Abstract
Major depressive disorder (MDD) is a debilitating disease with symptoms like persistent depressed mood and sleep disturbances. The prefrontal cortex (PFC) has been implicated as an important structure in the neural circuitry of MDD, with pronounced abnormalities in blood flow and metabolic activity in PFC subregions, including the subgenual cingulate cortex (sgACC, or Brodmann area 25). In addition, deep brain stimulation in the sgACC has recently been shown to alleviate treatment-resistant depression. Depressed patients also show characteristic changes in sleep: insomnia, increased rapid-eye-movement (REM) sleep and shortened REM sleep latency. We hypothesized that sleep changes and depressive behavior may be a consequence of the abnormal PFC activity in MDD. The rat ventromedial PFC (vmPFC, prelimbic and infralimbic cortices) is considered to be the homolog of the human sgACC, so we examined the effect of excitotic lesions in the vmPFC on sleep-wake and depressive behavior. We also made lesions in the adjacent dorsal region (dmPFC) to compare the effect of this similar but distinct mPFC region. We found that both dmPFC and vmPFC lesions led to increased REM sleep, but only vmPFC-lesioned animals displayed increased sleep fragmentation, shortened REM latency and increased immobility in the forced swim test. Anatomic tracing suggests that the mPFC projects to the pontine REM-off neurons that interact with REM-on neurons in the dorsal pons. These results support our hypothesis that neuronal loss in the rat vmPFC resembles several characteristics of MDD and may be a critical area for modulating both mood and sleep.
Keywords: Ibotenic acid, REM sleep latency, forced swim test, neural circuit
Introduction
Major Depressive Disorder (MDD) is one of the leading causes of disability worldwide and continues to increase in prevalence (World Health Organization, 2002, 2012). MDD is diagnosed by the presence of at least five symptoms, which must include depressed mood or anhedonia (Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), 1994). Despite the prominence of MDD and other mood-related disorders, its etiology remains unclear.
MDD likely involves dysfunction in a network of structures in the brain. Based on human imaging studies, this network is believed to include regions of the limbic system: the prefrontal cortex, hippocampus, nucleus accumbens, and amygdala (Nestler et al, 2002). The medial prefrontal cortex (mPFC) has been of particular interest because subregions of the mPFC show marked changes in neural activity in depressed patients compared to healthy controls. For instance, the ventral mPFC (vmPFC) of MDD patients exhibits increased cerebral blood flow (Drevets et al, 1992) and metabolism (Mayberg, 1997), while the subgenual anterior cingulate cortex (sgACC) (Drevets et al, 1997; Jahn et al, 2010; Ongür et al, 1998; van Tol et al, 2010), shows increases in glucose metabolism in depression (Drevets et al, 1997, 2008) that normalize with successful antidepressant treatment, including deep-brain stimulation near this area (Mayberg et al, 2000, 2005). In addition, responders to acute sleep deprivation exhibited decreased activity in the ventral (including subgenual) region of the anterior cingulate cortex (Clark et al, 2006). The involvement of the mPFC in MDD suggests a key role for this structure in regulating the affective and behavioral aspects of depression.
Recent animal studies have also implicated the mPFC as a possible key site for depression etiology. Chronic stress protocols, which induce depression-like behaviors, led to decreased glial cell count (Banasr and Duman, 2008) and dendritic atrophy in this region (Brown, 2005). Glial cell ablations in the rat mPFC produced depression-like behaviors, including decreased sucrose preference and increased immobility in the forced swim test (Banasr and Duman, 2008). Excitotoxic neuronal lesions in the rat mPFC led to ‘learned helplessness’ upon exposure to inescapable footshocks (Klein et al, 2010). Interestingly, deep brain stimulation in the mPFC induced an antidepressant-like response in the forced swim test (Hamani et al, 2010), and optogenetic stimulation in the mPFC of mice susceptible to social defeat stress increased their sucrose preference (Covington et al, 2010), although 100 Hz stimulation may be mostly inhibitory.
These studies suggest the mPFC may regulate a variety of depressotypic behaviors, including abnormal sleep. Depressed patients complain of sleep disturbances and have increases in REM sleep, along with decreased REM latency (the interval of time between sleep onset and REM sleep onset; Berger and Riemann, 1993; Reynolds and Kupfer, 1987; Steiger and Kimura, 2010), and many antidepressant drugs suppress REM sleep (Rijnbeek et al, 2003). Animals that undergo a chronic mild stress protocol demonstrate changes in sleep similar to those observed in depressed humans (Grønli et al, 2004). Furthermore, several longitudinal studies confirm that poor sleep quality and decreased (or significantly increased) quantity of sleep increases the risk of developing an affective disorder (Ford and Kamerow, 1989; Hohagen et al, 1993; Katz and McHorney, 1998), regardless of family history. Because the basic structure of sleep is similar in humans and rodents, and because the underlying circuitry regulating sleep is also thought to be similar, sleep disturbances represent an important marker for testing the role of neural circuits in depression. We hypothesized that prefrontal cortex dysfunction may be critical in producing depression-associated behaviors, including changes in sleep patterns. Previous studies have described conflicting roles of the rat mPFC subregions, the anterior cingulate (dmPFC) and the prelimbic/infralimbic (vmPFC) cortices (Bissiere et al, 2006; Hamani et al, 2010; Klein et al, 2010; Scopinho et al, 2010). As the vmPFC especially projects to limbic areas involved in the control of emotion (Sesack et al, 1989) and has thus been suggested to be the homolog of the human sgACC, we hypothesized that this region in particular may play a key role in depressotypic behavior and sleep changes in rodents. To test this hypothesis, we made neuronal lesions in the ventral and dorsal subdivisions of the medial prefrontal cortices of rats, measured their sleep behaviors, and tested the animals for depressive-like behavior in the forced swim test (FST) (Detke et al, 1995; Porsolt et al, 1977). We subsequently propose a model of sleep modulation via the prefrontal cortex.
