Abstract
The orexin/hypocretin system has recently been implicated in reward-seeking, especially for highly salient food and drug rewards. Given that eating disorders affect women more than men, we reasoned that the orexin system may be strongly engaged in female rats, and during periods of food restriction as we recently reported in male rats. Therefore, the present study examined the involvement of the orexin system in operant responding for sucrose, and in cue-induced reinstatement of extinguished sucrose-seeking, in ad libitum fed vs. food-restricted female subjects. Female Sprague Dawley rats were trained to self-administer sucrose pellets, and we determined the effects of pretreatment with the OxR1 receptor antagonist SB 334867 (SB; 10-30 mg/kg) on fixed ratio (FR) sucrose self-administration, and on cue-induced reinstatement of extinguished sucrose-seeking. SB decreased sucrose self-administration in food-restricted but not in ad libitum-fed females. SB did not alter active lever responding during cue-induced reinstatement of sucrose-seeking in either feeding group. These results confirm our previous results in male rats that signaling at the OxR1 receptor is involved in the sucrose reinforcement and self-administration in food-restricted subjects. However, the finding that SB is ineffective at attenuating cue-induced reinstatement in females, but was effective in food-restricted males, leads us to conclude that food seeking induced by conditioned stimuli engages the orexin system differentially in males and females.
Keywords: orexin, obesity, reward-based feeding, palatable food, conditioned stimuli, females
1.1 Introduction
Like many eating disorders, obesity affects women more often than men (Amer Psy Assoc, 1994, Flegal et al., 2002; Klump et al., 2006; Ogden et al., 2003). The underlying difference precipitating this sex difference remains poorly understood, and there are few treatments that are effective for obesity in men or women. Estrogen regulates food intake by acting on hypothalamic nuclei in humans and animals, and the lack of estrogen in postmenopausal women contributes substantially to the development of obesity. However, estrogen replacement therapies in women produce adverse side effects. Therefore, better therapeutic interventions are needed to treat the growing obesity epidemic.
Sex differences in taste preferences for palatable foods and regulation of food intake may contribute to the sexual dimorphism in obesity rates. Several studies show that females prefer sweets compared to males (Valenstein et al., 1967; Wade et al., 1969; Zucker 1969). Furthermore, it is well known that food intake is regulated by the estrous cycle of the female rats (Asarian et al., 2006 for review). Other findings indicate that females, but not males, show a rebound increase in food intake when returned to ad libitum access following acute food restriction (Funabashi et al., 2009). These findings indicate that females may be more sensitive than males to changes in homeostatic factors that influence feeding.
Previous work also indicates that sexual dimorphisms in the orexin system may contribute to the difference in obesity rates in men and women. Orexins are implicated specifically in reward based feeding (Borgland et al., 2009; Cason et al., 2010; Cason and Aston-Jones, 2013a; Cason and Aston-Jones, 2013b; Choi et al., 2010; Choi et al., 2012; Nair et al., 2008), and female rats express higher levels of the orexin A peptide, and higher levels of OxR1s in the hypothalamus, than male rats (Johren et al., 2001; Taheri et al., 1999). In addition, activation of lateral hypothalamic orexin neurons is increased in female rats, but not in male rats, following acute food restriction (Funabashi et al., 2009).
Recent studies from our laboratory and others have implicated the orexin system in food-reinforced behaviors in addition to homeostatic feeding processes (Borgland et al., 2009; Cason et al., 2010; Cason and Aston-Jones, 2013a; Cason and Aston-Jones, 2013b; Choi et al., 2010; Choi et al., 2012; Nair et al., 2008). Central administration of orexin increases operant responding for sweets, and pharmacological blockade of OxR1s decreases responding for caloric rewards including high fat chocolate or sucrose (Borgland et al., 2009; Cason et al., 2010; Cason and Aston-Jones, 2013a), and non-caloric rewards such as saccharin (Cason and Aston-Jones, 2013b). Furthermore, pharmacological disruption of orexin signaling decreases cue-induced reinstatement of sucroseand saccharin-seeking, as well as binge eating of a high fat diet (Cason et al., 2010; Cason and Aston-Jones, 2013a; Cason and Aston-Jones, 2013b; Piccoli et al., 2012). Notably, the effects of orexin signaling on motivated behavior seem to be specific for palatable foods with high reinforcing value (Borgland et al., 2009; Piccoli et al., 2012). Moreover, ingestion and conditioned responding for the non-nutritive food saccharin is orexin-dependent (Cason and Aston-Jones, 2013b). These studies indicate that the orexin system plays a role in hedonically motivated feeding and in conditioned responding for highly rewarding foods in addition to homeostatically driven feeding (Borgland et al., 2009; Cason et al., 2010; Cason and Aston-Jones, 2013a; Cason and Aston-Jones, 2013b; Harris et al., 2005). More importantly, these studies link the orexin system to hedonically motivated eating and stimulus-induced food-seeking, both of which are major contributors to obesity.
