Abstract
Electrophysiological studies show that distinct subsets of nucleus accumbens (NAc) neurons differentially encode information about goal-directed behaviors for intravenous cocaine versus natural (food/water) rewards. Further, NAc rapid dopamine signaling occurs on a timescale similar to phasic cell firing during cocaine and natural reward-seeking behaviors. However, it is not known whether dopamine signaling is reinforcer specific (i.e., is released during responding for only one type of reinforcer) within discrete NAc locations, similar to neural firing dynamics. Here, fast-scan cyclic voltammetry (FSCV) was used to measure rapid dopamine release during multiple schedules involving sucrose reward and cocaine self-administration (n=8 rats) and, in a separate group of rats (n = 6), during a sucrose/food multiple schedule. During the sucrose/cocaine multiple schedule, dopamine increased within seconds of operant responding for both reinforcers. Although dopamine release was not reinforcer specific, more subtle differences were observed in peak dopamine concentration [DA] across reinforcer conditions. Specifically, peak [DA] was higher during the first phase of the multiple schedule, regardless of reinforcer type. Further, the time to reach peak [DA] was delayed during cocaine-responding compared to sucrose. During the sucrose/food multiple schedule, increases in dopamine release were also observed relative to operant responding for both natural rewards. However, peak [DA] was higher relative to responding for sucrose than food, regardless of reinforcer order. Overall, the results reveal the dynamics of rapid dopamine signaling in discrete locations in the NAc across reward conditions, and provide novel insight into the functional role of this system in reward-seeking behaviors.
Keywords: fast-scan cyclic voltammetry, self-administration, reward, rat, addiction, behavior
1. Introduction
Learning about rewards and appropriately directing behavior to obtain them is critical for survival. These processes are subserved by a distributed network of brain nuclei including the NAc and its dopaminergic input. The NAc receives convergent glutamatergic afferents from limbic areas including the prefrontal cortex, hippocampus, and basolateral amygdala (Zahm and Brog, 1992; Brog et al., 1993) and impacts behavior through its projections to motor-related regions such as the ventral pallidum (Zahm, 1999). Further, dopamine functions as a neuromodulator within the NAc, influencing the activity of the glutamatergic afferents onto NAc neurons that ultimately alters output to motor structures (O'Donnell et al., 1999; Nicola et al., 2000). This connectivity supports the classic view of the NAc as a “limbic-motor integrator” that translates motivation into goal-directed actions (Mogenson et al., 1980).
Consistent with this role, in vivo electrophysiology studies have shown that NAc neurons encode goal-directed behaviors for both natural and drug rewards. That is, NAc neurons exhibit phasic changes in activity (excitations and/or inhibitions in firing rate) within seconds before, during, and after operant responses for natural as well as drug rewards (Carelli and Deadwyler, 1994; Chang et al., 1998; Janak et al., 1999; Carelli, 2002; Nicola et al., 2004; Peoples et al., 2004). In order to track the activity of the same NAc neurons across different reinforcer conditions, studies from this lab employed multiple schedule designs. This work revealed that subsets of NAc neurons exhibit largely differential, nonoverlapping firing patterns during operant responding for natural rewards (food, water, or sucrose) versus intravenous cocaine (Carelli et al., 2000; Carelli, 2002; Carelli and Wondolowski, 2003; Carelli and Wondolowski, 2006; Cameron and Carelli, 2012). In contrast, NAc neurons exhibit similar types of neuronal firing patterns during responding for two natural reinforcers (food versus water) (Carelli et al., 2000). This pattern holds true even when one of the natural reinforcers is highly palatable sucrose (Roop et al., 2002). Collectively, these findings support the contention that the NAc is comprised of discrete, functionally segregated ‘microcircuits’ that process particular types of reinforcement-related information to influence goal-directed actions (Alexander et al., 1986; Pennartz et al., 1994; Groenewegen et al., 1996; Carelli and Wightman, 2004).
Importantly, studies employing electrochemical methods have revealed that rapid (subsecond) dopamine release in the NAc is also observed within seconds of goal-directed actions for cocaine and natural rewards. Critically, these dynamic changes in dopamine signaling occur on a timescale similar to NAc phasic cell firing (Phillips et al., 2003b; Roitman et al., 2004; Stuber et al., 2005; Saddoris et al., 2013). The similarity in temporal dynamics between NAc cell firing and rapid dopamine release support the contention that dopamine functions to modulate NAc cell firing that encodes and ultimately influences goal-directed actions. However, it is not known whether rapid dopamine release is reinforcer specific within discrete locations in the NAc in a manner similar to NAc cell firing observed in our previous electrophysiology studies (e.g., Carelli et al., 2000). To address this issue, we used fast scan cyclic voltammetry (FSCV) to measure rapid dopamine release in the NAc core during performance of two different tasks: a sucrose/cocaine or sucrose/food multiple schedule. This design allowed us to track and directly compare dopamine release dynamics in specific locations in the NAc during operant responding for a natural reward and intravenous cocaine, as well as two natural rewards.
2. Material and Methods
2.1. Subjects
Male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN, USA; n = 14) aged 90-120 days and weighing 260–350 g were used as subjects and individually housed with a 12/12 h light/dark cycle. Body weights were maintained at no less than 85% of pre-experimental levels by food restriction (10–15 g of Purina laboratory chow each day). Water was available ad libitum. This regimen was in place for the duration of the experiment, except during the postoperative recovery period when food was given ad libitum. Animal procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee (IACUC).
2.2. Surgery and Behavioral Training
All training was conducted in custom-made experimental chambers that consisted of a 43 × 43 × 53 cm Plexiglass chamber housed within a commercial sound-attenuated cubicle (Med Associates Inc., St Albans, VT, USA). One side of the chamber was equipped with two retractable levers (Coulbourn Instruments, Allentown, Pennsylvania) 17 cm apart, corresponding cue lights positioned 6 cm above each lever, and a reward receptacle located equidistantly between the levers.
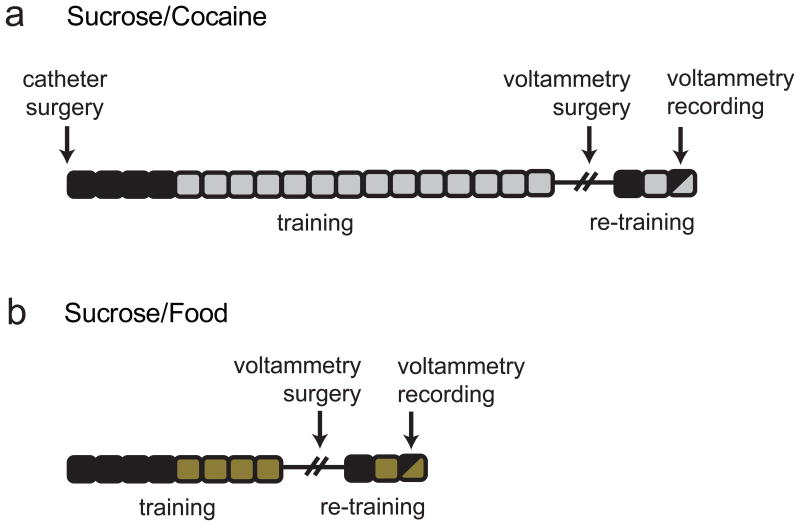
Figure 1 shows the experimental timeline for each study. In the first experiment (sucrose/cocaine multiple schedule; Fig. 1a), rats (n = 8) were surgically implanted with an intravenous catheter following established procedures, described in detail previously (Carelli et al., 2000). Following recovery from surgery, rats were first trained to press one lever for sucrose (45 mg pellet; TestDiet, St. Louis, MO, USA) on a fixed-ratio 1 schedule of reinforcement. The start of the sucrose training session was signaled by the onset of the cue light positioned above the active lever and extension of the lever into the chamber. Lever depression resulted in delivery of a sucrose pellet to the reward receptacle, onset of a tone (65 dB, 2900 Hz, 20 s), and retraction of the lever (20 s). Rats underwent daily 30 min training sessions until they reached criterion (at least 50 presses per session). Rats were then trained to self-administer cocaine on a fixed-ratio 1 schedule of reinforcement during daily 2 h sessions. The start of the self-administration session was signaled by the onset of the cue light positioned above the active lever and extension of the lever into the chamber. The cocaine-associated lever was spatially distinct from the lever previously used during sucrose training. Lever depression resulted in intravenous cocaine delivery (0.33 mg/infusion, approximately 1 mg/kg/infusion, 6 s) via a computer-controlled syringe pump, onset of a different tone (65 dB, 800 Hz, 20 s), and retraction of the lever (20 s). The tones and levers (left or right) associated with cocaine vs. sucrose were counterbalanced across animals.