General Experimental Design
In our experiments, adult male rats were surgically lesioned in two different regions of the medial prefrontal cortex. Saline-injected animals were used as controls. EEG and EMG electrodes were also surgically implanted, and sleep-wake behavior was observed for the experimental groups and controls to observe the effects the lesions had on the animals’ sleep and REM sleep latency (a measure often used in human and rodent depression studies). In addition, the forced swim test was performed to investigate the rats’ behavior with respect to a standard rodent depression model.
Methods and Materials
Animals
All animals used were pathogen-free adult male Sprague-Dawley rats (350-400g) purchased from Taconic (Hudson, NY). They were housed in individual cages (Allentown Inc, New Jersey) in rat-specific holding rooms controlled for temperature (22±1°C) and humidity. Food (Cat. No 5008, Formulab, USA) and water were available ad libitum, and lights were automatically switched on and off according to a 12:12 L:D cycle (lights on 8:00am – 8:00pm). The animals were cared for in accordance with National Institutes of Health standards, and all procedures were pre-approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
Animal Surgery and EEG/EMG Implantation
Prior to surgery, animals were anesthetized with ketamine-xylazine (i.p., 800 mg/kg ketamine, 80 mg/kg xylazine, Med-Vet, Mettawa, IL) and then placed in a stereotaxic frame so that their head was fixed. Injections of ibotenic acid (IBO, Tocris, Ellisville, MO), 0.9% saline (Med-Vet, Mettawa, IL) or neural tracer (BD, Molecular Probes, Grand Island, NY or CTB, List Biological, Campbell, CA) were administered directly into the brain using a fine glass pipette (1 mm glass stock, tapering slowly to a 10-20 μm tip) connected to an air compression system. A series of 20-40psi puffs of air were used to deliver the compounds into and with the following coordinates and volumes: dmPFC: AP+3.0 mm to 3.5 mm DV-1.4 mm RL+/−0.6 mm, 66nL 1 - 5% IBO; vmPFC: AP+3.0 mm to 3.5 mm, DV-3.4 mm RL+/−0.6 mm, 66-99nL 5% IBO, 16.5nL 1% CTB; vlPAG: AP-7.2 mm, DV-5.6 mm, RL-1.2 mm, 33nL 1% CTB; SLD: AP-9.4 mm, DV-6.3 mm, RL-1.2 mm, 16.5nL 1% CTB (Paxinos and Watson, 2007).
On the same day some of the animals received four EEG screw electrodes (Plastics One, Roanoke, VA) that were screwed into skull (Two front screws at AP=0.0 mm, ML= 3.0 mm; two rear screws at AP = −2.0 mm, ML=3.0 mm), and two flexible EMG wire electrodes were also placed on the left and right nuchal muscles. The free ends of the leads were placed in a plastic electrode pedestal that was cemented onto the skull using Jet Denture Repair Powder and Jet Liquid (Henry Schein, Melville, NY). Any animals that did not receive electrodes had their incision closed with wound clips. Upon completion of the procedure, animals were given a subcutaneous injection of the analgesic meloxicam (1.0 mg/kg, Med-Vet, Mettawa, IL) and allowed to recover on a warm plate until awakened from anesthesia.
Sleep Recordings and Analysis
Rats typically resume visibly normal activity within 48 hours post surgery. In our experiments animals were given at least a week of post-surgical recovery before being placed in isolated recording chambers in preparation for sleep recordings. Flexible cables that were mounted to fixed commutators were attached to the electrode pedestals, and the cages were placed such that the animals could move freely. As before, food and water were available ad libitum, ambient temperature was controlled, and the light:dark cycle was 12:12. Video cameras were placed to capture movement in the entire cage, and the animals were habituated without disturbance for at least two days and then recorded for 48h using VitalRecorder (Kissei Comtec Co., Nagano, Japan). Upon completion of the recordings, animals were detached from the cables and returned to the holding room.
The EEG/EMG recordings were analyzed using SleepSign (Kissei Comtec Co., Nagano, Japan). The recordings were divided into 12s epochs and each epoch scored manually as wake, REM, or NREM sleep. Wake was identified by high-frequency, desynchronized EEG accompanied by frequent EMG activity and observed behaviors on the video playback. Deep NREM sleep was identified by the dominant presence of high-amplitude, low-frequency (<4 Hz) EEG activity and little muscle tone on the EMG recording, and light sleep was identified by low EMG and sleep posture (video) as well as slow EEG. REM sleep was identified by theta waves (4-7 Hz) of consistent low amplitude on the EEG recording accompanied by very low EMG activity (Video recordings were used to confirm active/inactive states and are especially critical in identifying quiet wake and light sleep). REM sleep latency was defined as the interval of time between sleep onset and REM sleep onset averaged over 24 hours. This is the standard definition used for rodents, in contrast to a single latency measure (as for humans), because rodents do not have as consolidated sleep as humans. Sleep-wake percentages, bout numbers, bout durations and REM latency were analyzed using unpaired t-test and adjusted using Bonferroni’s correction, using a significance threshold p<0.05.
Forced swim test
The forced swim test procedure was conducted as described previously (Castagn’ et al, 2011; Detke et al, 1995). The test was conducted over two days using acrylic cylinders (20cm x 40cm; Northeast Plastics, Philadelphia, PA) that were filled 30 cm with 25°C water. On the first day, animals were placed in the cylinder for 15 min while being video recorded using a computer running Ethovision (Noldus, Leesburg, VA). The animals were subsequently removed and gently handled and dried before being returned to their cages. On the second day, the swim test was repeated for 5 min. All animals were habituated to the room at 12:00 and the swim tests were completed between 13:00 and 15:00 to limit any circadian influences. Total amounts of immobility during the 5-min test session were scored using Ethovision and parameters validated for rats (EthoVision, 2004) (sampling rate 5 Hz; immobility threshold 11.5%).