The present study investigated the role of OxR1s in operant responding for sucrose and in cue-induced reinstatement of extinguished sucrose-seeking, as a function of food restriction in female rats. Previous evidence indicates that the orexin system regulates food-seeking associated with food restriction. For example, orexin signaling mediates food restriction-induced increases in operant responding (Vialou et al., 2011), and orexin mRNA expression is elevated in calorically restricted rats (Pankevich et al., 2010). Moreover, we recently reported that reinstatement of extinguished sucrose-seeking was orexin-dependent in food restricted male rats (Cason et al., 2010; Cason and Aston-Jones, 2013a). These studies highlight the potential of orexin signaling to modulate food-seeking behavior in the context of caloric restriction and dieting. Here, we examined whether the orexin system in females is engaged by food-associated stimuli in food-restricted subjects to increase motivational responding for sucrose.
1.2 Materials and Methods
1.2.1 Subjects
Female Sprague Dawley rats (Charles River, Wilmington, MA, USA) were singly housed under a reversed 12h/12h light/dark cycle (lights off 0600 h). Rats were divided into two groups: ad libitum fed and food-restricted. Ad libitum rats had free access to food and water; food-restricted rats had free access to water but were fed once daily (at 1500 h) to maintain their body weight at ~85% of the ad libitum rats’ body weights. All test procedures were conducted between 0700 -1200h. Rats were housed in the animal facility at the Medical University of South Carolina (AAALAC-accredited). All experiments were approved by the Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health specifications outlined in their Guide for the Care and Use of Laboratory Animals.
1.2.2 Experiment 1: Fixed ratio responding for sucrose
Self-administration sessions were conducted in standard operant chambers housed in sound attenuating cubicles and controlled via MED-PC IV software (Med-Associates, St Albans, VT, USA) as described previously (Cason and Aston-Jones, 2013a; Cason and Aston-Jones, 2013b). Rats were trained to lever press for sucrose reward (45 mg sucrose pellets, Test Diet, Richmond, IN, USA) on a fixed ratio 1 (FR1) schedule of reinforcement during daily 1 h sessions. Presses on the inactive lever had no programmed consequences. Pellet delivery was accompanied by a discrete light + tone compound cue (78 dB, 2900 Hz; white stimulus light above the active lever), and was accompanied by a 20 s timeout. The red house light (on the wall opposite the levers) was turned off during the sucrose reward and time-out. Rats were trained in self-administration until they produced 10 sessions in which they earned ≥ 10 sucrose pellets. Once such stable responding was established, rats were given a total of 3 injections (vehicle or SB) 30 min prior to a self-administration session. All rats received the vehicle injection first, 24h following self-administration day 10. All rats also received two SB injections (10, 20, or 30 mg/kg) prior to self-administration sessions. Only two doses of SB were tested in each rat, and 12-14 ad libitum and food-restricted rats were tested at each dose of SB. The order of SB injections was counterbalanced so that some rats received the higher dose of SB first and others received the lower dose first. Two or more daily sessions separated SB injections to allow responding to return to baseline levels of responding.
1.2.3 Experiment 2: Progressive ratio responding for sucrose
Following self-administration sessions, 16 rats (8 ad libitum fed, 8 food-restricted rats) from Experiment 1 were tested for progressive ratio responding for sucrose. 24 h following the final FR1 session, rats were transferred to a progressive ratio (PR) schedule of reinforcement where sucrose reward was dependent upon an increasing number of responses based on the following equation: Response ratio = [5e(pellet number × 0.2)] - 5. This equation produced the following sequence of required lever presses: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, etc. (Richardson and Roberts 1996). A single sucrose pellet was delivered each time the required response ratio was achieved. PR test sessions lasted until 1 h elapsed in which the rat did not obtain reward. The last response ratio achieved before 1 h with no reward was considered the breakpoint. Stable baseline PR responding was established across 6 test sessions. The following day rats were given injections of vehicle or SB (10 or 30 mg/kg) 30 min prior to PR testing. The order of injections was counterbalanced such that half of the rats received SB 10 mg/kg first and the other half received SB 30 mg/kg first. Two or more days separated injections to allow responding to return to baseline levels.