Figure 1.
Experimental timeline. (a) Timeline for the sucrose/cocaine multiple schedule. Each box represents one day of behavioral training. Black boxes indicate days of sucrose responding. Gray boxes indicate days of cocaine self-administration. On the recording day, animals completed a multiple schedule for sucrose and cocaine. (b) Timeline for the sucrose/food multiple schedule. Each box represents one day of behavioral training. Black boxes indicate days of operant responding for sucrose and gold boxes indicate days of food responding. On the recording day, animals completed a multiple schedule for sucrose and food.
Following 14 days of cocaine self-administration, rats were surgically prepared for voltammetric recording in the NAc core as previously described (Phillips et al., 2003a). Animals were anesthetized with a ketamine hydrochloride (100 mg/kg) and xylazine hydrochloride (10 mg/kg) mixture (intramuscular) and placed in a stereotaxic frame. A guide cannula (Bioanalytical Systems, West Lafayette, IN) was positioned dorsally to the NAc core (+1.3 mm anterior, -1.3mm lateral from bregma). An Ag/AgCl reference electrode was placed contralateral to the stimulating electrode in the left forebrain. The bipolar stimulating electrode (Plastics 1 Inc., Roanoake, VA) was placed dorsally to the VTA (-5.2 mm posterior, -1.0 mm lateral from bregma and -7 mm ventral from brain surface). Stainless steel skull screws and dental cement were used to secure all items. The bipolar stimulating electrode was lowered in 0.2 mm increments until physical responses to electrical stimulation diminished, indicative of proper electrode placement. The stimulating electrode was then fixed with dental cement.
After recovering from surgery, rats were retrained for two consecutive days (one session of sucrose operant responding followed the next day by one session of cocaine self-administration) while tethered to the headstage. These retraining sessions were identical to the training sessions that took place prior to surgery, and allowed the animals to habituate to the headstage and reestablish normal operant responding. Following retraining, rats underwent voltammetric recording (see below) during a multiple schedule of reinforcement for sucrose and cocaine. Specifically, rats had access to the sucrose-reinforced lever (fixed-ratio 1; 15 min) followed by a 20 s time-out period (no lever extended; dark chamber) and extension of the second cocaine-reinforced lever (fixed-ratio 1; 2 h). Illumination of a cue light above each lever signaled the phase (sucrose or cocaine) of the multiple schedule. Lever depression resulted in delivery of the designated reinforcer (sucrose or cocaine), onset of the reinforcer-associated tone, and retraction of the lever (20 s). The order of reinforcer availability was varied across animals such that 4 animals self-administered sucrose followed by cocaine while 4 other animals self-administered cocaine followed by sucrose. Importantly, rats only received both reinforcers in the multiple schedule during the test day. Therefore, one reinforcer never came to predict access to the other.
In a second experiment (sucrose/food multiple schedule; Fig. 1b), another set of rats (n = 6) were trained in a similar manner; however, food (45 mg pellet, TestDiet, St. Louis, MO, USA; fixed-ratio 1; 30 min) was substituted for cocaine in training and during the multiple schedule. Following acquisition of sucrose responding, these animals underwent at least two additional days of training on operant responding for food. On test day, the rats performed a sucrose/food multiple schedule that consisted of two 15 minute periods of operant responding separated by a brief 20 s time-out. All other aspects of the multiple schedule (reward delivery, tone, lever retraction) were as described above. As in the sucrose/cocaine multiple schedule, the lever and tone associated with each reinforcer was counterbalanced across animals. Further, the order of reinforcer availability was varied across animals such that 4 recordings were obtained in which sucrose was self-administered followed by food, and 5 recordings in which food was self-administered followed by sucrose.
2.3. Fast Scan Cyclic Voltammetry
Changes in dopamine concentration ([DA]) during behavior were assessed using FSCV as previously described (Roitman et al., 2004; Day et al., 2007). On the experimental day, a detachable micromanipulator containing a carbon-fiber electrode (90-110 μm length) was inserted into the guide cannula and lowered into the NAc core. The carbon-fiber and Ag/AgCl reference electrodes were connected to a head-mounted voltammetric amplifier attached to a commutator (Med-Associates, St. Albans, VT) at the top of the test chamber. Voltammetric recordings were made every 100 msec by applying a triangular waveform (-0.4 to +1.3 V, 400 V/sec). Data were digitized and stored to a computer using software written in LabVIEW (National Instruments, Austin, TX). Dopamine release within the NAc core was electrically evoked by stimulating the VTA (24 biphasic pulses, 60 Hz, 120 μA, 2 msec per phase) to ensure that carbon-fiber electrodes were in close proximity to dopamine release sites. The electrode position was optimized at a location with maximal dopamine release. To create a training set for principal component analysis for the detection of dopamine and pH changes during the behavioral session, additional stimulations at various parameters were performed (2-24 biphasic pulses, 20-60 Hz, 120 μA, 2 msec/phase). After the session, electrical stimulation was repeated to ensure that the site could still support dopamine release. A second computer and software system (Med Associates) controlled behavioral events and sent digital outputs for each event to the voltammetry recording computer to be time-stamped along with the electrochemical data. In some cases (two animals that performed the sucrose/food multiple schedule), a second or third recording session was completed on another day in which an electrode was lowered to a new location in the NAc core.
2.4. Data Analysis
All lever press events were recorded during performance of the multiple schedule. The number of lever presses as well as inter-response interval (INT) was calculated for each reinforcer during each behavioral session. The number of lever presses and INT were compared across reinforcers in each multiple schedule (sucrose/cocaine and sucrose/food) with two-way mixed design ANOVAs (reinforcer type, within subjects factor × reinforcer order, between subjects factor). To eliminate any effect of an uneven number of responses for one reinforcer (sucrose, cocaine or food) over another, lever press responses were randomly selected to allow for an equal number of trials within a recording session. The number of random trials that were selected from each session were equivalent to the number of trials of the reinforcer for which the animal responded less. Thus, if an animal responded 40 times for sucrose and 20 times for cocaine during a sucrose/cocaine multiple schedule, 20 trials were selected each for sucrose and cocaine. All trials were then averaged to obtain an average trace for each reinforcer within a behavioral session. This procedure was used for all analyses described below.
Dopamine signals from FSCV were identified as previously described (Roitman et al., 2004). For analyte identification, current during a voltammetric scan is plotted against applied potential to yield a cyclic voltammogram (the chemical signature of the analyte; see examples in Figures 2a,b and 4a,b insets). Cyclic voltammetric data were analyzed on stimulation trials before and after each experiment, and ±10 s relative to the important behavioral events (lever press). A background signal from 1 voltammetric scan (100 msec time bin) before a stimulation or behavioral trial was subtracted from the remainder of the scans to reveal changes in [DA], as opposed to absolute values.
Figure 2.