Statistical Analysis of Forced Swim Test
FST immobility between groups were analyzed using unpaired t-test and adjusted using Bonferoni’s correction, using a significance threshold p<0.05. Both dmPFCx and vmPFCx lesion groups were included in the correlation analysis comparing FST and REM sleep latency.
Perfusion and fixation
Animals were anesthetized with 7% chloral hydrate (i.p. 500 mg/kg, Sigma, St. Louis, MO). The body cavity was opened using surgical scissors and a 16G needle was inserted into the left ventricle of the heart. The top of the right atrium was cut to allow blood to be drained. About 100 mL of saline was flushed through the vascular system using an intravenous line, followed by 500mL of 10% buffered formalin (Fisher Scientific, Pittsburgh, PA). Upon fixation of the tissue the brain was removed from the skull and stored in 10% formalin for 4-5 hours. The brains were then moved to 20% sucrose and 0.02% azide solution overnight.
Histology and Immunohistochemistry
Brains were sliced into four series of 40um sections using a freezing microtome. The sections were stored in PBS-0.02% azide in 20°C.
Immunohistochemical staining was completed as follows: tissue sections were rinsed in PBS three times, 3-5 min each. They were then incubated for 30 min in 0.3% H2O2 (Sigma, St. Louis, MO) in PBT (phosphate buffer with Triton X-100; Sigma, St. Louis, MO) to oxidize any remaining blood. The sections were again rinsed in PBS and then incubated in primary antibody diluted in PBT-Azide for 1-2 nights, depending on the antibody (NeuN, MAB377, mouse monoclonal, 1:20,000, Chemicon, Billerica, MA). Tissue were then rinsed in PBS three times, and incubated in secondary antibody (1:1000, biotin SP-conjugated against appropriate species IgG, Jackson ImmunoResearch Laboratories, West Grove, PA) for 60-90 min. Sections were again rinsed in PBS and placed in ABC solution (1:1000 each Vectastain solutions A and B, Vector Laboratories, Burlingame, CA) for 60-90 min. Sections were rinsed in PBS and stained for 5 min in a solution consisting of: 1% DAB, 0.3% H2O2 (and 0.01% Ni, 0.005% CoCl2 if desired a black stain). Staining procedure for BD started with ABC solution because the tracer is biotinylated. Tissue were then rinsed in PBS and mounted on microscope slides in gelatin.
Slides were counterstained by placing them in ddH2O for 5 min, followed by 10-30 sec in 0.1% thionin (Sigma, St. Louis, MO). The slides were dehydrated step-wise by incubating in 50% EtOH, 70% EtOH, 95% EtOH, and 100% EtOH for 2 min each. Slides were then placed in xylene for several hours before covering with glass coverslips.
For CTB and BD double immunofluorescence staining, sections were rinsed and incubated in primary antibody as stated previously. Following rinses in PBS, sections were incubated in Alexa Fluor 488 (A11055, anti-goat, 1:1000, Molecular Probes, Grand Island, NY) and Cy3-conjugated streptavidin (016-160-084, 1:500, Jackson ImmunoResearch Laboratories, West Grove, PA). Sections were rinsed and mounted on microscope slides under dim light. The fluorescent cells were imaged on a confocal microscope (Zeiss, Thornwood, NY) at 63x magnification, at a single optical layer.
Results
Lesion results
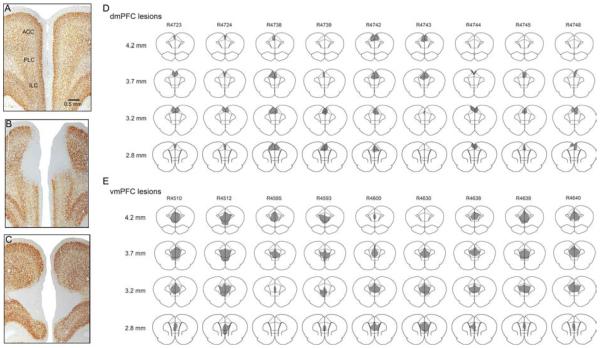
Bilateral ibotenic acid lesions were placed in the dorsal (anterior cingulate cortex, dmPFCx) or ventral mPFC (infralimbic and prelimbic cortex, vmPFCx) of adult male Sprague-Dawley rats. Photomicrographs of the lesions are shown in Fig. 1A-C, and schematic drawings of lesions in a representative set of brains are shown in Fig. 1D-E. Lesions that significantly extended into the premotor cortex (M2) or orbital frontal cortex were excluded from the analyses (11 animals excluded), resulting in n=9 for dmPFCx group and n=24 for vmPFCx group. Control animals received injections of 0.9% saline into the vmPFC (n=10).
Figure 1.
Histology of mPFC lesion and control. mPFC histology of saline-injected control (A), ibotenic acid injection in dmPFC (B) and vmPFC (C).. Bregma +3.5mm. D and E. Lesion areas in representative cases from each lesion group. Levels indicated on the left are with respect to Bregma.