1.2.4 Experiment 3: Cue-induced reinstatement of extinguished sucrose-seeking
Following self-administration sessions, the remaining rats from Experiment 1 that were not tested for progressive ratio responding underwent daily extinction sessions during which sucrose reward and cues were withheld. Presses on either lever had no consequences. These sessions continued until rats met the criteria of two consecutive sessions with < 25 active lever presses each. Then, rats were tested for cue-induced reinstatement of sucrose-seeking by delivering the tone + light cues (previously associated with sucrose administration) for active lever presses; no sucrose reward was delivered.
To test the effects of the OxR1 antagonist SB-334867 (SB) on cue-induced reinstatement of sucrose-seeking, rats (n = 18 ad libitum, n = 14 food-restricted) were given four test sessions in a within-subjects design: two late extinction sessions with no cues (vehicle or SB pretreatment) and two cue-induced reinstatement sessions (vehicle or SB pretreatment). The order of sessions was counterbalanced so that the vehicle vs SB test sessions were presented in different orders to different rats. Vehicle or SB (30 mg/kg, ip) was given 30 min prior to test sessions. The SB dose was chosen from our previous studies with this paradigm showing that lower doses of SB did not significantly reduce cue-induced reinstatement of sucrose seeking in males (Cason and Aston-Jones, 2013a).
1.2.4 Drugs
SB [1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl urea hydrochloride, generously by the National Institute of Drug Abuse, Research Triangle Park, NC,USA], was suspended in 2% dimethylsulfoxide and 10% 2-hydroxypropyl-b-cyclodextrin (Sigma) in sterile water; SB was given in a volume of 4 ml/kg (i.p.) 30 min prior to self-administration, extinction or cue-induced reinstatement test sessions.
1.2.5 Data Analysis
Two-way ANOVAs, one-way ANOVAs and student's t-tests were utilized for analyses, with test day as a repeated measure when appropriate. Post hoc analyses were computed with the Tukey-Kramer test. All data are presented as mean ± SEM.
1.3 Results
1.3.1 Experiment 1A: Fixed ratio responding
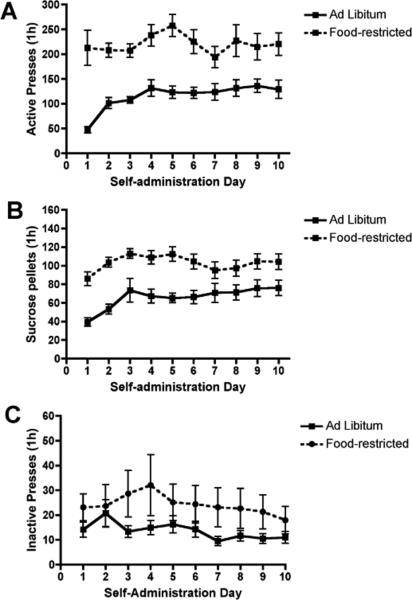
The number of days to meet the acquisition criteria for sucrose self- administration was similar in ad libitum (11.83 ± 0.40) and food-restricted (10.2 ± 0.09) female rats. The mean number of lever presses and sucrose pellets earned during the last 10 days of self-administration are shown in Figure 1. Repeated measures ANOVA did not reveal a significant group (food-restricted vs. ad libitum) × test day (1-10) interaction for active presses or the number of sucrose reinforcements earned. There was a significant main effect of group [F(1,58) = 42.58, p < 0.001] and test day [F(9,522) = 5.27, p < 0.001] on active presses and significant main effects of group [F(1,58) = 34.36, p < 0.001] and test day [F(9,522) = 15.31, p < 0.001] on the number of sucrose reinforcements earned. As expected, food-restricted rats performed significantly more active lever presses (Fig 1A) and earned more sucrose reinforcements (Fig 1B) than ad libitum fed rats. Active pressing and the number of sucrose reinforcements earned were increased on test days 2-10 compared to the first test day (Fig 1A, B). There was no significant group × test day interaction on inactive lever presses. There was a significant main effect of group [F(1,58) = 4.03, p = 0.05] and test day [F(9,522) = 2.00, p < 0.05] on inactive lever presses. Food-restricted rats exhibited slightly more inactive presses compared to ad libitum fed rats; and in contrast to active presses, inactive presses decreased across test days (Fig 1C).
Figure 1.
Operant lever responding for sucrose in food-restricted and ad libitumfed female rats. Food-restricted female rats (n = 20) exhibited more active presses (p < 0.001) and obtained more sucrose pellets (p < 0.001) than ad libitum females (n = 24) during FR1 self-administration. There was no difference in the number of inactive presses between groups.
