporters individually does not eliminate the rewarding effect of cocaine (Rocha et al., 1998, Sora et al., 1998, Xu et al., 2000). These studies clearly demonstrate that none of these transporters are absolutely required for cocaine reward at least under certain circumstances. In addition, double knock-out mice lacking DAT and SERT do not show cocaine reward, suggesting that there might be redundant pathways or mechanisms for cocaine reward (Sora et al., 2001, Uhl et al., 2002). However, the knock-out mice showed very significant adaptive changes to compensate for the lack of an important transporter (Giros et al., 1996, Bengel et al., 1998, Rocha et al., 1998, Sora et al., 1998, Li et al., 1999, Rioux et al., 1999, Xu et al., 2000). In addition, fluoxetine and nisoxetine, selective inhibitors for SERT and NET respectively, produce CPP in DAT knockout mice but not in WT mice (Hall et al., 2002). These results suggest that the mechanisms of cocaine action in these knockout mice with very substantial adaptive changes are quite different from normal mice.
To avoid strong adaptive changes, we made a knock-in mouse line carrying a functional but cocaine-insensitive DAT (DAT-CI mice) (Wu and Gu, 2003, Chen et al., 2006). In these mice, cocaine no long stimulates locomotion and does not produce reward as measured by CPP and self-administration (Chen et al., 2006, Tilley et al., 2007, Tilley and Gu, 2008a, Thomsen et al., 2009, Tilley et al., 2009). In addition, cocaine actually produces conditioned place aversion (O’Neill et al., 2013). However, the mutant DAT in DAT-CI mice has significantly reduced DA uptake function and the mice show significantly higher basal locomotor activity (Chen et al., 2006, Tilley and Gu, 2008b, Tilley et al., 2009). The altered DAT function in DAT-CI mice can also cause some adaptive changes and it is possible that these adaptive changes may be responsible to the lack of cocaine responses in these mice. To test this possibility, we use viral vectors to reintroduce the wild type DAT back into the brains of fully developed DAT-CI mice. The AAV infection in adult mice should not reverse any adaptive changes that have already occurred during development, although 4 weeks of AAV-DAT expression may cause some adaptive changes. The virus mediated cocaine sensitive DAT will be expressed in addition to the endogenous cocaine-insensitive DAT, creating a situation similar to heterozygous DAT-CI mice in the virus infected brain regions. We have shown that AAV mediated DAT expression contributes to DA homeostasis and cocaine slows down DA clearance on the virus injected side but not on the uninjected side of DAT-CI mouse brain (O’Neill et al., 2014). In the current study, we found that virus mediated partial restoration of cocaine inhibition of DAT can restore cocaine reward and stimulation in fully developed adult DAT-CI mice. Therefore, the lack of cocaine responses in DAT-CI mice is not due to adaptive changes but due to the lack of cocaine inhibition of the mutant DAT. These results strengthen the conclusion that cocaine inhibition of DAT is necessary for the stimulating and rewarding effects of cocaine.
The dopaminergic circuitry originating in the midbrain has been traditionally divided into a nigrostriatal motor system and a mesolimbic reward and motivational system (Ungerstedt, 1971). Abundant evidence indicates that the mesolimbic dopamine system, containing dopaminergic projections from ventral tegmental area (VTA) to the nucleus accumbens (NAc) and other forebrain structures, plays a critical role in reward / reinforcement and that most addictive drugs elevate extracellular dopamine in the NAc (Carboni et al., 1989, Cass et al., 1992, Di Chiara, 1995, Koob, 1998). However, there is now compelling evidence suggesting that the substantia nigra dopamine neurons also play a significant role in reward and addiction in addition to DA neurons of the mesolimbic system (Wise, 2009) and there are also conflicting results about the brain regions important for cocaine stimulation or reward. It was shown that rats self-administer amphetamine directly into the NAc (Hoebel et al., 1983). However, intra-accumbens infusions of cocaine produced conditioned locomotor activity but not conditioned place preference (Hemby et al., 1992) (Delfs et al., 1990). Cocaine was self-administered into the medial prefrontal cortex but not into NAc or VTA (Goeders and Smith, 1983). Therefore, further studies are needed to clarify the roles of different brain regions in mediating the effects of cocaine and other drugs. AAV mediated wild type DAT expression in selected brain regions of DAT-CI mice provide a tool to test directly how cocaine inhibition of DAT in various brain regions contributes to different behavioral responses induced by cocaine. Recombinant AAV vectors can infect a wide range of cells, including non-dividing cells such as neurons. AAV vector genomes can persist in circular episomal forms in the host cell nucleus and often remain intact for the life of the host cells (Carter, 2004). AAV injection in DA neuron projection areas such as CPu or NAc will result in DAT expression mostly in neurons and cells other than DA neurons which have their nucleus located far away from the injection sites. AAV mediated DATwt expressions in the CPu or NAc do not contribute to the recycling of released DA back to DA terminals but they contribute to the clearance of extracellular DA (O’Neill et al., 2014), and thus should contribute to DA signaling and cocaine responses.