Cell body-specific lesions of the rat mPFC increase REM sleep and sleep fragmentation
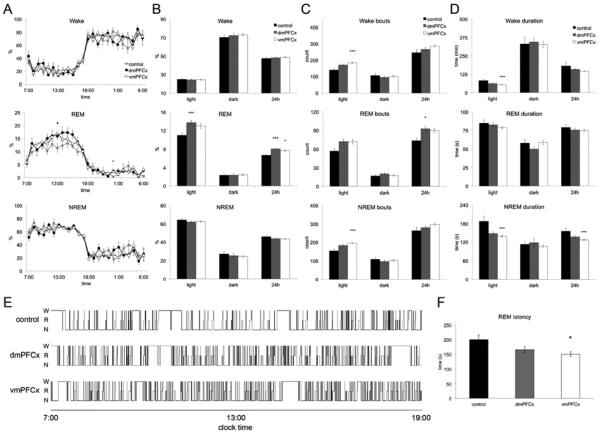
Repeated measures ANOVA was used to determine any differences in sleep-wake activity between the dmPFC-lesioned, vmPFC-lesioned, and control groups. No differences were observed in wake (F(2,40) = 0.474, p>0.05) or NREM sleep (F(2,40) = 1.421, p>0.05). REM sleep amount was significantly different between the three groups (F(2,40) = 4.950, p<0.05). Pairwise comparisons demonstrate that both dmPFC- and vmPFC-lesioned groups had significantly more REM sleep than the control group (p<0.05 for both) but were not different compared to each other.
Repeated measures ANOVA was also used to compare sleep-wake bout durations during the light and dark phase, as well as over 24 hours, between the three groups. Bout counts between the groups were determined to be different over the 24 hour timeframe F(2,40)=4.289, p<0.05 and during the light phase (inactive phase) F(2,40)=12.945, p<0.001, but not during the dark phase F(2,40)=0.272. The pairwise comparisons showed that over 24 hours, bout numbers were different for just vmPFC-lesioned animals compared to controls p<0.05. During the light phase, both experimental groups were different compared to controls (p<0.05, p<0.001 for dmPFC-lesioned and vmPFC-lesioned groups, respectively). Similarly, in examining the average duration of bouts, bout numbers were found to be different between the groups over the 24-hour window F(2,40)=4.617, p<0.05 and during the light phase F(2,40)=11.019, p<0.001, but not during the dark phase, F(2,40)=0.072. Again, the difference over 24 hours was due to the vmPFC-lesioned animals compared to the control group, whereas the difference during the light phase can be attributed to both experimental groups (p<0.05 and p<0.001).
Animals in the dmPFC-lesioned group were found to have a 20.7% increase in REM sleep time compared to controls, t(17)=3.95, p<0.001 (Fig. 2B). During the light phase, none of the individual measures of wake, REM sleep, or NREM sleep bout or were significantly different than in control animals. In addition, animals with dmPFC lesions did not have significantly altered REM sleep latency compared to control animals, t(17)=1.04, p>0.05.
Figure 2.
Summary of the sleep-wake behavior for the dmPFCx, vmPFCx and control group. A. The average wake, REM, and NREM sleep per hour over 24h shows a trend in increased REM sleep in both experimental groups. Symbols indicate significance using Bonferroni adjusted p-values: ‘*’, p<0.05 for dmPFCx group; ‘+’, p<0.05 for vmPFCx group. B, C, D. Percentage of time, bout numbers, and average bout duration of wake, REM, and NREM sleep are summarized for each the light phase, dark phase, and over 24h. Asterisks indicate p-values: ‘*’, p<0.05; ‘***’, p<0.005. E. Hypnograms of individual cases from each group exemplify increased fragmentation (shorter and increased number of bouts) during the rest (lights on) period in the lesion groups compared to controls. F. The vmPFCx, but not the dmPFCx, animals had shortened REM latency compared to controls. This measure was calculated by averaging the interval of time between the onset of NREM sleep and REM sleep for each sleep episode over 24h. ‘*’, p <0.05.
Rats with lesions to the vmPFC had a 16% increase in REM sleep compared to sham-lesion controls, t(31)=2.05, p<0.05, and REM sleep bout number increased compared to controls but did not reach statistical significance (t(31)=2.05, p=0.057). The vmPFC-lesioned animals had 33.5% shorter wake bouts, t(31)=3.23, p<0.001, and 26.0% shorter NREM sleep bouts, t(31)=3.86, p<0.001, during the light period (Fig. 2B), and 18.3% shorter NREM bouts over a 24 hour period compared to sham-lesioned animals, t(31)=3.23, p<0.001 (Fig. 2D). In addition, wake and NREM sleep average bout durations were significantly shorter during the light phase for vmPFC-lesioned animals (t(31)=3.61, p<0.05 and t(31)=3.86, p<0.001). These results overall suggest increased sleep fragmentation during the light period (typically rest period) for vmPFC-lesioned animals, which is also observed in the hypnograms in Fig. 2E. In addition, in contrast to dmPFC-lesioned animals, vmPFC-lesioned animals had significantly reduced (24.7%) REM sleep latency, t(31)=2.69, p<0.005 (Fig. 2F). While this result achieves statistical significance, it should be noted that the difference in group size between the dmPFC-lesioned and vmPFC-lesioned groups is large and may bias these data. Despite this, altogether the results from the sleep-wake analyses suggest that the ventral and dorsal mPFC both influence REM sleep amount, but only lesions in the vmPFC lead to a pronounced increase in sleep fragmentation and shortened REM sleep latency.