1.3.2 Experiment 1B: Effects of pretreatment with the OxR1 antagonist SB-334867 on FR responding
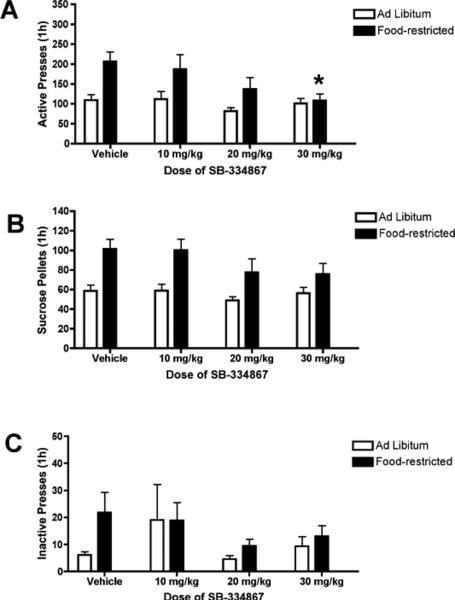
Figure 2 shows the effects of pretreatment with SB on established sucrose self-administration. For active presses, a 2-way ANOVA did not reveal a significant group (food-restricted vs. ad libitum) × dose (0,10,20,30 mg/kg) interaction [F(3,112) = 1.59, p = 0.20]. There was a significant main effect of group [F(1,112) = 13.391, p < 0.001] and a significant main effect of dose (Fig 2A). Post hoc analyses showed that food-restricted females exhibited more active presses than ad libitum fed females (p < 0.001), and pretreatment with SB (20 and 30 mg/kg) decreased active pressing compared to vehicle pretreatment (p < 0.05). However, the effect of SB on active pressing appeared mainly due to the large decrease in active presses following pretreatment with SB in food-restricted rats. When separate one-way ANOVAs within groups (ad libitum or food-restricted) were used to compare the effects of dose of SB on active lever presses, ANOVAs revealed that SB decreased active lever pressing in food-restricted rats [F(3,55) = 2.82, p < 0.05], but not in ad libitum rats. Post hoc analysis in food-restricted rats indicated that only the highest dose of SB (30 mg/kg) significantly decreased active presses during sucrose self-administration (p = 0.01).
Figure 2.
Attenuation of fixed ratio responding for sucrose in food-restricted female rats by the OxR1 antagonist SB-334867 (SB). Rats were pretreated with SB or vehicle 30 min prior to the self-administration session. SB 30 mg/kg reduced active lever presses in food-restricted rats (p < 0.05) compared to vehicle treated rats. SB had no significant effect on the number of sucrose pellets obtained or on inactive lever presses in food-restricted or ad libitum fed rats. * p < 0.01 versus vehicle injection.
Additionally, two-way ANOVA revealed there was not a significant group (ad libitum or food restricted) × dose (0,10,20,30 mg/kg) interaction, or a significant main effect of dose, for the number of sucrose pellets earned (Fig 2b). There was a significant main effect of group [F(1,112) = 25.05, p < 0.001]. Post hoc analysis showed that food-restricted females earned more sucrose rewards than ad libitum fed rats. Responding on the inactive lever was minimal regardless of group, and there was not a significant group × dose interaction or a significant main effect of group or dose for inactive presses (Fig 2c).
1.3.3 Experiment 2: Progressive ratio responding for sucrose
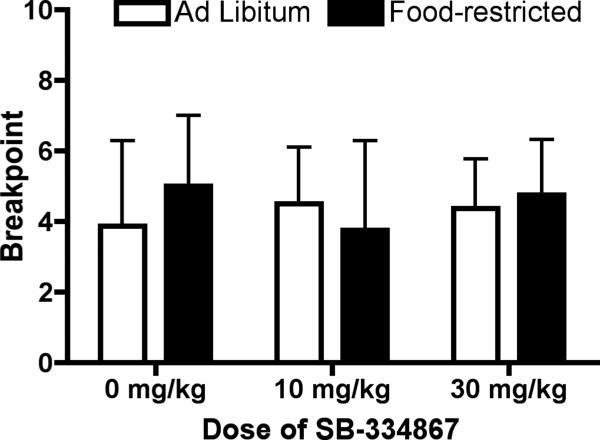
Student's t-test revealed that there was no difference in PR breakpoint between ad libitum fed and food-restricted rats during baseline PR testing. The effects of pretreatment with vehicle or SB (10 or 30 mg/kg) on breakpoints for sucrose during PR testing are shown in Figure 3. Two-way ANOVA for group (ad libitum or restricted) × dose (0, 10, 30) results did not show a significant group × dose interaction or main effect of group or dose.
Figure 3.