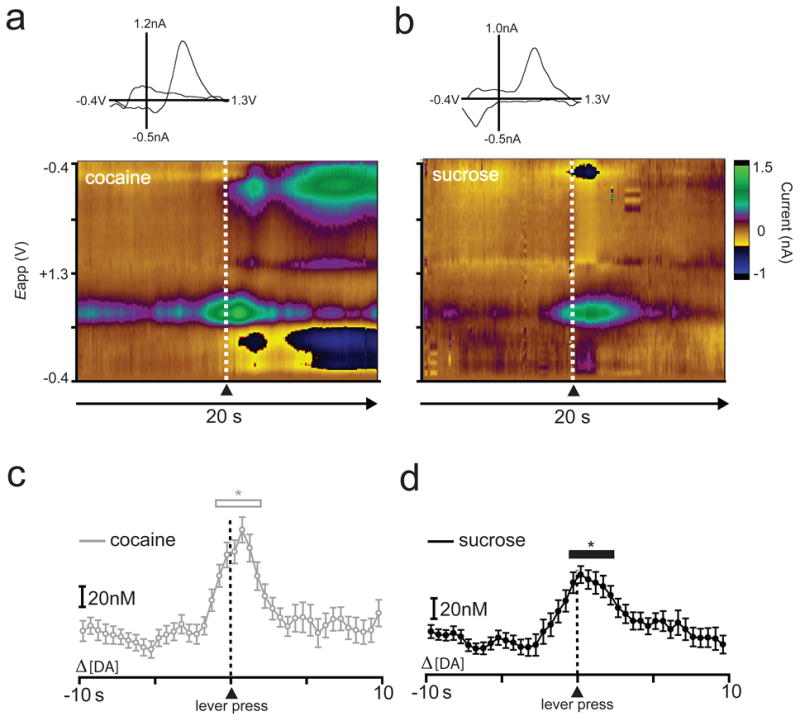
Example of dopamine release dynamics for a representative animal during the sucrose/cocaine multiple schedule (phase 1 = cocaine, phase 2 = sucrose). The rat completed 29 responses for cocaine and 48 responses for sucrose; 29 trials for each reinforcer were included in the analysis. Lever press is indicated by dotted line at time zero. (a,b) Two-dimensional color representation of cyclic voltammetric data collected for 20 s around lever pressing for cocaine (a) or sucrose (b). The ordinate is the applied voltage (Eapp) and the abscissa is time (s). Changes in current at the carbon-fiber electrode are indicated in color. Insets: cyclic voltammograms at the time of lever press. (c,d) Differential dopamine concentrations determined via principal component analysis for cocaine (c) and sucrose (d). Data are plotted in 500 msec bins (mean ± S.E.M.). Asterisks above open (c) or closed (d) bars indicate significant increases in [DA] from baseline, p < 0.05.
Figure 4.
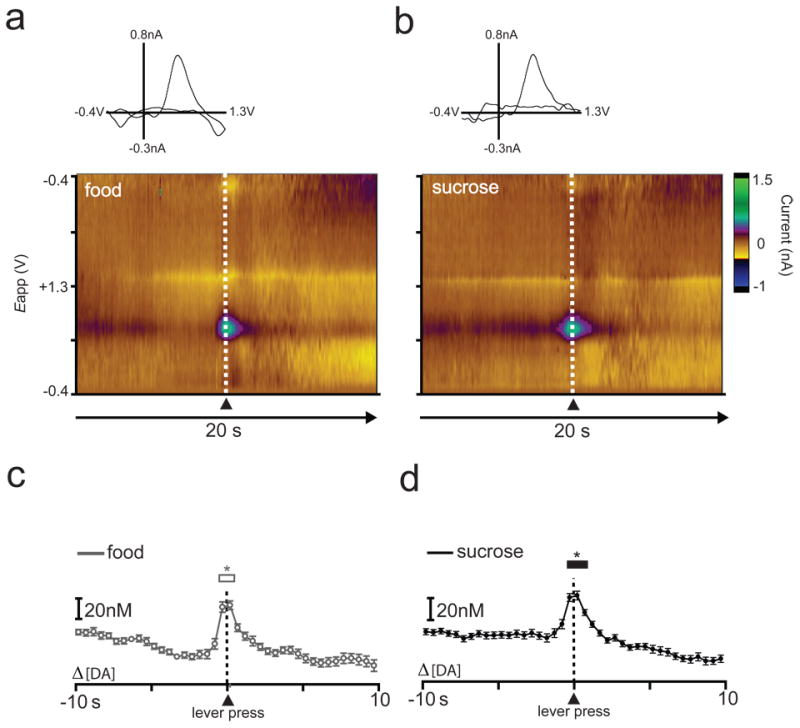
Example of dopamine release for a representative animal during performance of the sucrose/food multiple schedule (phase 1 = food, phase 2 = sucrose). The rat completed 45 responses for food and 40 responses for sucrose; 39 trials for each reinforcer were included in the analysis. Lever press is indicated by dotted line at time zero. (a,b) Two-dimensional color representation of cyclic voltammetric data collected for 20 s around lever pressing for food (a) or sucrose (b). The ordinate is the applied voltage (Eapp) and the abscissa is time (s). Changes in current at the carbon-fiber electrode are indicated in color. Insets: cyclic voltammograms at the time of lever press. (c,d) Differential [DA] determined via principal component analysis for food (c) and sucrose (d). Data are plotted in 500 msec bins (mean ± S.E.M.). Asterisks above open (c) or closed (d) bars indicate significant increases in [DA] from baseline, p < 0.05.
[DA] changes were then quantified using principal component regression (Heien et al., 2005; Keithley et al., 2010). Training sets were constructed from representative, background subtracted cyclic voltammograms for dopamine and pH. These training sets were then used to perform principal component regression on data collected during behavioral sessions. Principal components were selected such that at least 99% of the variance in the training set was accounted for by the model. Current was converted to [DA] using a calibration factor of 10nA/μM, a standard value obtained by calibrating numerous electrodes from different studies in this laboratory.
Changes in extracellular [DA] during behavior were assessed by aligning [DA] traces to lever press events. Data were averaged into 500 msec bins, and analyzed 10 s before to 10 s after lever press. To account for any differences in baseline between reinforcers within a behavioral session, the baseline bin (-10 to -9.5 s) was set to an equal value for each animal. Changes in NAc [DA] from baseline in response to lever presses were evaluated independently for each reinforcer (sucrose, cocaine, or food) using a one-way repeated measures ANOVA with Newman-Keuls post hoc tests. This analysis compared the baseline bin (-10 to -9.5 s) to each subsequent 500 msec bin.
The pattern of rapid dopamine release during lever pressing for sucrose versus cocaine (or sucrose versus food) during the multiple schedule was compared with two-way repeated measures ANOVAs (reinforcer × time) followed by Newman-Keuls post hoc tests of significant effects. For these analyses, data were averaged into 500 msec bins, and analyzed 10 s before to 10 s after lever press.
Next, we examined peak [DA] elicited by operant responding for sucrose, cocaine, or food. Peak [DA] was defined as the highest DA concentration within a 3 s window surrounding a lever press (100 msec bins), and was determined for each reinforcer (sucrose, cocaine, or food) for each animal. Again, differences in baseline between reinforcers within a behavioral session were removed by setting the baseline bin (-10 to -9.9 s) to an equal value for each animal. Average peak [DA] across rats were then compared across reinforcer types (sucrose/cocaine or sucrose/food) using paired t tests. The influence of reinforcer order and reinforcer type on peak [DA] was analyzed with a two-way mixed design ANOVA.
The time to reach peak [DA] was also determined for each reinforcer within a behavioral session. For this analysis, [DA] across all trials for a particular reinforcer within a recording session were averaged. Again, analysis was restricted to a 3 s window surrounding the lever press (100 msec bins). The highest [DA] in this window was noted and the corresponding time relative to the lever press considered the “time to peak.” For this analysis, time zero corresponded to lever press. Thus, negative values represent a peak that occurred prior to a response, while positive values represent a peak that occurred after a response. Time to peak was compared across reinforcer types using paired t tests. The influence of reinforcer order and reinforcer type on time to peak was analyzed with a two-way mixed design ANOVA.
For all analyses, the alpha level for significance was 0.05. All statistics were performed with commercially available software (Statistica, Tulsa, Oklahoma; GraphPad Software, La Jolla, CA).
2.5. Histology
On completion of each experiment, rats were deeply anesthetized with a ketamine/xylazine mixture (100 mg/kg and 10 mg/kg, respectively). To mark the placement of electrode tips, a 209 μA current was passed through a stainless steel electrode for 10 s. Brains were removed and post-fixed in 10% formalin. After post-fixing and freezing, 50 μm coronal brain sections were taken and mounted throughout the rostral–caudal extent of the NAc. The specific position of individual electrodes was assessed by visual examination of successive coronal sections for electrolytic lesions.
