Abstract
The aims of this study were to determine (i) the effects of intracerebroventricular (i.c.v.) injections of 5-hydroxytryptamine (5-HT, 10 µg) on mean arterial blood pressure (MAP), heart rate (HR) and mesenteric (MR), renal (RR) and hindquarter (HQR) vascular resistances of conscious rats, (ii) the central 5-HT receptor subtype which mediates these effects, and (iii) the role of nitric oxide (NO) in the expression of these responses. The i.c.v. injection of 5-HT had minor effects on MAP but produced a decrease in HR (−18 ± 4%), which lasted for 20 min. The i.c.v. injection of 5-HT elicited marked increases in MR (+50 ± 7%) and reductions in HQR (−31 ± 3%). These responses occurred promptly and lasted for 25–35 min. 5-HT also produced a transient decrease in RR (−26 ± 8% at 10 min). All of these responses were prevented by the prior i.c.v. injection of the 5-HT1/5-HT2-receptor antagonist, methysergide (10 µg). The intravenous injection of the NO synthesis inhibitor, L-NAME (25 µmol/kg), produced a sustained pressor response, bradycardia and increases in MR, RR and HQR. Subsequent i.c.v. injection of 5-HT produced a minor pressor response (+7 ± 2%), bradycardia (−18 ± 3%), an increase in MR (+52 ± 8%) but no decreases in RR or HQR. This study demonstrates that i.c.v. 5-HT differentially affects peripheral vascular resistances by activation of central 5-HT1/5-HT2-receptors. It appears that L-NAME did not interfere with the central actions of 5-HT as it did not prevent the 5-HT-induced bradycardia or mesenteric vasoconstriction. Since the 5-HT-induced falls in RR and HQR were abolished by L-NAME, it is possible that these responses are mediated by an active neurogenic process involving the release of NO within the vasculature.
Keywords: 5-hydroxytryptamine (5-HT), intracerebroventricular, arterial blood pressure, heart rate, regional vascular blood flows, regional vascular resistances, methysergide, 5-HT1/5-HT2-receptors, nitric oxide, conscious rats
Introduction
There is substantial evidence that central neurons using 5-hydroxytryptamine (5-HT) as their neurotransmitter regulate cardiovascular function (Kristic and Djurkovic, 1981; Dalton, 1986; Coote, 1990; Conner and Higgins, 1990; Valenta et al., 1990; Dreteler et al., 1991; Sporton et al., 1991; Bagdy et al., 1992). This evidence includes that central injections of 5-HT or 5-HT receptor agonists alter mean arterial blood pressure (MAP), heart rate, and parasympathetic and sympathetic nerve activity (Baum and Shropshire, 1975; Yusof and Coote, 1988; Inoue and Bunag, 1989; Nosjean and Guyenet, 1991; Anderson et al., 1992). For example, Inoue and Bunag (1989) reported that intracerebroventricular (i.c.v.) injection of 5-HT in anesthetized rats elicited a pressor response, which was associated with bradycardia and sympathoinhibition. This pressor response was attenuated by i.c.v. administration of the non-selective 5-HT receptor antagonist, methysergide, or systemic injection of an arginine vasopressin (AVP) V1-receptor antagonist (Inoue and Bunag, 1989). The pressor response elicited by i.c.v. 5-HT in anesthetized rats is also attenuated by cervical spinal cord transection, adrenalectomy, or systemic injection of adrenergic blocking drugs or α-adrenoceptor antagonists and (Kristic and Djurkovic, 1980). Furthermore, i.c.v. 5-HT causes a natriuresis that is dependent upon intact renal nerves (Montes and Johnson, 1990). This suggests that i.c.v. 5-HT increases blood pressure by activation of the sympathetic nervous system although Inoue and Bunag (1989) found that i.c.v. 5-HT did not increase splanchnic sympathetic nerve activity. However, a later study in anesthetized rats found that i.c.v. 5-HT increased renal sympathetic nerve activity via activation of central 5-HT1A-receptors and stimulated AVP release via activation of central 5-HT2 receptors (Anderson et al., 1992). It is also established that i.c.v. injections of 5-HT elicit pressor responses and bradycardia in conscious rats (Dalton, 1986; Sukamoto et al., 1984; Montes and Johnson, 1990). However, the peripheral hemodynamic mechanisms, which underlie the arterial blood pressure responses elicited by i.c.v. 5-HT in conscious Sprague-Dawley rats are unknown. As such, a major aim of this study was to examine the time-course of effects of i.c.v. injection of 5-HT (10 µg) on vascular resistances in mesenteric, renal and hindquarter beds of conscious rats and to establish whether i.c.v. injection of methysergide (10 µg) blocks the responses. The dose of 5-HT (10 µg, which is approximately 25 nmol) was chosen on the basis that it produced robust and consistent changes in regional vascular resistances. The 25 nmol dose is within the range of doses (0.3 to 100 nmol) used by others in conscious rats (Dalton, 1986; Sukamoto et al., 1984; Montes and Johnson, 1990; Watts et al., 2012).
Endothelium-derived nitric oxide (NO) and other NO-containing factors such as S-nitrosothiols (together referred to as nitrosyl factors) play essential roles in the regulation of vascular resistance (see Moncada et al., 1991; Davisson et al., 1996a; Lewis et al., 2006; Moncada and Higgs, 2006; Lima et al., 2010). Moreover, we found that active sympathetic neurogenic vasodilation in the hindlimb of conscious rats is also mediated by the release of nitrosyl factors (Davisson et al., 1994, 1996b,c; Davisson et al., 1997). We therefore sought to establish the role of nitrosyl factors in the hemodynamic responses produced by i.c.v. administration of 5-HT. In these studies, we examined the hemodynamic responses produced by the i.c.v. injection of 5-HT in rats pretreated systemically with a 25 µg/kg dose of the NO synthesis inhibitor, NG-nitro-L-arginine methylester (L-NAME) (Moncada et al., 1991). This dose of L-NAME elicits robust hemodynamic responses, most likely by actions within microvasculature (see Davisson et al., 1996a), but does not appear to act in the brain since it did not interfere with central processing of afferent input regulating hemodynamic function (Possas et al., 1997, 2006).