Progressive ratio responding for sucrose was not affected in females by pretreatment with the OxR1 antagonist SB-334867 (SB). Rats were pretreated with SB or vehicle 30 min prior to the progressive ratio (PR) session. SB had no effect on PR breakpoint in food-restricted or ad libitum fed rats.
1.3.4 Experiment 3: Cue-induced reinstatement of extinguished sucrose-seeking
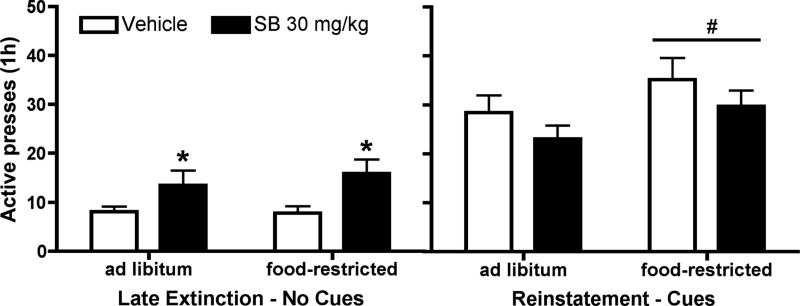
Both ad libitum fed and food-restricted female rats met extinction criterion within five extinction sessions, and subsequently showed cue-induced reinstatement. Figure 4 shows the mean number of active lever presses during late extinction sessions vs. during cue-induced reinstatement of sucrose-seeking following pretreatment with SB (30 mg/kg) or vehicle. A two-way ANOVA revealed that there was not a significant group (ad libitum vs food-restricted) × treatment (vehicle vs SB) interaction during extinction or a significant main effect of group on active pressing during extinction. There was a significant effect of treatment [F(1,60) = 8.31, p < 0.01]. Post hoc analysis showed SB increased active pressing during extinction. A two-way ANOVA revealed that during reinstatement there was not a significant group (ad lib vs restricted) × treatment (vehicle vs SB) interaction or significant main effect of treatment. There was a significant main effect of group [F(1,60) = 4.06, p < 0.05] on active pressing during reinstatement; food-restricted rats exhibited more active presses than ad libitum fed rats.
Figure 4.
Cue-induced reinstatement of sucrose-seeking in female rats was not affected by pretreatment with the OxR1 antagonist SB-334867 (SB). In a within-subject design, female rats were pretreated with SB (30 mg/kg) or vehicle 30 min prior to late extinction sessions (no cues or sucrose) or cue-induced reinstatement (tone + light cues). SB increased active pressing during extinction (p < 0.05) regardless of food-restriction. Both ad libitum-fed and food-restricted females showed increased active lever responding following vehicle + cues (reinstatement session) compared to vehicle + no cues (late extinction session), and food-restricted rats showed greater reinstatement of active lever pressing compared to ad libitum fed rats (p < 0.05). Reinstatement of active lever pressing was not significantly affected in either group by pretreatment with 30 mg/kg SB (n = 18, ad libitum; n = 14 food-restricted). * p < 0.05 versus vehicle injection, # p < 0.05 versus ad libitum fed rats.
1.4 Discussion
The present study indicates that signaling at the OxR1 receptor is involved in sucrose seeking and reinforcement in female rats. These findings are consistent with the view that orexin neurons are involved in the motivation induced by highly salient rewards (as discussed in the Introduction). Pretreatment with the OxR1 antagonist, SB, significantly attenuated operant responding for sucrose during self-administration in food-restricted female rats; however, pretreatment with SB had no effect on cue-induced reinstatement of extinguished sucrose-seeking in female rats regardless of food-restriction.
1.4.1 OxR1 antagonist (SB-334867) effects on FR and PR responding for food
SB attenuated responding for food in female rats, and the reduction in responding was dependent of food restriction. Our findings in females are consistent with previous findings in male rats that SB decreases FR responding for palatable food rewards including sucrose and high-fat food in food-restricted rats (Borgland et al., 2009; Cason et al., 2010; Cason and Aston-Jones, 2013a), but not in ad libitum fed rats (Cason and Aston-Jones 2013a; Richards et al., 2008). Responding for sucrose during FR self-administration was lower in food-restricted females compared to previously published levels in male rats, while responding for sucrose was similar in ad libitum fed female and male rats (Cason and Aston-Jones 2013a). Furthermore, higher doses of SB (30 mg/kg) were required to attenuate self-administration responding in female rats compared to previously published doses (10-30 mg/kg) in male rats (Cason and Aston-Jones, 2013a). Similarly, female rats exhibited lower breakpoints for sucrose reinforcement during PR testing when compared to findings in male rats (Cason and Aston-Jones, 2013a). In contrast to male rats, food restriction failed to increase this measure of motivation to obtain sucrose reinforcement; and SB did not decrease motivation for sucrose reinforcement in female rats. Together these findings indicate that food-restriction increases motivation for sucrose reinforcement to a lesser extent in female compared to male rats, and that OxR1 signaling may be involved in this higher motivation in male rats.