3. Results
3.1. Behavior during the multiple schedules
Behavioral data was obtained from 8 recordings (n = 8 rats) during performance of the sucrose/cocaine multiple schedule and from 9 recordings (n = 6 rats) during the sucrose/food multiple schedule. Independent of reinforcer order, during the sucrose portion of the sucrose/cocaine multiple schedule, rats completed a mean of 39.75 ± 3.18 lever presses with a mean inter-response interval (INT) of 30.14 ± 6.23 s. During the cocaine portion of the multiple schedule, rats completed a mean of 23.00 ± 1.75 lever presses with a mean INT of 5.40 ± 0.39 min. A two-way mixed design ANOVA with a within-subjects factor of reinforcer type and a between-subject factor of reinforcer order conducted on the number of lever presses revealed a main effect of reinforcer type (F1,6 = 37.935, P < 0.001), but no main effect of reinforcer order (F1,6 = 0.072, P > 0.05) and no reinforcer type × order interaction (F1,6 = 1.023, P > 0.05). Further, a two-way mixed design ANOVA (reinforcer type × reinforcer order) conducted on INT revealed a main effect of reinforcer type (F1,6 = 166.023, P < 0.0001), but no main effect of order (F1,6 = 0.005, P > 0.05) and no reinforcer type × order interaction (F1,6 = 0.474, P > 0.05). Thus, rats pressed more often and faster for sucrose than cocaine, and this pattern held regardless of order of reinforcer presentation in the multiple schedule. These data reflect typical self-administration patterns previously observed in this lab (e.g., Cameron and Carelli, 2012). Further, under a fixed-ratio schedule, cocaine INTs are tightly correlated with levels of DA uptake inhibition (Oleson et al. 2009). Thus, the INT for cocaine observed in this study (5.40 ± 0.39 min) is predicted by the self-administered dose of cocaine (∼1mg/kg/infusion).
During performance of the sucrose portion of the sucrose/food multiple schedule, rats completed a mean of 37.56 ± 3.47 lever presses with a mean INT of 24.64 ± 1.57 s. During responding for food in the multiple schedule, rats completed a mean of 43.22 ± 1.71 lever presses with a mean INT of 21.81 ± 0.059 s. A two-way mixed design ANOVA (reinforcer type × reinforcer order) conducted on number of lever presses revealed no main effect of reinforcer type (F1,7 = 2.221, P > 0.05) or order (F1,7 = 0.002, P > 0.05), and no reinforcer type × order interaction (F1,7 = 4.403, P > 0.05). Further, a two-way mixed design ANOVA (reinforcer type × reinforcer order) conducted on INT revealed no main effect of reinforcer type (F1,7= 2.780, P < 0.05) or order (F1,7 = 1.389, P > 0.05), but a significant reinforcer type × order interaction (F1,7 = 6.836, P > 0.05). Newman-Keuls post hoc tests on the reinforcer type × order interaction revealed that rats had a slightly longer INT for sucrose when it was presented in phase 2 of the multiple schedule compared to when sucrose was presented in phase 1. Collectively, these data indicate that rats pressed a similar number of times for sucrose and food regardless of order of presentation, but order of presentation did influence the rate of responding for sucrose.
3.2. Dopamine release during performance of the sucrose/cocaine multiple schedule
To compare extracellular [DA] during lever pressing for sucrose pellets versus intravenous infusions of cocaine (i.e., drug self-administration), [DA] traces were aligned to the execution of lever press responses. Figure 2 (a,b) shows example cyclic voltammograms and color representations (colorplots) of a set of background-subtracted cyclic voltammograms and the corresponding [DA] traces (c,d) for one animal that completed the cocaine/sucrose multiple schedule. In this example, cocaine was the reinforcer in phase 1 followed by sucrose in phase 2. Dopamine release was observed during both phases of the multiple schedule. During cocaine self-administration (phase 1), a one-way repeated-measures ANOVA revealed that [DA] during lever pressing for cocaine was significantly higher than during baseline (F39,1092 = 10.698, P < 0.0001; Fig. 2c). Newman-Keuls post hoc tests revealed a significant increase in dopamine release from baseline beginning 1 s before and ending 2 s after the lever press (P < 0.05). Likewise, a one-way repeated measures ANOVA revealed that [DA] was also increased from baseline during responding for sucrose (F39,1092 = 9.501, P < 0.0001; Fig. 2d). Newman-Keuls post hoc tests revealed significant increases in dopamine release events that began 0.5 s before the press and ended 2 s after response completion (P < 0.05). These findings are consistent with our previous electrochemistry reports showing increased [DA] relative to lever pressing for either sucrose (Roitman et al., 2004) or cocaine (Phillips et al., 2003b).
This pattern of increased dopamine release was observed across all animals. Separate one-way repeated measure ANOVAs were performed to examine when [DA] increased from baseline during responding for cocaine versus sucrose. These statistics revealed that [DA] increased relative to baseline during both the cocaine (F39,273 = 5.152, P < 0.0001) and sucrose (F39,273 = 8.864, P < 0.0001) portions of the multiple schedule. Newman-Keuls post hoc tests revealed that for the cocaine portion of the multiple schedule [DA] increased beginning at lever press, or time 0, and ended 1.5 s after response completion (P < 0.05). Likewise, during the sucrose portion of the multiple schedule, Newman-Keuls post hoc tests revealed that [DA] was significantly elevated beginning 1 s before the response and returned to baseline levels 1.5 s after the lever press (P < 0.05).
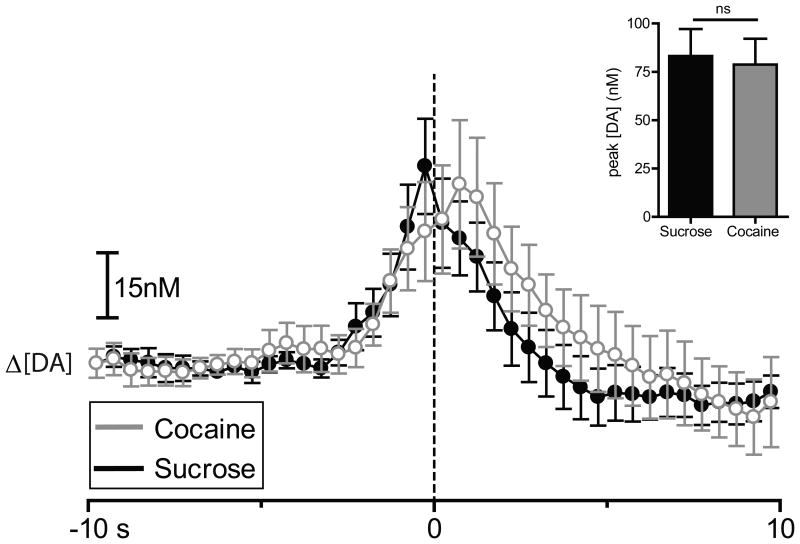
In order to directly compare changes in DA signaling dynamics across reinforcer types relative to lever press, a two-way repeated measures ANOVA was conducted that examined reinforcer type (sucrose versus cocaine) and time on [DA] (Fig. 3). This statistic revealed a main effect of time (F39,273 = 10.995, P < 0.0001), but no main effect of reinforcer type (F1,7 = 0.525, P > 0.05) and no reinforcer type × time interaction (F39,273 = 0.846, P > 0.05). Thus, [DA] increased from baseline during responding for both sucrose and cocaine, but there were no significant differences in [DA] between the two types of reinforcers.
Figure 3.
Sucrose responding and cocaine self-administration evoke rapid dopamine release in the NAc core across all rats (n=8). [DA] is averaged into 500 msec bins (mean ± S.E.M) and aligned to lever press (dotted line, time 0 s) for cocaine (gray) and sucrose (black). Inset: comparison of peak [DA] within a 3 sec window surrounding lever press for sucrose (black) versus cocaine (gray). Peak [DA] is calculated with 100 msec bins. ns = non significant.