MATERIALS AND METHODS
Animals
All procedures were in accordance with the National Institutes of Health (1986) Guides for the Care and Use of Laboratory Animals and were approved by the University of Iowa Animal Care and Use Committee. All studies were performed in conscious male Sprague-Dawley rats (Harlan, Inc., Madison, Wisconsin) weighing 300–350 grams (n = 40). The rats were individually housed in and maintained on a daily 12 hour light/dark cycle with rat chow and tap water available ad libitum.
Implantation of i.c.v. cannulae, catheters and Doppler flow probes
The rats were anesthetized with acepromazine maleate (12 mg/kg, i.p.) and ketamine (120 mg/kg, i.p.) and placed in a stereotaxic apparatus (Kopf) for implantation of a guide cannula (20 gauge, 10 mm in total length) into the left cerebral ventricle. The guide cannula was placed into the ventricle and cemented into position using screws to anchor the injection assembly into the skull. An obturator, which protruded 0.5 mm past the tip of the guide cannula, was then inserted. Catheters (PE-50) were implanted into the left common carotid artery to measure pulsatile and mean (MAP) arterial blood pressure and heart rate, and into the right jugular vein to administer drugs. After catheterization, a midline laparotomy was performed and Doppler flow probes were placed on the superior mesenteric artery, left renal artery and lower abdominal aorta to measure mesenteric, renal and hindquarter blood flow velocities, respectively, and for the calculation of mesenteric, renal and hindquarter vascular resistances. The probes were sutured in place, and the leads and catheters were tunneled subcutaneously and exteriorized between the scapulae. The wounds were sutured closed. To protect the probe wires and polyethylene tubing while allowing animals unrestricted movement, the free ends of the catheters and Doppler leads were led through a stainless steel skin button spring swivel assembly that was mounted to a ring stand clamp and suspended above the cage. The skin button was attached to the skin incision in the scapular region using stainless steel sutures. Details of the Doppler technique, including construction of probes, reliability of the method for estimation of flow velocity, and quantitative determination of percent changes in resistances, have been described previously by Haywood et al (Haywood et al., 1981).
In vivo protocols
After a 7-day recovery period, the rats were connected to a Beckman Dynograph coupled pressure transducer (Cabe Lab, Inc.) and Doppler flowmeter (Bioengineering, University of lowa) for the recording of HR, PP, MAP and blood flows, respectively. The stylus was removed from the guide cannula positioned into the cerebral ventricle and a microinjection needle (25 gauge, 10.5 mm in total length) connected to a glass Hamilton micro syringe (5 µL) via PE-10 tubing was then inserted into the guide cannula. The syringe, tubing and needle were filled with solutions of the test agents. The rats were given several minutes to relax before the i.c.v. injections were given. In study 1, the time-course of effects of i.c.v. injections of artificial cerebrospinal fluid (aCSF, 2 µL) or 5-HT (10 µg in 2 µL of aCSF) on hemodynamic variables were determined. In study 2, the responses elicited by i.c.v. 5-HT (10 µg) were examined in rats which had received i.c.v. injections of aCSF (2 µI) or methysergide (10 µg) 10 min earlier. In study 3, the responses elicited by i.c.v. 5-HT (10 µg) were examined in rats pretreated with an intravenous injection of saline (150–200 µl) or L-NAME (25 µmol/kg). 5-HT was given 15–20 min after saline or L-NAME, when stable values had been reached.
Drugs
5-HT creatinine sulphate and L-NAME were obtained from Sigma Chemical Company (St. Louis, MO). Ketamine HCl was obtained from Aveco Company (Fort Dodge, IA). Methysergide maleate was obtained from Sandoz Pharmaceuticals (East Hanover, NJ).
Statistical Analyses
The data are presented as mean ± SEM. The single SEM value displayed on each dose-response curve in figs. 2–4 were determined by the formula S.E. = (EMS/n)1/2 where EMS is the error mean square term from the analysis of variance (ANOVA) and n is the number of rats in each of the experimental groups (see Davisson et al., 1996a). The data were analyzed by repeated-measures ANOVA followed by Student's modified t-test with the Bonferroni correction for multiple comparisons between means using the EMS terms from the ANOVA (see Davisson et al., 1996a).
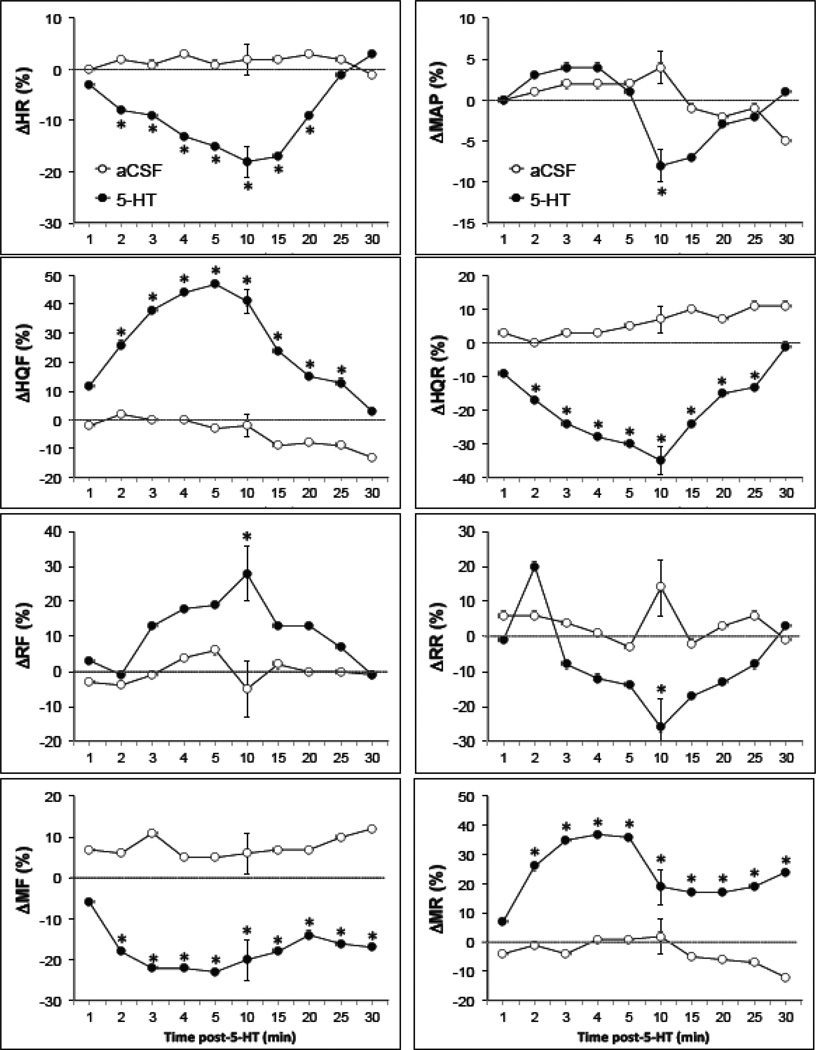
Fig. 2.