Cell body-specific lesions of the rat vmPFC increase immobility in the FST
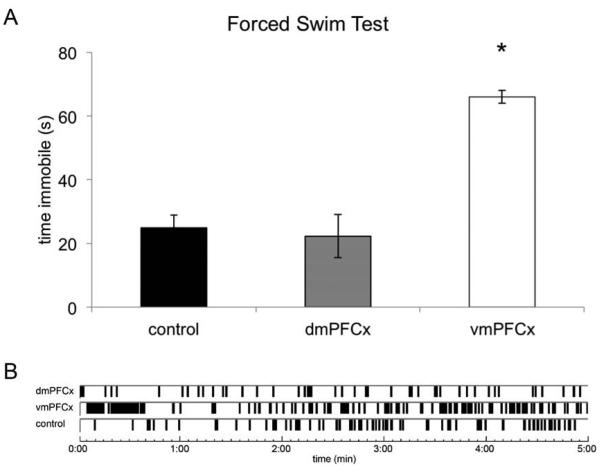
To investigate if the dmPFC or vmPFC may modulate depression-like behaviors other than sleep, we tested the lesioned and sham-lesioned animals under the forced swim test (FST) paradigm, a model of depression in which immobility is believed to be indicative of a depression-like state (Porsolt et al, 1977). vmPFC-lesioned animals had 165.3% more immobility time compared to sham-lesioned animals (t(26)=2.66, p=0.004), while dmPFC-lesioned animals were not statistically different from controls (t(11)=-1.27, p>0.05; Fig. 3). It has been shown that lesions in the rat mPFC do not impair locomotor activity (Klein et al, 2010), and we confirmed during the day 1 swim session that neither experimental group displayed movement deficits.
Figure 3.
A. Average time animals in each experimental group were immobile during the forced swim test. The vmPFCx, but not the dmPFCx animals had increased immobility compared to control animals. ‘*’, p <0.05. B. Example schematic of immobility bouts during the forced swim test as scored by Ethovision. The software compares dynamic pixel changes frame-to-frame, and if less than the threshold percentage of pixels differs between frames the animal is considered immobile. Parameters were validated by manual scorers (EthoVision, 2004).
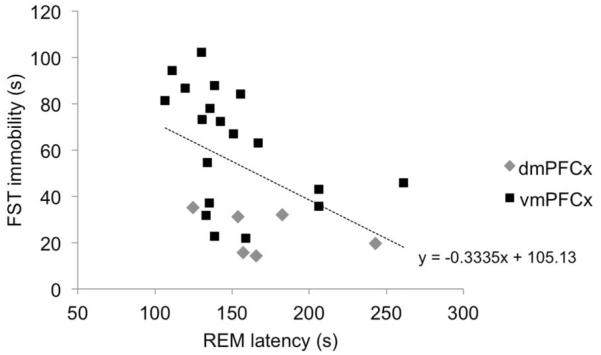
As REM sleep latency and FST immobility are both characteristic of depression-like states, we examined whether the severity of these behaviors were associated within the lesioned animals. FST immobility time and REM sleep latency were significantly negatively correlated (r=-0.464, p=0.019; Fig. 4). Animals with less REM sleep latency also had greater FST immobility time, suggesting that these two measures may share common neural bases. Correlation analysis of the dmPFC- (n=7) and vmPFC-lesioned (n=19) separately does not reveal significant correlations (p>0.05), although this may be due to the small sample sizes or variance in the lesions. The negative relationship between REM latency and FST immobility in the larger vmPFC-lesioned group nearly reaches statistical significance (r=-0.445, p=0.056).
Figure 4.
The time an animal was immobile during the FST was plotted v. their REM latency measure during sleep. A significant correlation between these measures was found (R=-0.464, p=0.019), suggesting a possible biological relationship between these two measures.
No relationship was found between lesion area and FST immobility time nor lesion area and REM sleep latency in either experimental group. However, this is not unexpected as quality and placement of the lesions are likely just as (or more) important as lesion size, but those two parameters are challenging to characterize quantitatively.
Neural circuit of mPFC regulating REM sleep
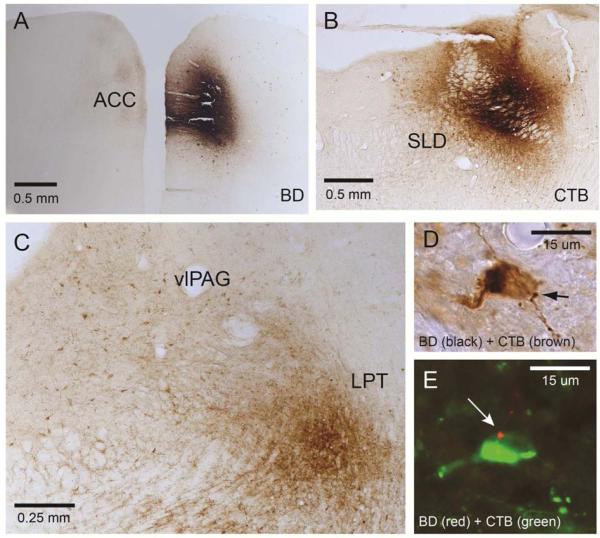
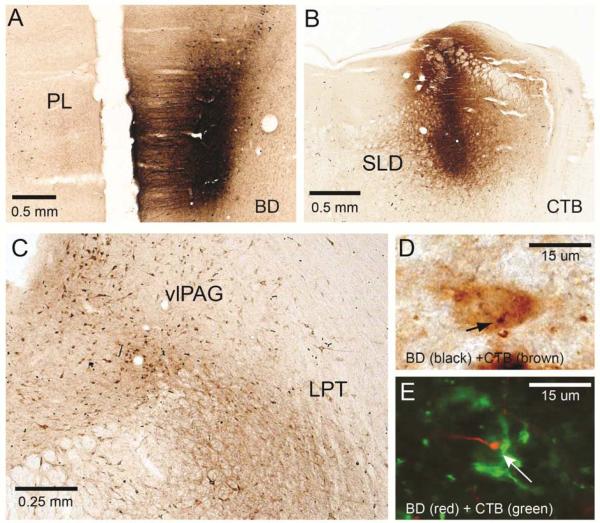
To determine the neuroanatomic substrate of REM sleep suppression by the vmPFC and dmPFC, we examined the projections from these regions to REM sleep control regions. Previous work has shown that the ventrolateral periaqueductal gray and adjacent lateral pontine tegmentum (vlPAG-LPT) contain GABAergic neurons that inhibit the sublaterodorsal nucleus (SLD), which promotes REM sleep (Lu et al, 2006), suggesting that the vlPAG-LPT suppresses REM sleep. To investigate whether each mPFC region may be modulating sleep via direct projections to this REM control site, we injected the anterograde tracer biotin dextran (BD) into the vmPFC (Fig. 5) or dmPFC (Fig. 6) and retrograde tracer cholera toxin B (CTB) into the SLD in the same animals (n=4 each).