1.4.2 OxR1 antagonist (SB-334867) effects on cue-induced reinstatement of extinguished food-seeking
The present study is the first to investigate a role of orexin in cue-induced reinstatement of extinguished food seeking in female rats. Our finding that signaling at the Ox1R is not necessary for cued reinstatement of sucrose-seeking in females is in stark contrast to our previous results in male rats showing that the OxR1 antagonist significantly attenuated cue-induced reinstatement of sucrose seeking in food restricted male rats (Cason and Aston-Jones, 2013a). Importantly, male and female rats pretreated with vehicle show similar levels of cue-induced reinstatement, show similar levels of SB in the brain following systemic SB injections (Zhou et al., 2012), and show similar responses to SB during sucrose self-administration (Cason and Aston-Jones, 2013a). Therefore, the present finding that SB does not attenuate cue-induced reinstatement of extinguished sucrose-seeking in females, in combination with our contrasting findings in males (Cason and Aston-Jones, 2013a), indicate that signaling at the OxR1 is sexually dimorphic during cue-driven food seeking. Similarly, a recent study by Zhou et al (2012) demonstrated that SB is ineffective at reducing cue-induced reinstatement of cocaine seeking in female rats although it attenuates this behavior in males. Together, these findings indicate that orexin's influence on cue-induced seeking of both drugs and natural rewards is sex-dependent.
It is interesting that SB increased late extinction responding in both ad lib and food-restricted females. This was not seen in males and reflects another sex difference in the role of orexin in food-seeking (Cason and Aston-Jones, 2013a,b). The functional implications of this increased extinction responding are not clear at present, but might indicate that orexin plays a larger role in memory for reward devaluation in females than in males. Additional studies are needed to test this and other possibilities.
Our findings that OxR1 signaling is not necessary for cue-induced food seeking in females indicate that other systems are likely driving cue-induced food-seeking in females. Other feeding peptides like leptin, insulin and ghrelin are involved in reward-driven feeding and may potentially modulate cue-induced food-seeking (Monteleone and Maj, 2013; Davis et al., 2012; Kanoski et al., 2012; Perello and Zigman, 2012; Daws et al., 2011; Clegg et al., 2007). Additionally, changes in the hormonal mileu of the female rat that influence homeostatic feeding could also influence cue-induced food-seeking (Asarian and Geary, 2006). Future studies are needed to determine if additional peptides like insulin, leptin or ghrelin independent of orexin drive cue-induced reinstatement in females and to determine if estrogen levels of female rats influence cue-driven food seeking potentially protecting females from disruptions in OxR1 signaling. Finally, it is possible that these internal signals interact with the orexin system rendering male rats more sensitive than female rats to disruptions in OxR1 signaling during cue-driven food-seeking.
1.4.3 Orexin and reward circuitry
It is unknown where orexin acts to influence sucrose-motivated behavior, but orexin targets in the midbrain dopaminergic or basal forebrain cholinergic systems are plausible sites of action. Previous studies have shown that cortical acetylcholine release in response to food or associated stimuli requires signaling via the OxR1 (Fadel at al., 1996; Frederick-Duus et al., 2007; Inglis et al., 2004; Petrovich et al., 2002), and that midbrain dopaminergic projections to hypothalamus stimulate orexin neurons to increase ingestion of palatable food (Stratford et al. 1997; Stratford and Kelley 1999; Zhang and Kelley 2000; Zheng et al. 2007). Findings from more recent studies indicate that central manipulation of OxR1 in ventral tegmental area and the paraventricular nucleus of the thalamus disrupts other reward driven feeding behaviors (Zheng et al., 2007; Choi et al., 2012). Central administration of other pharmacological agents in the nucleus accumbens shell, a region activated by food-associated cues, modulate cue-driven reinstatement to food-seeking and may also be involved in these behaviors (Floresco et al, 2008; Guy et al., 2011); thus, this is one area to examine for sex differences in orexin signaling identified in our studies. Interestingly, limbic areas such as the amygdala and bed nucleus of the stria terminalis are implicated in food-seeking and food-cue interactions and are sexually dimorphic (Stefanova and Ovtscharoff, 2000). Importantly, all of the nuclei described above receive dense orexin projections (Peyron et al., 1998; Sutcliffe and de Lecea, 2002). Future studies examining the effects of SB on Fos expression in orexin targets following cue-induced reinstatement of extinguished sucrose-seeking will further elucidate orexin's specific site of action and distinguish circuits where orexin actions are sexually dimorphic.