Time-course of effects of i.c.v. injections of either aCSF (2µL, n = 8) or 5-HT (10 µg in 2 µL, n = 8) on mean arterial blood pressures (MAP), heart rates (HR), hindquarter (HQF), renal (RF) and mesenteric (MF) blood flows, and hindquarter (HQR), renal (RR) and mesenteric (MR) vascular resistances in conscious freely-moving rats. Each value is the mean ± SEM of the percentage changes in these parameters. *P < 0.05, significant response.
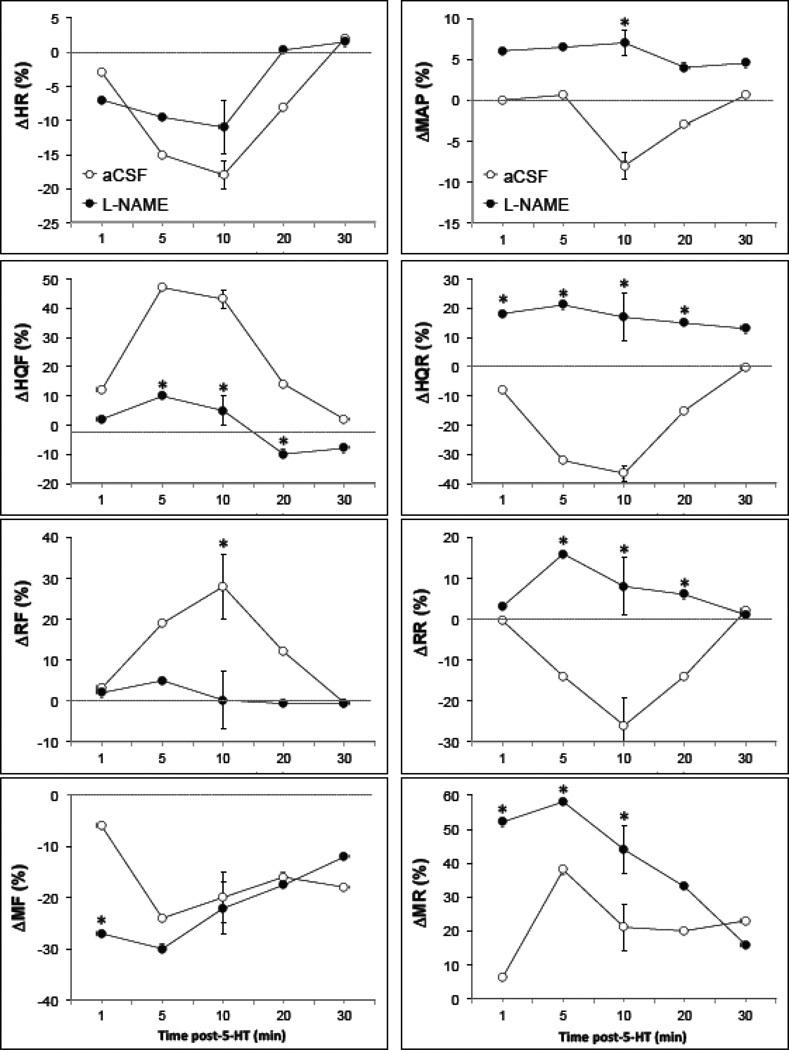
Fig. 4.
Time-course of effects of i.c.v. 5-HT (10 µg) on mean arterial blood pressures (MAP), heart rates (HR), hindquarter (HQF), renal (RF) and mesenteric (MF) blood flows, and hindquarter (HQR), renal (RR) and mesenteric (MR) vascular resistances in conscious freely-moving rats which had received i.v. injections of saline (n = 7) or L-NAME (25 µmol/kg, n = 7). Each value is the mean± SE of the percentage changes in these parameters. *P < 0.05, L-NAME versus saline.