Figure 5.
The projections of the mPFC to REM sleep circuit. To investigate a possible pathway by which the dmPFC may be modulating REM sleep, two tracers were injected (both unilaterally) into an individual animal: A BD, an anterograde tracer, into the dmPFC (Bregma 3.5mm); B CTB, a retrograde tracer, into the REM-on SLD (Bregma −9.4mm). C Cells in the vlPAG-LPT (Bregma −7.2mm) were then sought that were stained for CTB (brown) and also had BD boutons (black) (D). E. A series from the same case was stained with CTB (AlexaFluor488 green) and BD (Cy3 red) and viewed under a confocal microscope (63x). A cell in the vlPAG is stained green, indicating it projects to the SLD area, and has a red bouton from the vmPFC (arrow). This image was taken in a single optical plane.
Figure 6.
As in Fig. 5, two tracers were injected into an individual animal: A BD, an anterograde tracer, into the vmPFC (Bregma 3.5mm); B CTB, a retrograde tracer, into the REM-on SLD (Bregma −9.4mm). C Cell bodies in the vlPAG-LPT (Bregma −7.2mm) were then sought that were stained for CTB (brown) and also had BD boutons (black) (D). E. A series from the same case was stained with CTB (AlexaFluor488, green color) and BD (CY3 red color) and viewed under a confocal microscope (63x). A cell in the vlPAG is stained green, indicating it projects to the SLD area, and has a red bouton from the vmPFC (arrow). This image was taken in a single optical plane.
Of the cell bodies in the vlPAG-LPT that were labeled with the retrograde tracer in the SLD, and thus project to the SLD, 24.2% also had appositions stained from the anterograde tracer in the dmPFC, while 16.0% had appositions from the vmPFC. A series from each case was labeled with fluorescent antibodies and viewed under a confocal microscope to confirm the appositions (Figs. 5E and 6E). These results suggest that the mPFC innervates some neurons in the REM-off vlPAG-LPT that also project to the REM-on SLD. As the mPFC projection is glutamatergic, lesions of the mPFC would be expected to disinhibit REM sleep, consistent with the increased REM sleep found in both vmPFC and dmPFC lesions.
Discussion
While previous studies identified the mPFC as an important region underlying MDD, how this region affected depression-associated behaviors was unknown. In the present study, we found that ventral and dorsal mPFC lesions increased REM sleep, but only ventral mPFC lesions led to decreased REM latency, pronounced sleep fragmentation, and increased immobility in the forced swim test. Furthermore, REM latency and FST measures were significantly negatively correlated. We also show that neurons in the mPFC terminate on cells in the brainstem implicated in suppressing REM sleep, suggesting a possible mechanism of cortical REM modulation. These results suggest that mPFC neurons, and in particular vmPFC neurons, are critical for regulating depressive and sleep behavior.
Previous studies examining the FST in mPFC-lesioned animals have found that dmPFC-lesioned animals do exhibit a depression-like phenotype, contrary to our results (Bissiere et al, 2006). This difference may be due to variance in lesion size and location, as their lesion encompassed less than half of the dmPFC and little of the rostral sections, or due to measurements at different circadian phases (Kelliher et al, 2000). We avoided such effects by testing all our animals within the same 90-minute window each day. Other researchers (Hamani et al, 2010) have found that vmPFC-lesioned animals did not show increased immobility in the FST. However, this particular study targeted the infralimbic cortex, and the lesion histology provided was located more caudally than our target. As our lesions encompassed the prelimbic and infralimbic cortices, our larger lesions, particularly along the dorsal-ventral axis and more rostrally, may account for the difference in results.
A recent paper by Slattery and colleagues (Slattery et al, 2011) pharmacologically inactivated the infralimbic cortex of the rat, and found that FST immobility decreased as a result (in contrast to our results). There are a few possible explanations for this discrepancy: 1) They used the GABAA agonist muscimol to inhibit the region acutely and it is not clear at this does whether the GABAergic interneurons are more sensitive than pyramidal glutamatergic neurons, whereas in our methods we killed all the neurons in the region. Both approaches undoubtedly have a combination of local and downstream effects, and thus it is difficult to conclude that they are activating and inactivating the same regions. 2) No histology was provided with the publication, but the coordinates they used were very rostral (AP +4.5 mm) in contrast to ours (AP +3.5 – 3.0 mm). The “infralimbic cortex” at this level (1.0 mm rostral) may be distinct from the “infralimbic cortex” we included in the vmPFC lesions, and in the latest Paxinos and Watson rat atlas the more rostral "infralimbic cortex" is referred to as the “medial orbital cortex” (Paxinos and Watson, 2007). Finally, cannular injection often reaches much bigger regions than the intended ones.
The rat mPFC and sleep circuitry
The dmPFC, also called the anterior cingulate cortex, primarily projects to the vmPFC, premotor regions, and parts of the medial and mediodorsal thalamus (Sesack et al, 1989) to control motor and cognitive function. The vmPFC, encompassing the prelimbic and infralimbic cortices, primarily projects to limbic, hypothalamic, and brainstem areas (Vertes, 2003) involved in affect. The only region that both dmPFC and vmPFC project to that modulates REM sleep is the vlPAG-LPT (Hurley et al, 1991; Vertes, 2006). Therefore, we hypothesized that REM sleep changes following dmPFC and vmPFC lesions were caused by loss of direct projections to the area. Descending cortical neurons are predominantly excitatory, so loss of excitatory projections to the REM-off vlPAG-LPT could disinhibit and increase REM sleep. However, the finding that shortened REM sleep latency is unique to vmPFC-lesioned animals suggests that entry into REM sleep may be modulated by neural circuitry that is specific to the vmPFC. As REM sleep latency has been associated with affect disorders including MDD, the vmPFC may be a critical neural substrate for this biomarker. How the vmPFC regulates REM sleep latency in addition to the behaviors regulated by the dmPFC is an intriguing area for future investigation.