Next, we examined if there were significant differences in peak [DA] across the two reinforcer types. Peak [DA] was calculated with 100 msec bins restricted to a 3 s analysis window surrounding lever press. No significant difference was observed in average peak [DA] across the two reinforcer types (t7 = 0.2792; P > 0.05; Fig. 3, inset), indicating that rapid dopamine release does not occur selectively to one component of the sucrose/cocaine multiple schedule. Therefore, unlike our previous electrophysiology studies that revealed reinforcer specific patterned cell firing in the NAc during food/cocaine multiple schedules (Carelli et al., 2000; Carelli, 2002; Carelli and Wondolowski, 2003; Carelli and Wondolowski, 2006; Cameron and Carelli, 2012), rapid dopamine signaling increases to the same extent in the NAc during responding for a natural reward versus intravenous cocaine.
3.3. Dopamine release during performance of the sucrose/food multiple schedule
In contrast, our prior electrophysiology studies also revealed that the same population of NAc neurons was similarly activated during lever press responding on a multiple schedule involving two natural reinforcers (food versus water) (Carelli et al., 2000), even when one of the natural reinforcers was highly palatable sucrose (Roop et al., 2002). Here, we extended that work to determine if similarities in dopamine release dynamics are observed during performance of a sucrose/food multiple schedule. To compare changes in extracellular dopamine in each phase of the sucrose/food multiple schedule, [DA] traces were aligned to the execution of the lever press response for each reinforcer. Figure 4 shows example cyclic voltammograms, colorplots (a,b) and dopamine traces (c,d) for one animal that completed the food/sucrose multiple schedule. Note in this session, food was the reinforcer in phase 1 and sucrose was the reinforcer in phase 2. Here, increases in dopamine release were observed during both portions of the multiple schedule. A one-way repeated-measures ANOVA revealed that lever-pressing for food significantly increased [DA] compared to baseline (F39,1482 = 16.646, P < 0.0001; Fig. 4c), 0.5 s before to 0.5 s after the lever press (Newman-Keuls post hoc tests, P < 0.05). Likewise, a one-way repeated-measures ANOVA revealed that [DA] increased from baseline from 0.5 s before to 1 s after lever press responding for sucrose (F39,1482 = 25.389, P < 0.0001; Newman-Keuls post hoc tests, P < 0.05; Fig. 4d).
This pattern of increased dopamine release relative to responding for both types of reinforcers was observed in all animals. Separate one-way repeated measures ANOVAs revealed that [DA] increased relative to baseline during both the food (F39,312 = 2.159, P < 0.001) and sucrose (F39,312 = 5.047, P < 0.0001) portions of the multiple schedule. Newman-Keuls post hoc tests revealed that for the food portion of the multiple schedule [DA] increased beginning 0.5 s before and ending at lever press, or time 0 (P < 0.05). Likewise, during the sucrose portion of the multiple schedule, [DA] was significantly elevated beginning 0.5 s before the response and returned to baseline levels 0.5 s after the lever press (P < 0.05).
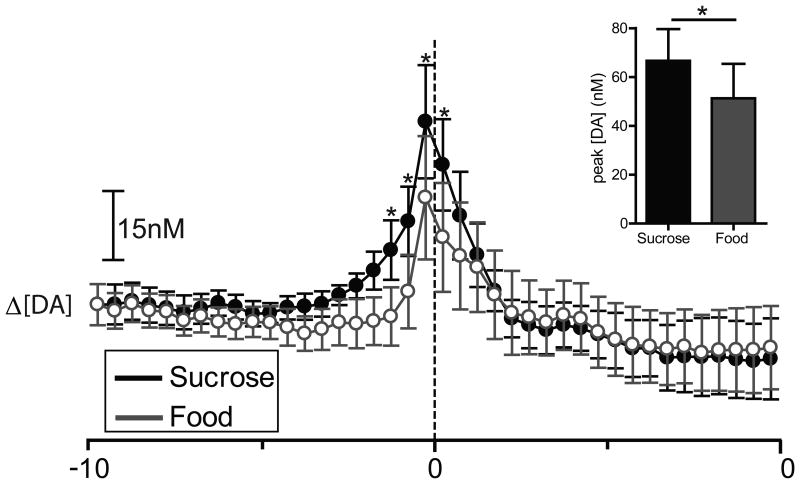
To compare dopamine release dynamics across the two reinforcer conditions (Fig. 5), a two-way repeated measures ANOVA (reinforcer type × time) was conducted on [DA]. There was no main effect of reinforcer type (F1,8 = 1.132, P > 0.05); however, there was a main effect of time (F39,312 = 3.856, P < 0.0001) and a significant reinforcer type × time interaction (F39,312 = 2.553, P < 0.0001). Newman-Keuls post hoc tests on the significant reinforcer type × time interaction revealed that [DA] was higher during responding for sucrose than during responding for food beginning 1.5 s before lever press and ending 0.5 s following lever press (P < 0.05).
Figure 5.
Sucrose and food responding evoke rapid dopamine release in the NAc core. [DA] is averaged into 500 msec bins (mean ± S.E.M.) and aligned to lever press (dotted line, time 0s) for food (gray) and sucrose (black). Data represent all animals (n = 6) that completed the sucrose/food multiple schedule. Asterisks above closed circles indicate significantly higher [DA] relative to sucrose-responding compared to food-responding within the indicated 500 msec bins, p < 0.05. Inset: comparison of peak [DA] within a 3sec window surrounding lever press for sucrose (black) versus food (gray). Peak [DA] is calculated with 100 msec bins, *p < 0.05.
As above, we also examined if there were differences in peak [DA] across the two reinforcer types, where peak [DA] was calculated with 100 msec bins restricted to a 3 s analysis window surrounding lever press. In this case, [DA] was significantly higher during lever pressing for sucrose than lever pressing for food (t8 = 2.552; P < 0.05; Fig. 5, inset).
3.4. Influence of reinforcer order and type on peak [DA] during performance of the sucrose/cocaine versus sucrose/food multiple schedules
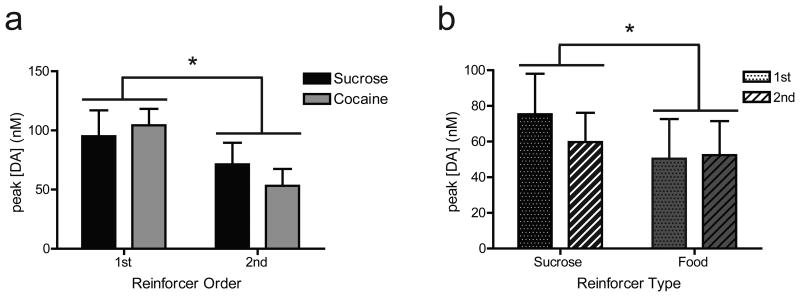
The order in which a reinforcer was presented during each multiple schedule was counterbalanced across animals such that one reinforcer did not predict access to the other. The influence of reinforcer order (i.e., self-administered 1st or 2nd) and reinforcer type (i.e., sucrose or cocaine) on peak [DA] was examined for each multiple schedule design. For the sucrose/cocaine multiple schedule, a two-way mixed design ANOVA with main effects of reinforcer type (sucrose vs. cocaine; between subjects factor), reinforcer order (1st or 2nd in multiple schedule; within subjects factor), and type × order interaction was performed on peak [DA]. This statistic revealed a main effect of reinforcer order (F1,6 = 24.114, P < 0.005), but no main effect of reinforcer type (F1,6 = 0.342, P > 0.05) or interaction (F1,6 = 0.335, P > 0.05). Therefore, peak [DA] was significantly higher for the reinforcer self-administered first compared to the reinforcer self-administered second in the sucrose/cocaine multiple schedule (Fig. 6a). These findings indicate that a higher average peak DA concentration was observed in phase 1, regardless of the type of reinforcer (sucrose or cocaine) obtained.
Figure 6.
The influence of reinforcer order and reinforcer type on peak [DA] during the multiple schedules. (a) Comparison of peak [DA] for the sucrose (black bars) versus cocaine (gray bars) multiple schedule. (b) Comparison of peak [DA] for the sucrose (dotted bars) versus food (striped bars) multiple schedule. *p < 0.05.