Results
Hemodynamic effects of i.c.v. injections of 5-HT
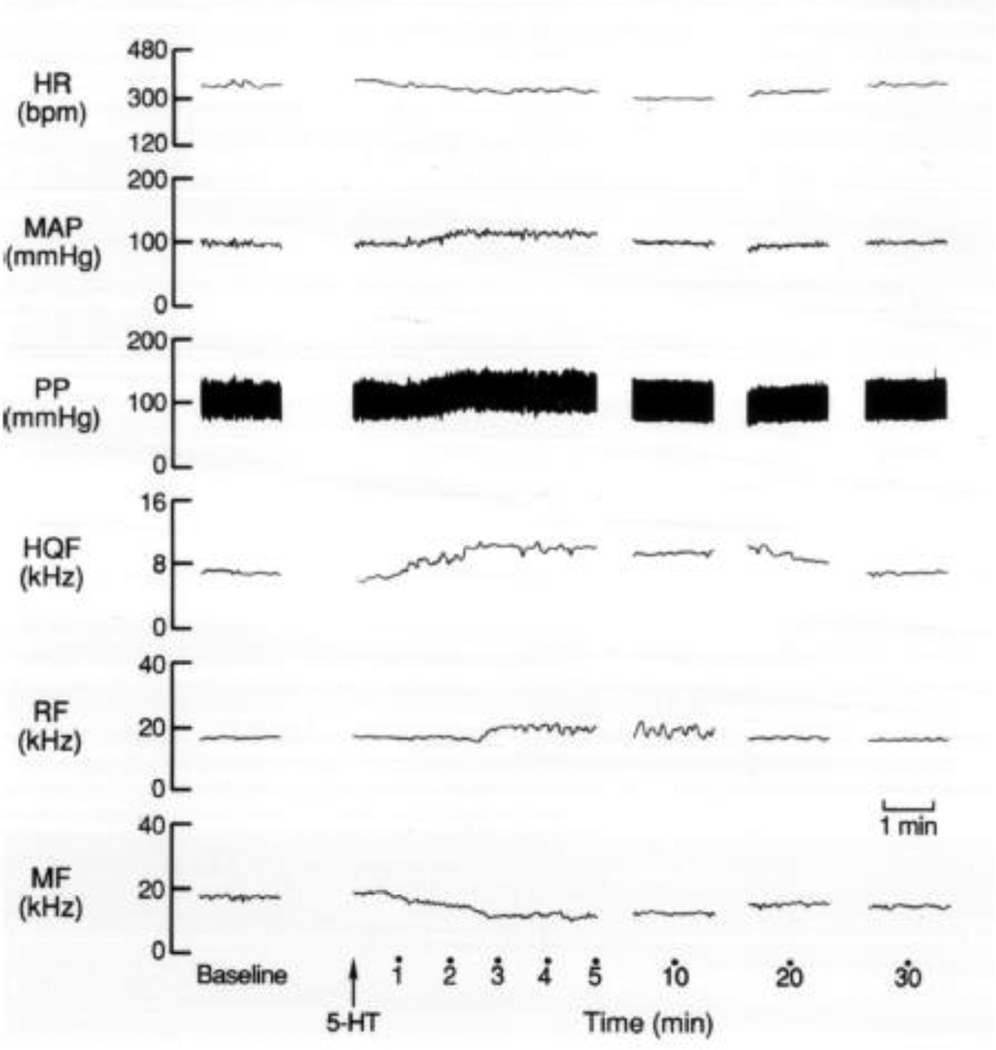
Resting hemodynamic variables in the groups of conscious rats, which received i.c.v. injections of either aCSF or 5-HT are summarized in Table 1. There were no between-group differences in these variables (P > 0.05, for all comparisons). A typical example of the time-course of effects of an i.c.v. injection of 5-HT on heart rate, MAP and regional blood flows in a conscious freely-moving rat is shown in Fig. 1. This dose of 5-HT produced a minor pressor response, which was associated with bradycardia, increases in hindquarter and renal blood flows and a decrease in mesenteric flow. The time-course of the hemodynamic responses elicited by i.c.v. injection of aCSF or 5-HT is summarized in Fig. 2. The injection of aCSF did not elicit responses. The i.c.v. injection of 5-HT did not affect MAP except for a minor decrease observed 10 min after injection. Despite the minor effects on MAP, i.c.v. 5-HT elicited an immediate fall in heart rate, which was sustained for 20 min. In addition, i.c.v. 5-HT produced prompt and pronounced increases in hindquarter blood flow and decreases in hindquarter vascular resistance. The vasodilation in the hindquarter bed lasted for 25 min. The i.c.v. injection of 5-HT produced more delayed but transient changes in renal blood flow and resistance. A small but statistically significant increase in renal blood flow and a decrease in renal resistance was observed at 10 min. The i.c.v. injection of 5-HT produced immediate and pronounced decrease in mesenteric blood flow and increases in mesenteric resistance. The vasoconstriction in the mesenteric bed was still evident 35 min after i.c.v. 5-HT.
Table 1.
A summary of the resting hemodynamic variables of two groups of conscious rats prior to the i. c.v. injection of either aCSF or 5-HT
| Parameter | aCSF | 5-HT |
|---|---|---|
| n | 8 | 8 |
| HR, beats/min | 346 ± 12 | 356 ± 14 |
| MAP, mmHg | 117 ± 4 | 121 ± 5 |
| HQF, kHz | 4.7 ± 0.6 | 4.3 ± 0.6 |
| HQR, mmHg/kHz | 26 ± 6 | 29 ± 5 |
| RF, kHz | 5.1 ± 1.7 | 4.6 ± 1.2 |
| RR, mmHg/kHz | 34 ± 8 | 37 ± 9 |
| MF, kHz | 6.9 ± 1.1 | 7.2 ± 0.9 |
| MR, mmHg/kHz | 19 ± 4 | 15 ± 3 |
Each value represents the mean± SEM. Note that there were no between group differences in any parameter at the P < 0.05 level.
Fig. 1.
A typical example of the effects of an i.c.v. injection of 5-HT (10 µg) on the heart rate (HR), mean (MAP) and pulsatile (PP) arterial blood pressures, and hindquarter (HQF), renal (RF) and mesenteric (MF) blood flows in a conscious freely-moving rat.