It seems unlikely that the effects of SB in the present studies were due to a generalized locomotor effect. SB did not attenuate inactive presses, nor did it decrease cue-induced reinstatement of sucrose seeking in ad libitum or food-restricted female rats. Similarly, other studies have shown that SB has little or no effect on responding for other rewards (Borland et al., 2009; Smith et al., 2009). These findings indicate that SB does not substantially impair lever-pressing behavior.
1.4.4 Implications for Obesity
Recent clinical studies have linked increased visual attention bias to food related cues and increased neuronal activation of reward centers in anticipation of food reward to obesity (Castellanos et al. 2009; Nijs et al 2012; Rothemund et al 2007; Stice et al. 2008). While our previous findings in male rats linked the orexin system with cue-induced food-seeking in food-restricted males, our current findings indicate that the orexin system does not modulate the same behavior in females. Notably, findings from a recent study indicate that males have increased neuronal activation compared to females in several brain regions implicated in taste processing and reward during food-restriction (Haase et al., 2011). Therefore, treatments that decrease conditioned orexin signaling may provide a novel treatment for obesity in a sex-dependent manner. Future studies are needed to identify the neural systems that elicit cue-induced food-seeking in female rats.
1.4.5 Conclusions
Our findings show that OxR1 activation regulates operant responding for sucrose reinforcement, but not motivation to work for sucrose or for sucrose-conditioned cues to drive sucrose-seeking in female rats. Together with our previous findings in male rats, these findings indicate that the OxR1 is more involved in highly motivated behaviors such as cue-induced reinstatement in male than in female rats, particularly during food restriction (Cason and Aston-Jones, 2013a). These sex differences indicate that signaling at the OxR1 has different roles with respect to sucrose food seeking in male vs. female rats.
Highlights.
OxR1 activation regulates responding for sucrose reward
OxR1 activation regulates cue-induced reinstatement of sucrose-seeking in male rats
The OxR1 is more involved in food seeking in food-restricted male rats
Acknowledgements
This research was supported by PHS grants DA 023354, DA 017289 and DA006214.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Cason has no biomedical financial interests or potential conflict of interests to disclose. Dr. Aston-Jones has no biomedical financial interests or potential conflict of interests to disclose.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: 1994. [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc London B Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell JE. Dose-response effects of orexin-A on food intake and the behavioural satiety sequence in rats. Regul Pept. 2000;96:71–84. doi: 10.1016/s0167-0115(00)00203-2. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/Hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100(5):419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking. Psychopharm. 2013a;226(1):155–165. doi: 10.1007/s00213-012-2902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G. Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharm. 2013b;228(3):499–507. doi: 10.1007/s00213-013-3051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes. 2009;33:1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167(1):11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience. 2012;210:243–248. doi: 10.1016/j.neuroscience.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexingenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- Davis JF, Perello M, Choi DL, Magrisso IJ, Kirchner H, Pfluger PT, Tshoep M, Zigman JM, Benoit SC. Goat induced ghrelin acylation regulates hedonic feeding. Horm Behav. 2012;62:598–604. doi: 10.1016/j.yhbeh.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Avison MJ, Robertson SD, Niswender KD, Galli S, Saunders C. Insulin signaling and addiction. Neuropharmacology. 2011;61:1123–1128. doi: 10.1016/j.neuropharm.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Moore H, Sater M, Bruno JP. Trans-synaptic stimulation of cortical acetylcholine release after partial 192 IgG-saporin-induced loss of cortical cholinergic afferents. J Neurosci. 1996;16(20):6592–6600. doi: 10.1523/JNEUROSCI.16-20-06592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. J Am Med Assoc. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience. 2008;154:877–884. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Frederick-Duus D, Guyton MF, Fadel J. Food-elicited increases in cortical acetylcholine release require orexin transmission. Neuroscience. 2007;149(3):499–507. doi: 10.1016/j.neuroscience.2007.07.061. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Hagiwara H, Mogi K, Mitsushima D, Shinihara K, Kimura F. Sex differences in the response of orexin neurons in the lateral hypothalamic area and feeding behavior to fasting. Neurosci Lett. 2009;463:31–34. doi: 10.1016/j.neulet.2009.07.035. [DOI] [PubMed] [Google Scholar]