The vmPFC but not dmPFC also project to the lateral hypothalamus including the orexin neurons and tuberomamillary nucleus (Wouterlood et al, 1987; Yoshida et al, 2006). This pathway may be responsible for sleep-wake fragmentation of the vmPFC lesions. We propose that sleep-wake fragmentation from mPFC dysfunction produces nighttime insomnia and daytime sleepiness in MDD.
Relationship between sleep and depression
An essential question in investigating the relationship between sleep disturbances and depression is whether one precedes the other (either in sequence or cause and effect), or if they occur simultaneously, due to a common underlying neural substrate. Animals subjected to chronic mild stress or regular inescapable footshocks demonstrate significantly fragmented sleep and increased REM sleep (Adrien et al, 1991; Grønli et al, 2004; Moreau et al, 1995), similar to the effects of mPFC lesions in the present study. Sleep changes are also associated with increased vulnerability to depression: for instance, a persistent short REM sleep latency appears to increase the risk of relapse (Giles et al, 1987). As REM sleep latency is calculated from the interval between NREM sleep onset and REM sleep onset, it is related to NREM sleep duration. Thus the mechanism underlying sleep fragmentation and shortened REM sleep latency are likely related. Furthermore, healthy, never-depressed subjects with a strong family history of depression, as well as remitted patients, display specific sleep markers including higher REM density (Lauer et al, 1995; Pillai et al, 2011). As the projections of the vmPFC suggest it may have a role in modulating REM sleep, aberrant activity in this region, as a result of negative life events (Mayberg, 1997) or other risk factors, may produce the REM sleep biomarkers of depression.
Our findings emphasize the importance of the mPFC neurons, particularly the vmPFC neurons, in modulating FST immobility and sleep. We also report an intriguing correlation between these two outcome measures. Indeed, humans several studies have found that REM latency is closely correlated with the Hamilton Depression Score (Kupfer and Foster, 1972; Kupfer, 1976; Kupfer et al, 1976; Spiker et al, 1978). One possible interpretation is that the same population of neurons in the deep layers of the vmPFC regulates REM sleep and depression-like behavior. This structure is therefore a possible cortical site for the sleep changes – increased fragmentation, increased REM sleep, and decreased REM latency – and other symptoms of MDD. As we also observed increases in REM sleep but not in other behaviors in dmPFC-lesioned animals, an overlapping set of neural circuits including the vmPFC may contribute differentially to sleep and other symptoms of depression.
Highlights.
We observed the effect of medial prefrontal cortex lesions on sleep and the forced swim test.
We found that vmPFC lesions led to sleep changes consistent with those observed in depressed humans.
We propose a circuitry model of how the vmPFC may me modulating the sleep-executive structures.
Acknowledgements
The authors thank Quan Ha and Xi Chen for their technical assistance in these experiments. This research was supported by the National Institute of Health Training Grant HL007901 and NS062727 and NS061841.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Permanent address: 1 Ash Place, Unit 2K, Great Neck, NY 11021
Financial Disclosures
The authors report no financial interests or potential conflicts of interest.
References
- Adrien J, Dugovic C, Martin P. Sleep-wakefulness patterns in the helpless rat. Physiol Behav. 1991;49:257–262. doi: 10.1016/0031-9384(91)90041-l. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial Loss in the Prefrontal Cortex Is Sufficient to Induce Depressive-like Behaviors. Biological Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Riemann D. Symposium: Normal and abnormal REM sleep regulation: REM sleep in depression-an overview. Journal of sleep research. 1993;2:211–223. doi: 10.1111/j.1365-2869.1993.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Bissiere S, McAllister KH, Olpe H-R, Cryan JF. The rostral anterior cingulate cortex modulates depression but not anxiety-related behaviour in the rat. Behavioural Brain Research. 2006;175:195–199. doi: 10.1016/j.bbr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Brown SM. Mild, Short-term Stress Alters Dendritic Morphology in Rat Medial Prefrontal Cortex. Cerebral Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Castagn’ V, Moser P, Roux S, Porsolt RD, et al. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Current protocols in neuroscience / editorial board, Jacqueline N Crawley. 2011 doi: 10.1002/0471142301.ns0810as55. Chapter 8: Unit 8.10A. [DOI] [PubMed] [Google Scholar]
- Clark CP, Brown GG, Frank L, Thomas L, Sutherland AN, Gillin JC. Improved anatomic delineation of the antidepressant response to partial sleep deprivation in medial frontal cortex using perfusion-weighted functional MRI. Psychiatry Research: Neuroimaging. 2006;146:213–222. doi: 10.1016/j.pscychresns.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. Antidepressant Effect of Optogenetic Stimulation of the Medial Prefrontal Cortex. Journal of Neuroscience. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EthoVision Evaluation of Porsolt Swim Test activity: Using the Mobility Parameter of EthoVision. EthoVision Application Note. 2004 [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA: The Journal of the American Medical Association. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Giles DE, Jarrett RB, Roffwarg HP, Rush AJ. Reduced rapid eye movement latency. A predictor of recurrence in depression. Neuropsychopharmacology. 1987;1:33–39. doi: 10.1016/0893-133x(87)90007-8. [DOI] [PubMed] [Google Scholar]