For the sucrose/food multiple schedule, a similar analysis was completed. Specifically, a two-way mixed design ANOVA with main effects of reinforcer type (sucrose vs. food; within subjects factor), reinforcer order (1st or 2nd in multiple schedule; between subject factor), and type × order interaction was performed on peak [DA]. This analysis showed a main effect of reinforcer type (F1,7 = 7.342, P < 0 .05), but no main effect of reinforcer order (F1,7 = 0.097, P > 0.05), and no interaction (F1,7 = 1.299, P > 0.05). Therefore, peak [DA] was higher relative to responding for sucrose than during responding for food, independent of order in the multiple schedule (Fig. 6b).
3.5. Time to peak [DA] during the sucrose/cocaine and sucrose/food multiple schedules
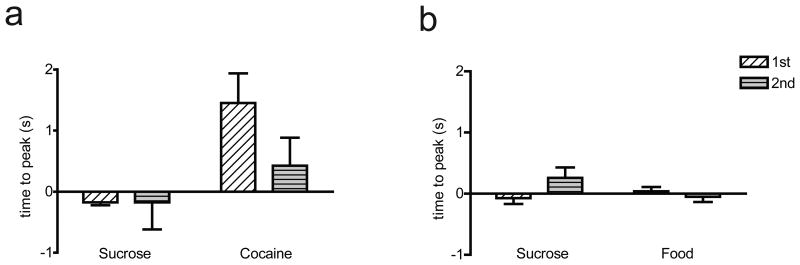
Finally, we examined the influence of both reinforcer type (cocaine, sucrose, or food) and order of reinforcer presentation (self-administered in phase 1 or phase 2) on the time to reach peak [DA] relative to lever press responding in each multiple schedule. These data are displayed in Figure 7 where negative values indicate time before the reinforced response (time 0) and positive values indicate time after the press. For the sucrose/cocaine multiple schedule, a two-way mixed design ANOVA was completed with main effects of reinforcer type (within subject factor), reinforcer order (between subject factor) and type × order interaction on peak [DA]. Results indicated no main effect of reinforcer type (F1,6 = 4.653, P = 0.07) or reinforcer order (F1,6 = 4.539, P > 0 .05), and no interaction (F1,6 = 0.987, P > 0 .05; Fig. 7a). Although not significant, there was a trend for the main effect of reinforcer type such that the time to reach peak [DA] was later for cocaine compared to sucrose (P = 0.074). These findings indicate that the time to reach peak [DA] concentration was slightly delayed when animals pressed for intravenous cocaine compared to when they responded for sucrose pellets, independent of phase.
Figure 7.
The influence of reinforcer type on time to peak [DA] within each multiple schedule. (a) Comparison of time to peak [DA] during the sucrose/cocaine multiple schedule. (b) Comparison of time to peak [DA] during the sucrose/food multiple schedule. Time 0 corresponds to lever press response. Positive numbers indicate the peak in [DA] occurred after lever press, negative numbers indicate that the peak in [DA] happened before lever press.
For the sucrose/food multiple schedule, a two-way mixed design ANOVA was completed with main effects of reinforcer type (within subjects factor), reinforcer order (between subjects factor) and type × order interaction on peak [DA]. Results revealed no main effect of reinforcer type (F1,7 = 1.028, P > 0 .05), no main effect of reinforcer order (F1,7 = 2.449, P > 0.05), and no interaction (F1,7 = 1.622, P> 0.05; Fig. 7b). Thus, unlike the sucrose/cocaine multiple schedule, there was no difference in time to peak [DA] relative to reinforcer type in the sucrose/food multiple schedule.
3.6. Histology
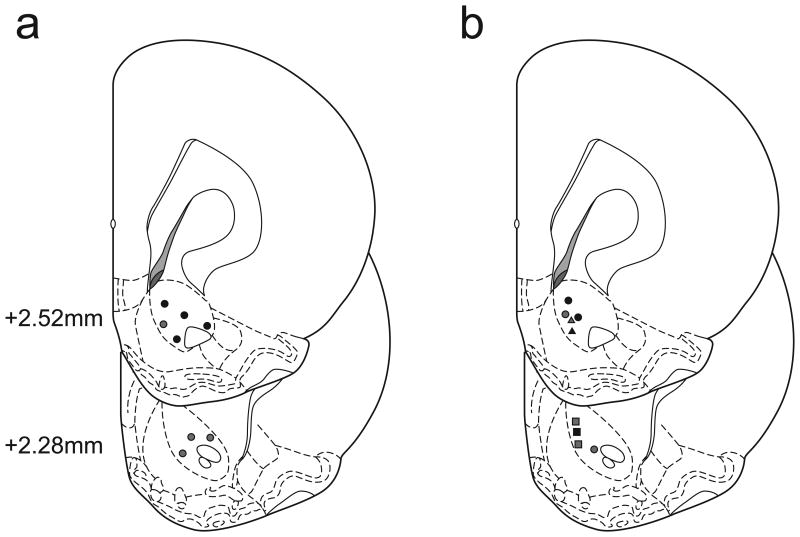
Histological reconstruction of carbon fiber electrode placements confirmed the location of recording sites in the NAc core (Fig. 8). Only data from electrode placements within the borders of the NAc core (Paxinos and Watson, 2007) were included in the analysis.
Figure 8.
Schematic representation of electrode tip placements in the NAc core. Numbers to the left of coronal sections indicate distance anterior to bregma (Paxinos & Watson, 2007). (a) Electrode placements for the sucrose/cocaine multiple schedule. Black dots indicate recording locations from animals that self-administered sucrose followed by cocaine. Gray dots represent recording locations from animals that self-administered cocaine followed by sucrose. (b) Electrode placements for the sucrose/food multiple schedule. Circles indicate recording locations from 4 separate animals. Triangles indicate 2 recording locations from the same rat over multiple days. Squares indicate 3 recording locations from the same rat over multiple days. Black symbols indicate recording locations in which animals self-administered sucrose followed by food. Gray symbols indicate recording locations in which animals self-administered food followed by sucrose.
4. Discussion
The present study incorporated multiple schedule designs to determine the dynamics of rapid dopamine release in the NAc core during operant responding for intravenous cocaine versus sucrose, or two natural rewards (sucrose and food). One advantage of using multiple schedules was that it enabled a direct comparison of dopamine release events in discrete NAc locations during lever pressing for different rewards within the same behavioral session. During the cocaine/sucrose multiple schedule, significant increases in rapid dopamine signaling were observed relative to operant responding during both phases of the task. Thus, unlike the selective cell firing of NAc neurons related to reinforcer type observed in previous electrophysiology studies (Carelli et al., 2000; Carelli, 2002; Carelli and Wondolowski, 2003; Carelli and Wondolowski, 2006; Cameron and Carelli, 2012), rapid dopamine release does not ‘turn on’ or ‘turn off’ across each phase of the cocaine/sucrose multiple schedule (i.e., is not reinforcer specific). However, more subtle differences were observed in peak [DA] across the drug versus natural reward conditions related to the order of reinforcer presentation and the time to reach peak [DA]. Likewise, similar dopamine release dynamics were observed during operant responding for two natural rewards, sucrose and food, also with some subtle differences in peak [DA] across reinforcer conditions. These findings are discussed in detail below.
4.1. Rapid dopamine release is not reinforcer specific during goal-directed behaviors for cocaine versus a natural reward
A major finding of the present study was that rapid dopamine release was not reinforcer specific during the sucrose/cocaine multiple schedule. That is, significant increases in [DA] were observed relative to operant responding for both sucrose and cocaine reward across both phases of the multiple schedule. The finding that rapid dopamine release increases in the NAc relative to lever pressing for a natural reward or intravenous cocaine is consistent with prior reports (Phillips et al., 2003b; Roitman et al., 2004). However, since electrophysiology studies from this laboratory have repeatedly shown that separate populations of NAc neurons selectively encode goal-directed actions for a natural reinforcer (food, water, or sucrose) versus cocaine (Carelli et al., 2000; Carelli, 2002; Carelli and Wondolowski, 2003; Carelli and Wondolowski, 2006; Cameron and Carelli, 2012), and that dopamine is considered a neuromodulator that may drive the activation of discrete populations of NAc neurons during behavior (O'Donnell et al., 1999; Nicola et al., 2000; Pennartz et al., 1994; Carelli and Wightman, 2004), the lack of reinforcer specificity in dopamine release dynamics reported here was unexpected. These findings indicate a more complex relationship between NAc activity that encodes goal-directed actions and rapid dopamine release events than simply a one-to-one correspondence (i.e. changes in cell firing are not related solely to increases in rapid dopamine release at the same sites). Indeed, using a combined electrophysiology/electrochemistry method we previously showed that although rapid dopamine release occurs almost exclusively at locations where NAc neurons encode goal-directed behaviors, only excitatory (not inhibitory) cell firing by NAc neurons appears functionally linked to rapid dopamine release (Cacciapaglia et al., 2011).