We first examined the CPu and the NAc. We injected AAV-DATwt into these two regions separately but none of the injections restored cocaine stimulation of locomotion or cocaine reward (O’Neill et al., 2014). We then targeted both the ventral and dorsal striatum covering parts of NAc and CPu in a single injection but multiple infusions of viruses with the injection needle at different dorsal/ventral levels. The combined NAc and CPu injection restored cocaine stimulation but not cocaine reward (O’Neill et al., 2014). The result suggests that both NAc and CPu are involved in mediating cocaine stimulation.
None of the AAV-DATwt injections described above restored cocaine reward, which suggests that cocaine reward may require cocaine inhibition of DAT in multiple brain regions. Therefore, in the current study we expanded the targeted regions to cover the olfactory tubercle (Tu) and the ventral midbrain (vMB) in addition to NAc and CPu. The ventral striatum comprises the NAc and the Tu which is a multi-sensory processing center. It has been shown that rats self-administer cocaine into the Tu and infusions of cocaine directly into the Tu but not ventral pallidum or nucleus accumbens induces conditioned place preference (Ikemoto, 2003). It was proposed that the Tu is an integral part of the reward/motivation behavior system (Ikemoto, 2007). We also included VTA and SN because DAT is also expressed in these regions taking up dendritically released DA (Nirenberg, 1997). It has been shown that blockade of D1 receptors in the VTA or in the SN reduces the rewarding effects of cocaine, indicating the contribution of DA signaling in these regions (Ranaldi and Wise, 2001, Quinlan et al., 2004). The spread of AAV injection in vMB covers both the substantia nigra (SN) and the ventral tegmental area (VTA) (Figure 2B).
AAV-DATwt injections in the combined regions restored both cocaine stimulation and cocaine reward, indicating that cocaine inhibition of DAT in the combined regions is sufficient for both cocaine effects. The AAV mediated DAT expressions are mainly around the virus perfusion sites (Figure 2). However, it is possible that some DAT may be transported along axons and expressed in brain regions away from the injection sites. This is quite likely for AAV-DATwt injection in VTA/SN where DA neuron cell bodies are located and DA neurons have the endogenous mechanism to target DAT to the axon terminals in projected areas (Giros et al., 1991) (Giros et al., 1991, Kitayama et al., 1992, Shimada et al., 1992, Lee et al., 1996). Indeed, AAV-DATwt injections in the VTA and SN resulted in wide spread expression of HA-tagged wild type DAT in the DA neuron projection areas including NAc, CPu, Tu (Figure 4), and likely other dopaminergic projection areas. We tested the behaviors of DAT-CI mice with AAV-DATwt injections in the ventral midbrain alone and found that cocaine stimulation and cocaine reward were both restored in these mice. This is due to wild type DAT expression in the ventral midbrain and, likely more importantly, the distribution of the wild type DAT to the DA terminals in DA neuron projection areas.
5. Conclusions
We used AAV vector to re-introduce the cocaine-sensitive wild type DAT back into selective brain regions of fully developed adult DAT-CI mice that carry a cocaine-insensitive mutant DAT. In virus affected regions, the mutant DAT and wild type DAT both contribute to DA homeostasis similar to the situation in heterozygous DAT-CI mice. We found that both the stimulating and rewarding effects of cocaine can be restored in DAT-CI mice by AAV-DATwt injection in the ventral midbrain. This result indicate that the lack of cocaine stimulation and rewarding effects in DAT-CI mice is not due to adaptive changes but due to the lack of cocaine inhibition of DAT. This strengthens our conclusion that cocaine inhibition of DAT is necessary for the rewarding and stimulating effects of cocaine.