- Grønli J, Murison R, Bjorvatn B, Sørensen E, Portas CM, Ursin R. Chronic mild stress affects sucrose intake and sleep in rats. Behavioural Brain Research. 2004;150:139–147. doi: 10.1016/S0166-4328(03)00252-3. [DOI] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Macedo CE, Brandão ML, Shumake J, Gonzalez-Lima F, et al. Antidepressant-Like Effects of Medial Prefrontal Cortex Deep Brain Stimulation in Rats. BPS. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Hohagen F, Rink K, Käppler C, Schramm E, Riemann D, Weyerer S, et al. Prevalence and treatment of insomnia in general practice. A longitudinal study. Eur Arch Psychiatry Clin Neurosci. 1993;242:329–336. doi: 10.1007/BF02190245. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. The Journal of Comparative Neurology. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Jahn AL, Fox AS, Abercrombie HC, Shelton SE, Oakes TR, Davidson RJ, et al. Subgenual prefrontal cortex activity predicts individual differences in hypothalamic-pituitary-adrenal activity across different contexts. Biol Psychiatry. 2010;67:175–181. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Archives of internal medicine. 1998;158:1099–1107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- Kelliher P, Connor TJ, Harkin A, Sanchez C, Kelly JP, Leonard BE. Varying responses to the rat forced-swim test under diurnal and nocturnal conditions. Physiology & behavior. 2000;69:531–539. doi: 10.1016/s0031-9384(00)00213-4. [DOI] [PubMed] [Google Scholar]
- Klein J, Winter C, Coquery N, Heinz A, Morgenstern R, Kupsch A, et al. Lesion of the medial prefrontal cortex and the subthalamic nucleus selectively affect depression-like behavior in rats. Behavioural Brain Research. 2010;213:73–81. doi: 10.1016/j.bbr.2010.04.036. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ. REM latency: a psychobiologic marker for primary depressive disease. Biol Psychiatry. 1976;11:159–174. [PubMed] [Google Scholar]
- Kupfer DJ, Foster FG. Interval between onset of sleep and rapid-eye-movement sleep as an indicator of depression. Lancet. 1972;2:684–686. doi: 10.1016/s0140-6736(72)92090-9. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Foster FG, Reich L, Thompson SK, Weiss B. EEG sleep changes as predictors in depression. Am J Psychiatry. 1976;133:622–626. doi: 10.1176/ajp.133.6.622. [DOI] [PubMed] [Google Scholar]
- Lauer CJ, Schreiber W, Holsboer F, Krieg JC. In quest of identifying vulnerability markers for psychiatric disorders by all-night polysomnography. Arch Gen Psychiatry. 1995;52:145–153. doi: 10.1001/archpsyc.1995.03950140063009. [DOI] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip–flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. The Journal of neuropsychiatry and clinical neurosciences. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Scherschlicht R, Jenck F, Martin JR. Chronic mild stress-induced anhedonia model of depression; sleep abnormalities and curative effects of electroshock treatment. Behavioural pharmacology. 1995;6:682–687. [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Waltham, MA: 2007. [Google Scholar]
- Pillai V, Kalmbach DA, Ciesla JA. A Meta-Analysis of Electroencephalographic Sleep in Depression: Evidence for Genetic Biomarkers. BPS. 2011 doi: 10.1016/j.biopsych.2011.07.016. 1– 8doi:10.1016/j.biopsych.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep. (3rd) 1987;10:199–215. doi: 10.1093/sleep/10.3.199. [DOI] [PubMed] [Google Scholar]
- Rijnbeek B, de Visser SJ, Franson KL, Cohen AF, van Gerven JMA. REM sleep effects as a biomarker for the effects of antidepressants in healthy volunteers. Journal of psychopharmacology (Oxford, England) 2003;17:196–203. doi: 10.1177/0269881103017002008. [DOI] [PubMed] [Google Scholar]
- Scopinho AA, Scopinho M, Lisboa SF, de Aguiar Correa FM, Guimarães FS, Joca SRL. Acute reversible inactivation of the ventral medial prefrontal cortex induces antidepressant-like effects in rats. Behavioural Brain Research. 2010;214:437–442. doi: 10.1016/j.bbr.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. The Journal of Comparative Neurology. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID, Cryan JF. Transient inactivation of the infralimbic cortex induces antidepressant-like effects in the rat. Journal of Psychopharmacology. 2011;25:1295–1303. doi: 10.1177/0269881110368873. [DOI] [PubMed] [Google Scholar]
- Spiker DG, Coble P, Cofsky J, Foster FG, Kupfer DJ. EEG sleep and severity of depression. Biol Psychiatry. 1978;13:485–488. [PubMed] [Google Scholar]
- Steiger A, Kimura M. Wake and sleep EEG provide biomarkers in depression. Journal of Psychiatric Research. 2010;44:242–252. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- van Tol M-J, van der Wee NJA, van den Heuvel OA, Nielen MMA, Demenescu LR, Aleman A, et al. Regional brain volume in depression and anxiety disorders. Archives of general psychiatry. 2010;67:1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- Vertes R. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2003;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- World Health Organization . The world health report 2002: reducing risks, promoting healthy life. WHO; Geneva, Switzerland: 2002. [Google Scholar]
- World Health Organization . World Health Organization Fact sheet: Depression. World Health Organization; Geneva, Switzerland: 2012. [Google Scholar]
- Wouterlood FG, Steinbusch HW, Luiten PG, Bol JG. Projection from the prefrontal cortex to histaminergic cell groups in the posterior hypothalamic region of the rat. Anterograde tracing with Phaseolus vulgaris leucoagglutinin combined with immunocytochemistry of histidine decarboxylase. Brain Res. 1987;406:330–336. doi: 10.1016/0006-8993(87)90802-x. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, España RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]