Another possibility is that phasic DA release during cocaine-responding was blunted through an increase in tonic extracellular DA levels. Repeated cocaine administration increases levels of tonic DA in the synapse, leading to presynaptic autoreceptor stimulation and decreased phasic DA release (Grace, 2000). However, this explanation can not account for the differences we observed in peak [DA] due to the order of reinforcer presentation, discussed in more detail below. Whether cocaine is self-administered prior to or after sucrose should have no effect on the autoreceptor-mediated feedback mechanism, yet the peak [DA] is higher when cocaine is self-administered first compared to when it is self-administered second.
Despite similarities in dopamine release dynamics across reinforcer conditions, more subtle differences in peak [DA] were revealed during the sucrose/cocaine multiple schedule. First, differences in rapid dopamine release events were observed as a function of reinforcer order in this task. Specifically, when cocaine was self-administered in phase 1, peak [DA] was higher relative to responding for it than during sucrose-responding in phase 2 (and vice versa for the opposite order of presentation). This finding was not observed when animals responded in the multiple schedule involving two natural rewards (sucrose and food). Thus, there was something unique about the sucrose/cocaine multiple schedule design (maybe related to having a history of cocaine training) that made dopamine release dynamics more responsive during presentation of the first reinforcer in the series.
A second subtle difference between dopamine release dynamics across the phases of the sucrose/cocaine multiple schedule was the time to reach peak [DA]. Prior work using FSCV indicated that increases in dopamine concentration typically begin within seconds prior to operant responding for cocaine (Phillips et al., 2003b) and sucrose (Roitman et al., 2004), and this increase in [DA] peaks near the execution of the lever press response. While similar in their temporal pattern, those prior studies did not compare the time to reach peak [DA] since measurements were completed in different animals. However, the multiple schedule designs incorporated here enabled a direct comparison of time to peak [DA] across reinforcer conditions. While not significant, we showed that the time to reach peak [DA] was delayed during cocaine-responding compared to responding for sucrose (Fig. 7a). It is likely that the relatively slower temporal resolution of FSCV (100 msec) compared to techniques such as in vivo electrophysiology prevented us from observing a statistically significant effect. However, this trend still suggests an interesting difference in the time to reach peak [DA]. This difference could be related to the delay of onset of interoceptive cues associated with cocaine versus food/sucrose. That is, in the present study intravenous cocaine infusion occurred over 6 s and typically takes another ∼10 s to result in general increases in dopamine release (transients) in the NAc due to its pharmacological actions (Stuber et al., 2005). In contrast, delivery of a pellet to a foodcup and its consumption typically occurs within ∼1-2s. Thus the difference in the temporal dynamics of peak [DA] is likely related to dissimilarities in the delivery, pharmacology, perception and consumption of the drug versus natural reward.
4.2. Rapid dopamine release is not reinforcer specific during goal-directed behaviors for two natural rewards
Rapid dopamine release dynamics were also similar relative to operant responding for two natural rewards in the sucrose/food multiple schedule. However, given previous findings from in vivo electrophysiological recordings showing that two natural reinforcers activate largely the same population of NAc neurons (Carelli et al., 2000; Roop et al., 2002), this later finding was not unexpected. Specifically, our earlier electrophysiology work found that the majority of NAc neurons recorded exhibited similar patterned cell firing (increases and/or decreases in activity) across two natural reinforcer conditions (Carelli et al., 2000). This overlapping pattern of phasic activity was maintained even when one of the natural reinforcers was of greater hedonic value (Roop et al., 2002). Thus, similarities in the temporal dynamics of rapid dopamine signaling and NAc cell firing should occur if the former functions as a neuromodulator of the later.
However, it is important to note that despite these similarities, more subtle differences in peak [DA] were observed during to sucrose/food multiple schedule. First, peak sucrose-evoked [DA] was significantly higher than peak food-evoked [DA] (Fig. 5), regardless of reinforcer order (Fig 6b). This finding may be related to differences in reward value for sucrose versus food. Indeed, prior electrochemistry studies revealed that the value of a reward is encoded by rapid dopamine release in the NAc, even when this value is subjective (Day et al., 2010; Sugam et al., 2012). In another study, sucrose-predictive cues evoked greater dopamine release in the NAc core than saccharin-predictive cues, and this difference in dopamine signaling was correlated with a behavioral preference for sucrose over saccharin (McCutcheon et al., 2012). The authors suggest that rats' preference for sucrose may be driven by several factors, including nutrition and taste; regardless, NAc dopamine appears to track this preference. While we did not directly test rats' preference for sucrose versus food in the present study, decades of research have shown that rats find sucrose more palatable than food (for a recent review, see (Berridge et al., 2010). Therefore, it seems likely that the greater dopamine release observed relative to sucrose compared to food responding in this study reflects a neural correlate of subjective hedonic value.
4.3. Insights into the functional organization of the NAc
It is often hypothesized that drugs of abuse exert their actions by ‘tapping into’ the brain reward system that has evolved to process natural reinforcers, causing aberrant reward processing and, ultimately, addiction (Wise, 1997). However, electrophysiological recordings from this laboratory have revealed that this notion may be an oversimplification, as goal-directed actions for natural and drug reinforcers are encoded by largely separate populations of NAc neurons (Carelli et al., 2000; Carelli, 2002; Carelli and Wondolowski, 2003; Carelli and Wondolowski, 2006), and appear to be influenced by time away from drug, or abstinence (Cameron and Carelli, 2012). Further, two natural reinforcers are encoded by largely overlapping populations of NAc neurons (Carelli et al., 2000; Roop et al., 2002). Collectively, these findings indicate that the NAc is part of a more complex neurocircuity underlying drug versus natural reward processing.
The anatomical organization of the NAc shows that this structure receives convergent synaptic inputs from a variety of cortical (e.g., PFC) and subcortical (e.g., BLA and hippocampus) structures, and in turn, sends efferent projections to motor areas, thereby supporting its role in limbic-motor integration (Mogenson et al., 1980). However, it is unlikely that the NAc as a whole sends a single integrated output to its target structures in order to initiate and modulate behavior. Theories of basal ganglia function suggest that the NAc is embedded in a larger system that is organized into several structurally and functionally discrete circuits that are essentially parallel in nature (Alexander et al., 1986; Alexander and Crutcher, 1990). Further, Pennartz et al. (1994) proposed that the NAc is composed of a collection of functionally heterogeneous ‘neuronal ensembles’ that are characterized by distinct afferent/efferent projections. Within this framework, dopamine acts as a neuromodulator, differentially influencing the discrete glutamatergic afferents onto specific neuronal ensembles (O'Donnell et al., 1999; Nicola et al., 2000; Carelli and Wightman, 2004) rather than exerting global actions across all NAc neurons.
Studies that employed a combined electrophysiology/electrochemistry method (i.e., enabling simultaneous measurements of rapid dopamine release and cell firing within discrete locations in the NAc) support this view. Those studies revealed that dopamine release occurs primarily at locations in the NAc where phasic cell activity was observed, and little to no dopamine release was observed at sites with nonphasic cell activity (Owesson-White et al., 2009; Cacciapaglia et al., 2011). While these findings might initially suggest that rapid dopamine release directly influences phasic cell firing, pharmacological manipulations show that this is not always the case. For example, reducing phasic DA release in the NAc has no effect on phasic inhibition of NAc neurons (Cheer et al., 2005; Cacciapaglia et al., 2011), but does reduce phasic excitation of NAc neurons (Cacciapaglia et al., 2011). Further, antagonism of D1-type dopamine receptors selectively reduces excitatory phasic activity of NAc neurons without altering DA release (Cheer et al., 2005). Together these findings suggest that rapid dopamine signaling plays a clear role in excitatory NAc cell firing, but is perhaps not functionally linked to inhibitory activity. As such, rapid dopamine release may not directly influence global NAc phasic cell firing, but make certain neurons more attuned to glutamatergic afferent inputs from brain regions such as the prefrontal cortex, basolateral amygdala and hippocampus.