- Guy EG, Choi E, Pratt WE. Nucleus accumbens dopamine and my opioid receptors modulate reinstatement of food-seeking behavior by food-associated cues. Behav Brain Res. 2011;219(2):265–272. doi: 10.1016/j.bbr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Haase L, Green E, Murphy C. Males and females show differential brain activatin to taste when hungry and sated in gustatory and reward areas. Appetite. 2011;57(2):421–434. doi: 10.1016/j.appet.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Day JC, Fibiger HC. Enhanced acetylcholine release in hippocampus and cortex during the anticipation and consumption of a palatable meal. Neuroscience. 2004;62(4):1049–1056. doi: 10.1016/0306-4522(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, et al. Differential effects of the selective orexin-1 receptor anagonist SB-334867 and lithium chloride on the behavioural satiety sequence in rats. Physiol Behav. 2004;81:129–140. doi: 10.1016/j.physbeh.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, et al. Satiety enhancement by selective orexin-1 receptor anagonist SB-334867: influence of test context and profile comparison with CCK8-S. Behav Brain Res. 2005;160:11–24. doi: 10.1016/j.bbr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Johren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P. Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology. 2001;142(8):3324–3331. doi: 10.1210/endo.142.8.8299. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry. 2013;73(9):915–923. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilduff TS, de Lecea L. Mapping of the mRNAs for the hypocretin/orexin and melanin-concentrating hormone receptors: networks of overlapping peptide systems. J Comp Neurol. 2001;435:1–5. doi: 10.1002/cne.1189. [DOI] [PubMed] [Google Scholar]
- Klump KL, Gobrogge KL, Perkins PS, Thorne D, Sisk CL, Breedlove SM. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychol Med. 2006;36:539–546. doi: 10.1017/S0033291705006653. [DOI] [PubMed] [Google Scholar]
- Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav. 2000;37:335–344. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Maj M. Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: beyond the homeostatic control of food intake. Psychoneuroendocrinology. 2013;38(3):312–330. doi: 10.1016/j.psyneuen.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154(2):406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs IM, Muris P, Euser AS, Franken IH. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite. 2010;54(2):243–254. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Flegal KM. Epidemiologic trends in overweight and obesity. Endocrinol Metab Clin North Am. 2003;32:741–760. doi: 10.1016/s0889-8529(03)00074-4. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Teegarden SL, Hedin AD, Jensen CL, Bale TL. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J Neurosci. 2010;30(48):16399–16407. doi: 10.1523/JNEUROSCI.1955-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Zigman JM. The role of ghrelin in reward-based feeding. Biol Psychiatry. 2012;72:347–353. doi: 10.1016/j.biopsych.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli L, Micioni Di Bonaventura MV, Cifani C, Costantini VJ, Massagrande M, Montanari D, Martinelli P, Antolini M, Ciccocioppo R, Massi M, Merio-Pich E, Di Fabio R, Corsi M. Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharm. 2012;37(9):1999–2011. doi: 10.1038/npp.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evan rats. Psychopharm. 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self administration studies in rats: a method to evaluate reinforcing efficancy. J Neurosci Methods. 1996;66(1):1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, et al. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410–21. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Smith R, See R, Aston-Jones G. Orexin/hypocretin signaling at the OX1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N, Ovtscharoff W. Sexual dimorphism of the bed nucleus of the stria terminalis and the amygdala. Adv Anat Embryol Cell Biol. 2000;158:III–X. 1–78. doi: 10.1007/978-3-642-57269-2. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2008;31(12):4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17(11):4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19(24):11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Taheri S, Mahmoodi M, Opacka-Juffry J, Ghatel MA, Bloom SR. Distribution and quantification of immunoreactive orexin A in rat tissues. FEBS Lett. 1999;457(1):157–161. doi: 10.1016/s0014-5793(99)01030-3. [DOI] [PubMed] [Google Scholar]
- Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharm. 2005;182(1):75–83. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Kakolewski JW, Cox VC. Sex differences in taste preference for glucose and saccharin solutions. Science. 1967;156:942–943. doi: 10.1126/science.156.3777.942. [DOI] [PubMed] [Google Scholar]
- Vialou V, Cui H, Perello M, Mahgoub M, Yu HG, Rush AJ, et al. A role for delta FosB in calorie restriction-induced metablic changes. Biol Psychiatry. 2011;70(2):204–207. doi: 10.1016/j.biopsych.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade GN, Zucker I. Hormonal and developmental influences on rat saccharin preferences. J Comp Physio Psy. 1969;2:291–300. doi: 10.1037/h0028208. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999;849:248–252. doi: 10.1016/s0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99(2):267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J Pharmacol Exp Ther. 2012;340(3):801–809. doi: 10.1124/jpet.111.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker I. Hormonal Determinants of sex differences in saccharin preference, food intake, and body weight. Physio Beh. 1969;4:595–602. [Google Scholar]