AAV injection in the vMB results in DATwt expression in ventral and dorsal striatum;
AAV-DATwt expression in adult DAT-CI mice restores cocaine locomotor stimulation.
AAV-DATwt expression also restores cocaine reward;
Cocaine inhibition of DAT is necessary for cocaine reward and simulation.
Acknowledgements
The authors would like to acknowledge Pauline Chen, and Michael Chee for their assistance with the mouse colony; and the NIDA drug supply program for cocaine and other reagents. This work was supported by grants from the NIH (DA014610 and DA20124) to HHG and by State Scholarship Fund from China Scholarship Council to HW.
Abbreviations
- 5-HT
serotonin
- AAV
adeno-associated virus
- AAV-DATwt
adeno-asssociated virus carrying cocaine-sensitive wild type DAT
- CPP
conditioned place-preference
- CPu
Caudate Putamen
- DA
dopamine
- DAT
dopamine transporter
- DAT-CI mice
cocaine-insensitive dopamine transporter knock-in mice
- HA-DAT
hemagglutinin-tagged DAt
- IHC
immunohistochemistry
- NAc
nucleus accumbens
- NE
norepinephrine
- SERT
serotonin transporter
- SN
substantia nigra
- Tu
olfactory tubercle
- VTA
ventral tegmental area
- vMB
ventral midbrain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Mayfield RD, Curella P, Zahniser NR. Differences in dopamine clearance and diffusion in rat striatum and nucleus accumbens following systemic cocaine administration. J Neurochem. 1992;59:259–266. doi: 10.1111/j.1471-4159.1992.tb08899.x. [DOI] [PubMed] [Google Scholar]
- CARTER BJ. Adeno-associated virus and the development of adeno-associated virus vectors: A historical perspective. Mol. Ther. 2004;10:981–989. doi: 10.1016/j.ymthe.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci U S A. 2006;103:9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KR, Liu X, McGrath JP, Johnson PR. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- Giros B, el Mestikawy S, Bertrand L, Caron MG. Cloning and functional characterization of a cocaine-sensitive dopamine transporter. FEBS Letters. 1991;295:149–154. doi: 10.1016/0014-5793(91)81406-x. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Gu H, Wall SC, Rudnick G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. Journal of Biological Chemistry. 1994;269:7124–7130. [PubMed] [Google Scholar]
- Hall FS, Li XF, Sora I, Xu F, Caron M, Lesch KP, Murphy DL, Uhl GR. Cocaine mechanisms: enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience. 2002;115:153–161. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Jones GH, Justice JB, Jr., Neill DB. Conditioned locomotor activity but not conditioned place preference following intra-accumbens infusions of cocaine. Psychopharmacology (Berl) 1992;106:330–336. doi: 10.1007/BF02245413. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Monaco AP, Hernandez L, Aulisi EF, Stanley BG, Lenard L. Self-injection of amphetamine directly into the brain. Psychopharmacology (Berl) 1983;81:158–163. doi: 10.1007/BF00429012. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci. 2003;23:9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama S, Shimada S, Xu H, Markham L, Donovan DM, Uhl GR. Dopamine transporter site-directed mutations differentially alter substrate transport and cocaine binding. Proc Natl Acad Sci U S A. 1992;89:7782–7785. doi: 10.1073/pnas.89.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Circuits, drugs, and drug addiction. Adv Pharmacol. 1998;42:978–982. doi: 10.1016/s1054-3589(08)60910-2. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Pristupa ZB, Ciliax BJ, Levey AI, Niznik HB. The dopamine transporter carboxyl-terminal tail. Truncation/substitution mutants selectively confer high affinity dopamine uptake while attenuating recognition of the ligand binding domain. J Biol Chem. 1996;271:20885–20894. doi: 10.1074/jbc.271.34.20885. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther. 1999;291:999–1007. [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. Immunogold localization of the dopamine transporter: an ultrastructural study of the rat ventral tegmental area. J Neurosci. 