4.4. Conclusions
The results of this study reveal that unlike NAc cell firing that is reinforcer specific during goal-directed behaviors for natural rewards versus cocaine, rapid dopamine release increases in a nonselective manner across reinforcer conditions. However, this study also reveals more subtle differences in peak [DA] across goal-directed behaviors for cocaine versus natural rewards related to factors such as the order of reinforcer presentation and the time to reach peak [DA]. As such, the exact relationship between dopamine release and phasic cell firing during operant responding for a natural versus drug reward remains to be established. Within distinct NAc microcircuits, rapid dopamine release likely modulates NAc cell activity based on a variety of factors such as dopamine receptor sub-type, phasic activity (excitatory versus inhibitory), reward type (sucrose versus food versus cocaine), and ongoing behavior. Additional studies are being completed that couple iontophoresis with FSCV and electrophysiology (Herr et al., 2010; Belle et al., 2013) to shed further insight into the causal relationship between rapid dopamine signaling and cell firing in the NAc during goal-directed behaviors.
Voltammetry was used to measure accumbens dopamine during goal-directed behaviors
Dopamine release was observed during responding for cocaine, sucrose, and food
Dopamine release during goal-directed behaviors was not reinforcer specific
However, more subtle disparities were noted in release dynamics across reinforcers
These findings shed light on the functional circuitry in the accumbens in reward
Acknowledgments
This research was supported by DA029978 to CMC and DA017318 to RMC. The authors thank Dr. Michael Saddoris for help with statistical analysis and Laura Ciompi and Fei Fei Wang for assistance in running experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in Neurosciences. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Belle AM, Owesson-White C, Herr NR, Carelli RM, Wightman RM. Controlled Iontophoresis Coupled with Fast-Scan Cyclic Voltammetry/Electrophysiology in Awake, Freely Moving Animals. ACS Chemical Neuroscience. 2013;4:761–771. doi: 10.1021/cn400031v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Research. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “Accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro-gold. The Journal of Comparative Neurology. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Cacciapaglia F, Wightman RM, Carelli RM. Rapid Dopamine Signaling Differentially Modulates Distinct Microcircuits within the Nucleus Accumbens during Sucrose-Directed Behavior. The Journal of Neuroscience. 2011;31:13860–13869. doi: 10.1523/JNEUROSCI.1340-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CM, Carelli RM. Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. European Journal of Neuroscience. 2012;35:940–951. doi: 10.1111/j.1460-9568.2012.08024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli R, Deadwyler S. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. The Journal of Neuroscience. 1994;14:7735–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’ reinforcement. Physiology & Behavior. 2002;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence That Separate Neural Circuits in the Nucleus Accumbens Encode Cocaine Versus “Natural” (Water and Food) Reward. The Journal of Neuroscience. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Current Opinion in Neurobiology. 2004;14:763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Selective Encoding of Cocaine versus Natural Rewards by Nucleus Accumbens Neurons Is Not Related to Chronic Drug Exposure. The Journal of Neuroscience. 2003;23:11214–11223. doi: 10.1523/JNEUROSCI.23-35-11214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Anatomic distribution of reinforcer selective cell firing in the core and shell of the nucleus accumbens. Synapse. 2006;59:69–73. doi: 10.1002/syn.20217. [DOI] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Comparison of Mesocorticolimbic Neuronal Responses During Cocaine and Heroin Self-Administration in Freely Moving Rats. The Journal of Neuroscience. 1998;18:3098–3115. doi: 10.1523/JNEUROSCI.18-08-03098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Heien MLAV, Garris PA, Carelli RM, Wightman RM. Simultaneous dopamine and single-unit recordings reveal accumbens GABAergic responses: Implications for intracranial self-stimulation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:19150–19155. doi: 10.1073/pnas.0509607102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM. Phasic Nucleus Accumbens Dopamine Release Encodes Effort- and Delay-Related Costs. Biological Psychiatry. 2010;68:306–309. doi: 10.1016/j.biopsych.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 95:119–128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Progress in brain research. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Heien MLAV, Khan AS, Ariansen JL, Cheer JF, Phillips PEM, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr NR, Daniel KB, Belle A, Carelli RM, Wightman RM. Probing presynaptic regulation of dopamine release with iontophoresis. ACS Chem Neurosci. 2010;1(9):627–638. doi: 10.1021/cn100056r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Chang JY, Woodward DJ. Neuronal spike activity in the nucleus accumbens of behaving rats during ethanol self-administration. Brain Research. 1999;817:172–184. doi: 10.1016/s0006-8993(98)01245-1. [DOI] [PubMed] [Google Scholar]
- Keithley RB, Carelli RM, Wightman RM. Rank Estimation and the Multivariate Analysis of in Vivo Fast-Scan Cyclic Voltammetric Data. Analytical Chemistry. 2010;82:5541–5551. doi: 10.1021/ac100413t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Beeler JA, Roitman MF. Sucrose-predictive cues evoke greater phasic dopamine release than saccharin-predictive cues. Synapse. 2012;66:346–351. doi: 10.1002/syn.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: Functional interface between the limbic system and the motor system. Progress in Neurobiology. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier DJ, Malenka RC. Dopaminergic Modulation of Neuronal Excitability in the Striatum and Nucleus Accumbens. Annual Review of Neuroscience. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-Evoked Firing of Nucleus Accumbens Neurons Encodes Motivational Significance During a Discriminative Stimulus Task. Journal of Neurophysiology. 2004;91:1840–1865. doi: 10.1152/jn.00657.2003. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Greene J, Pabello N, Lewis BL, Grace AA. Modulation of Cell Firing in the Nucleus Accumbens. Annals of the New York Academy of Sciences. 1999;877:157–175. doi: 10.1111/j.1749-6632.1999.tb09267.x. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Talluri S, Childers SR, Smith JE, Roberts DCS, Bonin KD, Budygin EA. Dopamine uptake changes associated with cocaine self-administration. Neuropsychopharmacology. 34:1174–1184. doi: 10.1038/npp.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Mark Wightman R, Carelli RM. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. European Journal of Neuroscience. 2009;30:1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th. New York: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: An integration of behavioural, electrophysiological and anatomical data. Progress in Neurobiology. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Lynch KG, Lesnock J, Gangadhar N. Accumbal Neural Responses During the Initiation and Maintenance of Intravenous Cocaine Self-Administration. Journal of Neurophysiology. 2004;91:314–323. doi: 10.1152/jn.00638.2003. [DOI] [PubMed] [Google Scholar]
- Phillips PEM, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods in molecular medicine. 2003a;79:443–464. doi: 10.1385/1-59259-358-5:443. [DOI] [PubMed] [Google Scholar]
- Phillips PEM, Stuber GD, Heien MLAV, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003b;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM. Dopamine Operates as a Subsecond Modulator of Food Seeking. The Journal of Neuroscience. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop RG, Hollander JA, Carelli RM. Accumbens activity during a multiple schedule for water and sucrose reinforcement in rats. Synapse. 2002;43:223–226. doi: 10.1002/syn.10041. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Cacciapaglia F, Carelli RM. Rapid dopamine dynamics in the accumbens core and shell: Learning and action. Frontiers in Bioscience. 2013;5:273–288. doi: 10.2741/e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PEM, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and non-contingent cocaine administration. Neuropsychopharmacology. 2005;30(5):853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Sugam JA, Day JJ, Wightman RM, Carelli RM. Phasic Nucleus Accumbens Dopamine Encodes Risk-Based Decision-Making Behavior. Biological Psychiatry. 2012;71:199–205. doi: 10.1016/j.biopsych.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Drug Self-Administration Viewed as Ingestive Behaviour. Appetite. 1997;28:1–5. doi: 10.1006/appe.1996.0059. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical Implications of the Nucleus Accumbens Core and Shell Subterritories. Annals of the New York Academy of Sciences. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]