Effects of i.c.v. methysergide on the hemodynamic responses produced by i.c.v. 5-HT
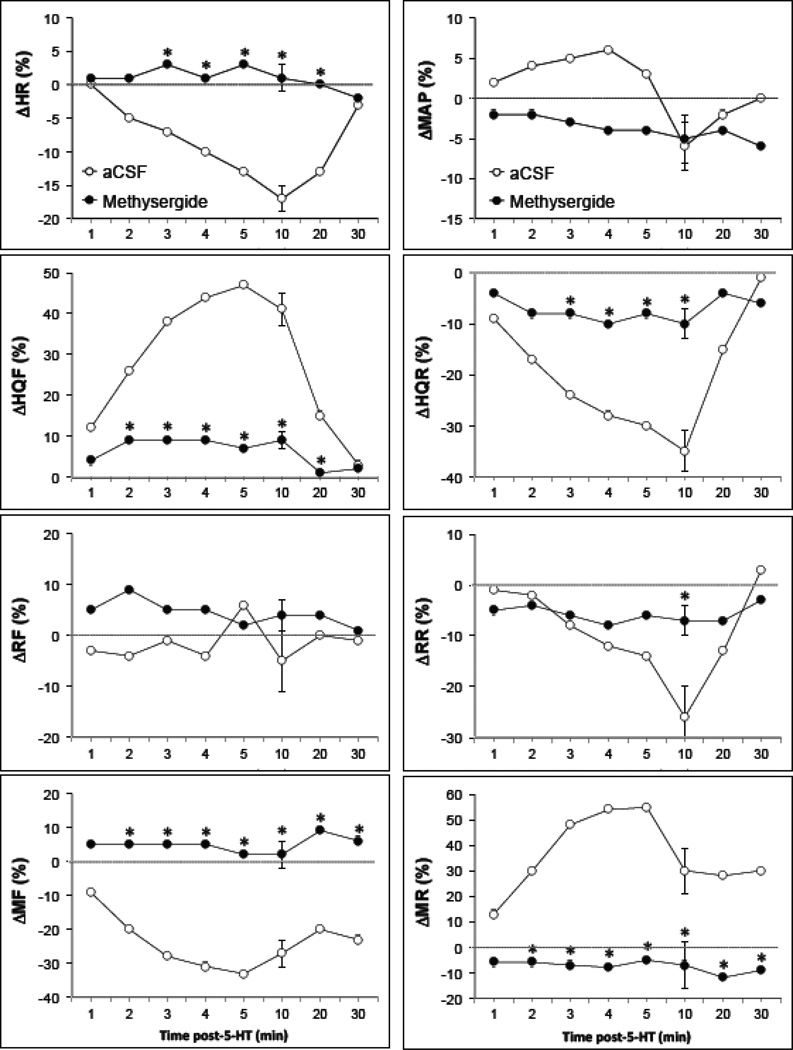
The effects of i.c.v. injection of methysergide (10 µg) on resting hemodynamic parameters are summarized in Table 2. None of the hemodynamic values were altered by administration of the 5-HT receptor antagonist. The effects of i.c.v. 5-HT (10 µg) on hemodynamic parameters of rats pretreated with i.c.v. injections of aCSF or methysergide are summarized in Fig. 3. As can be seen, pretreatment with methysergide prevented the hemodynamic responses elicited by 5-HT.
Table 2.
The effects of i.c.v. methysergide (10 µg) on resting hemodynamic parameters in conscious rats
| Parameter | Pre | Post | Δ (%Change) |
|---|---|---|---|
| HR(bpm) | 342 ± 8 | 336 ± 8 | −2 ± 2 |
| MAP (mm Hg) | 116 ± 4 | 118 ± 3 | +1 ± 3 |
| HQR (mm Hg/kHz) | 32 ± 6 | 32 ± 4 | 0 ± 7 |
| RR (mm Hg/kHz) | 69 ± 8 | 70 ± 7 | +1 ± 3 |
| MR (mm Hg/kHz) | 24 ± 5 | 27 ± 6 | +10 ± 6 |
Each value represents the mean ± SEM of data from 4 rats. Note that there were no between group differences in any parameter at the P < 0.05 level.
Fig. 3.
Time-course of effects of i.c.v. 5-HT (10 µg) on mean arterial blood pressures (MAP), heart rates (HR), hindquarter (HQF), renal (RF) and mesenteric (MF) blood flows, and hindquarter (HQR), renal (RR) and mesenteric (MR) vascular resistances in conscious freely-moving rats which had received i.v. injections of either aCSF (n = 6) or methysergide (10 µg, n = 4). Each value is the mean ± SEM of the percentage changes in these parameters. *P < 0.05, methysergide versus aCSF.
Effects of i.v. L-NAME on the hemodynamic responses produced by i.c.v. 5-HT
The effects of i.v. injection of saline or L-NAME (25 µmol/kg on resting parameters are summarized in Table 3. Saline did not modify any of these parameters. L-NAME produced a marked increase in MAP and vascular resistances and a bradycardia. These responses reached their maximum within 10 min and remained at these levels for 30–45 min. The responses elicited by i.c.v. 5-HT (10 µg) on hemodynamic variables in conscious rats pretreated with either saline or L-NAME (25 µmol/kg) are summarized in Fig. 4. As can be seen, i.c.v. 5-HT produced a minor pressor response in the L-NAME-treated rats, which was associated with a bradycardia of similar magnitude and duration to that observed in saline-treated rats. The pronounced hindquarter vasodilation produced by i.c.v. of 5-HT in saline-treated rats was not observed in L-NAME-treated rats. In addition, the transient 5-HT-induced vasodilation in the renal bed was also not observed in L-NAME-treated rats. The initial mesenteric vasoconstriction produced by i.c.v. 5-HT was enhanced in L-NAME-treated rats. The vasoconstrictor effects thereafter were equivalent in saline- or L-NAME-treated rats.
Table 3.
The effects i.v. injections of saline or L-NAME (25 µmol/kg) on resting cardiovascular parameters of conscious rat
| Saline | L-NAME | |||||
|---|---|---|---|---|---|---|
| Parameter | Pre | Post | Δ (%Change) | Pre | Post | Δ (%Change) |
| HR, beats/min | 338 ± 14 | 342 ± 16 | +1 ± 2 | 365 ± 8 | 262 ± 7 | −28 ± 3* |
| MAP, mmHg | 119 ± 5 | 120 ± 5 | +1 ± 1 | 123 ± 8 | 150 ± 4 | +24 ± 5* |
| HQF, kHz | 4.2 ± 0.7 | 4.3 ± 0.8 | +2 ± 4 | 4.9 ± 0.8 | 2.5 ± 0.6 | −52 ± 7* |
| HQR, mmHg/kHz | 31 ± 7 | 32 ± 8 | +4 ± 6 | +34 ± 9 | 96 ± 28 | +191 ± 58* |
| RF, kHz | 5.1 ± 0.9 | 4.9 ± 0.9 | −4 ± 3 | 5.5 ± 2.4 | 3.3 ± 1.3 | −45 ± 8* |
| RR, mmHg/kHz | 46 ± 12 | 49 ± 10 | +7 ± 8 | 70 ± 17 | 304 ± 122 | +171 ± 42* |
| MF, kHz | 7.3 ± 1.4 | 7.4 ± 1.5 | +1 ± 5 | 8.5 ± 1.8 | 4.7 ± 0.8 | −42 ± 4* |
| MR, mmHg/kHz | 17 ± 5 | 17 ± 6 | 0 ± 6 | 19 ± 3 | 37 ± 6 | +107 ± 15* |
Each value represents the mean± SEM of the values from the saline-treated (n = 7) and L-NAME-treated (n = 7) rats.