1997;17(14):5255–62. doi: 10.1523/JNEUROSCI.17-14-05255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill B, Gu HH. Amphetamine-induced locomotion in a hyperdopaminergic ADHD mouse model depends on genetic background. Pharmacol Biochem Behav. 2013;103:455–459. doi: 10.1016/j.pbb.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill B, Tilley MR, Gu HH. Cocaine produces conditioned place aversion in mice with a cocaine-insensitive dopamine transporter. Genes Brain Behav. 2013;12:34–38. doi: 10.1111/j.1601-183X.2012.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill B, Tilley MR, Han DD, Thirtamara-Rajamani K, Hill ER, Bishop GA, Zhou FM, During MJ, Gu HH. Behavior of knock-in mice with a cocaine-insensitive dopamine transporter after virogenetic restoration of cocaine sensitivity in the striatum. Neuropharmacology. 2014;79:626–633. doi: 10.1016/j.neuropharm.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan MG, Sharf R, Lee DY, Wise RA, Ranaldi R. Blockade of substantia nigra dopamine D1 receptors reduces intravenous cocaine reward in rats. Psychopharmacology (Berl) 2004;175:53–59. doi: 10.1007/s00213-003-1771-9. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Wise RA. Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: possible role for dendritically released dopamine. J Neurosci. 2001;21:5841–5846. doi: 10.1523/JNEUROSCI.21-15-05841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux A, Fabre V, Lesch KP, Moessner R, Murphy DL, Lanfumey L, Hamon M, Martres MP. Adaptive changes of serotonin 5-HT2A receptors in mice lacking the serotonin transporter. Neurosci Lett. 1999;262:113–116. doi: 10.1016/s0304-3940(99)00049-x. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice [see comments] [published erratum appears in Nat Neurosci 1998 Aug;1(4):330] Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Shimada S, Kitayama S, Walther D, Uhl G. Dopamine transporter mRNA: dense expression in ventral midbrain neurons. Brain Res Mol Brain Res. 1992;13:359–362. doi: 10.1016/0169-328x(92)90220-6. [DOI] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci U S A. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surratt CK, Wang JB, Yuhasz S, Amzel M, Kwon HM, Handler JS, Uhl GR. Sodium- and chloride-dependent transporters in brain, kidney, and gut: lessons from complementary DNA cloning and structure-function studies. Current Opinion in Nephrology & Hypertension. 1993;2:744–760. doi: 10.1097/00041552-199309000-00008. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2009;331:204–211. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JB, King JS, Bishop GA. Stimulation of the inferior olivary complex alters the distribution of the type 1 corticotropin releasing factor receptor in the adult rat cerebellar cortex. Neuroscience. 2008;153:308–317. doi: 10.1016/j.neuroscience.2008.01.076. [DOI] [PubMed] [Google Scholar]
- Tilley MR, Cagniard B, Zhuang X, Han DD, Tiao N, Gu HH. Cocaine reward and locomotion stimulation in mice with reduced dopamine transporter expression. BMC Neurosci. 2007;8:42. doi: 10.1186/1471-2202-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley MR, Gu HH. Dopamine transporter inhibition is required for cocaine-induced stereotypy. Neuroreport. 2008a;19:1137–1140. doi: 10.1097/WNR.0b013e3283063183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley MR, Gu HH. The effects of methylphenidate on knockin mice with a methylphenidate-resistant dopamine transporter. J Pharmacol Exp Ther. 2008b;327:554–560. doi: 10.1124/jpet.108.141713. [DOI] [PubMed] [Google Scholar]
- Tilley MR, O’Neill B, Han DD, Gu HH. Cocaine does not produce reward in absence of dopamine transporter inhibition. Neuroreport. 2009;20:9–12. doi: 10.1097/WNR.0b013e32831b9ce4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gu HH. Cocaine affinity decreased by mutations of aromatic residue phenylalanine 105 in the transmembrane domain 2 of dopamine transporter. Molecular Pharmacology. 2003;63:653–658. doi: 10.1124/mol.63.3.653. [DOI] [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]