P < 0.05, significant response.
Discussion
In this study, the i.c.v. injection of 5-HT (10 µg) elicited a relatively rapid, pronounced and long-lasting bradycardia in conscious rats that was associated with a delayed and relatively minor and transient fall in MAP. The rapid and robust nature of the bradycardia raises the possibility that 5-HT acts at sites close to the third ventricles. It is also unlikely that the bradycardia is of baroreflex in origin since the baroreflex would lower heart rate in response to an increase in MAP. However, it is not implausible that ICV administered 5-HT (1) reaches brain nuclei that process baroreflex information thereby promoting both a bradycardia and hypotension, and/or (2) reaches brainstem nuclei that directly control autonomic output to the heart. For example, Sporton et al (1991) found that microinjection of 5-HT1A agonists into the dorsal motor nucleus of the vagus elicit bradycardia in anesthetized rats. Our finding that prior i.c.v. treatment with methysergide (which did not affect resting heart rate in itself) prevented the bradycardia elicited by i.c.v. 5-HT in conscious rats is consistent with findings in anesthetized rats (Inoue and Bunag, 1989). Methysergide has multiple agonist and antagonist effects on 5-HT receptor sub-types (Hoyer et al., 1994; Boess and Martin, 1994; Saxena, 1995; Villalón and Centurión, 2007). For example, methysergide is an agonist at 5-HT1A receptors (Hoyer et al., 1994) but an antagonist of 5-HT2A-(Egan et al., 1998; Kovacs et al., 2003; Knight et al., 2004), 5-HT2B-(Glusa and Pertz, 2000; Knight et al., 2004) and 5-HT2C-receptors (Egan et al., 2000; Knight et al., 2004). In addition, the ICV injection of quipazine, which is a 5-HT2A and 5-HT3 receptor agonist, increases heart rate in anesthetized rats (Knowles and Ramage, 1999). As such, it is tempting to assume that the bradycardic responses elicited by i.c.v. 5-HT in our conscious rats is mediated via activation of 5-HT2B- and/or 5-HT2C-receptors.
The i.c.v. injection of 5-HT also produced changes in regional vascular resistances. These injections of 5-HT elicited a pronounced and long-lasting vasoconstriction in the mesenteric bed, a transient vasodilation in the renal bed and a pronounced and long-lasting vasodilation in the hindquarter bed. As such, it appears that the minimal effect of 5-HT on MAP is due to the counterbalancing influences of these opposing changes in vascular resistances. These findings differ to those of conscious Long-Evans rats in which ICV 5-HT (40 and 120 nmol/kg; approximately 10 to 12 nmol) elicited a substantial pressor response and renal vasoconstriction (unlike the minor falls in MAP and renal resistance observed in our Sprague-Dawley rats) although there was a decrease in heart rate, a mesenteric vasoconstriction and a hindquarter vasodilation of differing magnitude and duration that that observed in our rats. Although, the differences in the responses to 5-HT could be due to strain differences in the rats used in the two studies, the findings do provide compelling evidence that ICV 5-HT can have dramatically different effects on the neurogenic tone of systemic vascular beds. In our study, the rapid onset of the mesenteric vasoconstriction and the hindquarter vasodilation upon i.c.v. injection of 5-HT suggest that the responses are initiated by actions at sites close to the ventricles (Brody et al., 1978; Hoffman and Phillips, 1976). Smits and Struyker-Boudier (1976) found that microinjection of 5-HT into the anterior hypothalamic area causes an increase in MAP. This area, which lies close to the third ventricle, receives serotonergic input from the dorsal raphe nucleus (Coote, 1990). Since the hemodynamic responses produced by i.c.v. 5-HT were sustained for 25–35 min, it is also likely that these responses may involve actions of 5-HT on midbrain or brainstem sites. For example, microinjections of 5-HT1A receptor agonists into the raphe obscurus cause a pressor response in anesthetized rats (Dreteler et al., 1991). In addition, intrathecal 5-HT produces both excitation and inhibition of sympathetic nerve activity (Yusof and Coote, 1988). However, it is also known that activation of 5-HT1A receptors in other midbrain/brainstem sites such as the dorsal raphe (Conner and Higgins, 1990), raphe magnus and pallidus (Valenta and Singer, 1990) and rostral ventrolateral medulla (Nosjean and Guyenet, 1991) cause hypotension and sympathoinhibition.
Our observation that i.c.v. methysergide elicited minimal hemodynamic responses but prevented the hemodynamic responses produced by i.c.v. 5-HT in conscious rats is consistent with findings that methysergide attenuated the cardiovascular responses elicited by i.c.v. injections of 5-HT in anesthetized rats (Inoue and Bunag, 1989). Anderson et al (1992) reported that i.c.v. injection of 5-HT in anesthetized rats increased renal sympathetic nerve activity via stimulation of central 5-HT1A-receptors and stimulated AVP release via activation of central 5-HT2 receptors. As mentioned above, methysergide is an agonist at 5-HT1A receptors but an antagonist of 5-HT2A-, 5-HT2B- and 5-HT2C-receptors. As such, it is tempting to assume that the hemodynamic responses elicited by i.c.v. 5-HT in conscious rats due activation of 5-HT2-receptors, with the exact nature of the subtypes of 5-HT2 receptors to be determined.
The different pattern of hemodynamic changes produced by i.c.v. 5-HT could result from several mechanisms. Montes and Johnson (1990) and Inoue and Bunag (1989) provided evidence that i.c.v. 5-HT increases MAP, in part, by the release of AVP. As such, the vasoconstriction in the mesenteric bed produced by i.c.v. 5-HT may be mediated by the release of AVP, which constricts these vessels by activation of AVP V1-receptors (see Share, 1988). Moreover, there is now substantial evidence that the stimulation of AVP V1-receptors on vascular endothelial cells can produce vasorelaxation via the release of nitrosyl factors (see Yamada et al., 1993). Consequently, the hindlimb vasodilation produced by i.c.v. 5-HT may involve the AVP-mediated release of nitrosyl factors from the vascular endothelium in this bed. Another possibility is that the hemodynamic effects of i.c.v. 5-HT are mediated by the release of adrenal catecholamines. The i.v. injection of the selective 5-HT1A receptor agonist, 8-OH-DPAT, causes the release of epinephrine by the centrally-mediated increase in preganglionic input to the adrenal glands (Bagdy et al., 1992). The systemic administration of epinephrine produces a pressor response, which is associated with marked vasoconstriction in the mesenteric and renal beds, but a pronounced vasodilation in the hindquarter bed (see Kooy and Lewis, 1996). Therefore, except for the changes in resistance in the renal bed the hemodynamic effects of epinephrine are somewhat similar to those produced by 5-HT. In the present study, it was evident that the systemic injection of L-NAME prevented the hindlimb vasodilation produced by the i.c.v. injection of 5-HT. In contrast, we have reported that the NO synthesis inhibitor does not diminish the hindlimb vasodilation produced by the systemic injection of epinephrine (Davisson et al., 1994). However, Gardiner et al (1990) reported that L-NAME did partially attenuate the hindlimb vasodilation produced by the continuous infusion of epinephrine. Moreover, Gardiner et al (1990) demonstrated that the infusion of a high dose of epinephrine produces a vasodilation in the renal bed which is attenuated by L-NAME. Consequently, it is possible that epinephrine derived from the adrenal glands may contribute to the hemodynamic responses produced by the central administration of 5-HT. The loss of ICV 5-HT-mediated vasodilation in the renal and especially the hindquarter beds of L-NAME-treated rats is unlikely to be due to diminished efficacies of endogenous neural- or endothelium-derived vasodilators since the vasodilator responses to NO-donors are if anything exaggerated in these beds in L-NAME-treated rats (see Davisson et al., 1994, 1996a,b,c, 1997).
In light of evidence that central administration of 5-HT influences sympathetic nerve activity (Baum and Shropshire, 1975; Yusof and Coote, 1988; Montes and Johnson, 1990; Anderson et al., 1992), it is tempting to assume that the hemodynamic effects elicited by i.c.v. 5-HT involve changes in autonomic outflow. Central injections of 5-HT produce sympathoinhibition and/or sympathoexcitation in conscious and anesthetized rats (Baum and Shropshire, 1975; Kristic and Djurkovic, 1980, 1981; Yusof and Coote, 1988; Montes and Johnson, 1990; Nosjean and Guyenet, 1991; Anderson et al., 1992). Because the pressor response produced by i.c.v. 5-HT in anesthetized rats is attenuated by α-adrenoceptor antagonists and cervical transection of the spinal cord (Kristic and Djurkovic, 1980) it is likely that the mesenteric vasoconstriction produced by i.c.v. 5-HT is due to a centrally-mediated increase in sympathetic vasoconstrictor drive. Since i.c.v. 5-HT can also cause sympathoinhibition, we reasoned that the vasodilation observed in the renal and hindquarter beds may be due to the withdrawal of sympathetic vasoconstrictor drive. However, i.c.v. 5-HT-induced natriuresis in conscious rats is markedly attenuated by transection of efferent renal sympathetic nerves (Montes and Johnson, 1990) suggesting that central 5-HT causes an increase in renal sympathetic nerve activity. In addition, we provided evidence that activation of the lumbar sympathetic chain in conscious rats by air-jet stress elicits a pronounced vasodilation in the hindquarter bed (Davisson et al., 1994, 1996b) and that direct electrical stimulation of the lumbar sympathetic chain produces hindlimb vasodilation in anesthetized rats (Davisson et al., 1996c, 1997). These vasodilator responses were diminished by systemic administration of the NO synthesis inhibitor, L-NAME (Davisson et al., 1994, 1996a,b,c, 1997). Taken together, it is possible that the hindlimb vasodilation produced by i.c.v. of 5-HT is mediated by an active sympathetic vasodilator process. The finding that L-NAME inhibited the 5-HT-induced vasodilation in the renal bed suggests that these responses involve the release of nitrosyl factors within the vasculature.
Neuronal pathways in the brain synthesize and use nitrosyl factors as neurotransmitters and/or neuromodulators (see Dawson et al, 1992; Lipton et al., 1994; Do et al., 1996; Garthwaite, 2008; Virarkar et al., 2013; Wang and Golledge, 2013). Therefore, it is important to consider the possibility that L-NAME abolished the hindlimb vasodilation in response to i.c.v. 5HT by actions in the brain or spinal cord. This is especially important since systemically administered L-NAME may have important actions in the central nervous system (see Ma et al., 1995). Ma et al (1995) reported that the systemic injection of L-NAME (10 mg/kg, i.v.) influenced the activity of neurons in the nucleus tractus solitarius (the majority were inhibited) and that superfusion of rat brain slices with L-NAME decreased the firing rate of neurons in this nucleus (Ma et al., 1995). Therefore, it is possible that the loss of hindlimb vasodilation in rats treated with L-NAME may be due to the actions of the NOS inhibitor in the NTS. However, it was evident that L-NAME did not interfere with the ability of i.c.v. 5-HT to elicit bradycardia. In addition, i.c.v. 5-HT produced a pronounced vasoconstriction in the mesenteric bed of L-NAME-treated rats, despite the marked increase in resting mesenteric resistance produced by the NO synthesis inhibitor. This suggests that L-NAME did not interfere with the central actions of 5-HT or the central processing of autonomic outflow. We have also provided evidence that the systemic injection of a 25 µmol/kg dose of L-NAME did not interfere with central processing aortic depressor nerve or superior laryngeal afferent input (Possas et al., 1997, 2006). As such, it is unlikely that the ability of L-NAME to abolish the hindquarter vasodilation elicited by i.c.v. 5-HT involves actions in the brain.
Summary
Central neurons using 5-HT as their neurotransmitter play a major role in regulating hemodynamic (Watts et al., 2012) and chemosensory/ventilatory (Teran et al., 2014) control systems via many 5-HT receptor subtypes. Disorders within serotonergic pathways (e.g., altered 5-HT release and reuptake; receptor down-regulation) are implicated in the etiology of disorders of cardiovascular (Watts et al., 2012) and ventilatory (Hilaire et al., 2010) systems in humans, the interplay between these vital systems (Szczepanska-Sadowska et al., 2010) and in cardiorespiratory dysfunction in humans with sleep apnea Fenik and Veasey, 2003). Moreover, many humans take drugs that interfere with the central processing of 5-HT (e.g., metabolism, re-uptake) and therefore affect hemodynamic status. Indeed, reports of such drugs altering hemodynamic status (e.g., hypertension) and altering hemodynamic responsiveness (e.g., orthostatic hypotension) in humans and animals (Watts et al., 2012) are common. As such, elaborating the effects of central administration of 5-HT on regional systemic vascular resistances in normal subjects (and especially whether there is uniformity or non-uniformity in the changes in vascular tone between the beds) would be an important first step in understanding the hemodynamic consequences of aberrant serotonergic signaling. Our study demonstrates that whereas i.c.v. 5-HT (10 µg) produced minor changes in MAP, it elicited bradycardia and differential changes in vascular resistances including pronounced and sustained vasoconstriction in the mesenteric bed and vasodilation in the hindquarter bed and a transient renal vasodilation. It appears that the vasodilator responses produced by the i.c.v. 5-HT are mediated by the release of nitrosyl factors within these beds. The hemodynamic pattern produced by the i.c.v. 5-HT is somewhat similar to that produced by air-jet stress (Davisson et al., 1994, 1996b). This raises the possibility that serotonergic systems within the brain may play a role in regulating the hemodynamic responses of defense reactions.
Highlights.
ICV injections of 5-HT did not affect arterial blood pressure of conscious rats
ICV 5-HT elicited dramatic changes in systemic hemodynamics
ICV 5-HT increased mesenteric vascular resistance but decreased hindquarter resistance
All effects of ICV 5-HT were prevented by ICV injection of a 5-HT1/5-HT2-receptor antagonist
Nitric oxide synthase inhibition markedly affected the hemodynamic effects of ICV 5-HT
Acknowledgements
This study was supported by National Institutes of Health grants HL-14388 and 1P-01-HL-101871.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson IK, Martin GR, Ramage AG. Central administration of 5-HT activates 5-HT1A receptors to cause sympathoexcitation and 5-HT2/5-HT1C receptors to release vasopressin in anesthetized rats. Brit. J. Pharmacol. 1992;107:1020–1028. doi: 10.1111/j.1476-5381.1992.tb13401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IK, Ramage AG, Gardiner SM. Cardiovascular effects of serotonin and DP-5- CT in conscious Long-Evans and Brattleboro rats. Am. J. Physiol. 1996;271:R455–R463. doi: 10.1152/ajpregu.1996.271.2.R455. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Sved AF, Murphy DL, Szemeredi K. Pharmacological characterization of serotonin subtypes involved in vasopressin and plasma renin activity responses to serotonin agonists. Eur. J. Pharmacol. 1992;210:285–289. doi: 10.1016/0014-2999(92)90417-3. [DOI] [PubMed] [Google Scholar]
- Baum T, Shropshire AT. Inhibition of efferent sympathetic nerve activity by 5-hydroxytryptophan and centrally administered 5-hydroxytryptarnine. Neuropharmacology. 1975;14:227–233. doi: 10.1016/0028-3908(75)90010-6. [DOI] [PubMed] [Google Scholar]
- Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Brody MJ, Fink GD, Buggy J, Haywood JR, Gordon FJ, Johnson AK. The role of the anteroventral third ventricle (AV3V) region in experimental hypertension. Circ. Res. 1978;43:3–13. doi: 10.3109/10641967809068612. [DOI] [PubMed] [Google Scholar]
- Conner HE, Higgins GA. Cardiovascular effects of 5-HT1A receptor agonists injected into the dorsal raphe nucleus of conscious rats. Eur. J. Pharmacol. 1990;182:63–72. doi: 10.1016/0014-2999(90)90493-p. [DOI] [PubMed] [Google Scholar]
- Coote JH. The central antihypertensive action of 5-hydroxytryptarnine: the location of site of action. In: Saxena PR, Wallis DI, Wouters W, Bevan P, editors. Cardiovascular Pharmacology of 5-hydroxytryptamine; Prospective Therapeutic Applications. The Netherlands: Kluwer; 1990. pp. 259–266. [Google Scholar]
- Dalton DW. The cardiovascular effects of centrally administered 5-hydroxytryptarnine in the conscious normotensive and hypertensive rat. J. Auton. Pharmacol. 1986;6:67–75. doi: 10.1111/j.1474-8673.1986.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Johnson AK, Lewis SJ. Nitrosyl factors mediate active neurogenic hindquarter vasodilation in the conscious rat. Hypertension. 1994;23:962–966. doi: 10.1161/01.hyp.23.6.962. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Bates JN, Johnson AK, Lewis SJ. Use-dependent loss of acetylcholine and bradykinin-mediated vasodilation following NO synthase inhibition: Evidence for preformed stores of nitric oxide-containing factors in vascular endothelial cells. Hypertension. 1996a;28:354–360. doi: 10.1161/01.hyp.28.3.354. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Shaffer RA, Johnson AK, Lewis SJ. Use-dependent loss of active sympathetic neurogenic vasodilation following nitric oxide synthase inhibition in conscious rats. Hypertension. 1996b;28:347–353. doi: 10.1161/01.hyp.28.3.347. 1996. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Shaffer RA, Johnson AK, Lewis SJ. Stimulation of lumbar sympathetic nerves may produce hindlimb vasodilation via the release of pre-formed stores of nitrosyl factors. Neuroscience. 1996c;72:881–887. doi: 10.1016/0306-4522(96)00090-5. 1996. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Possas OS, Murphy SP, Lewis SJ. Neurogenically-derived nitrosyl factors mediate lumbar sympathetic vasodilation in the hindlimb vasculature of the rat. Am. J. Physiol. 1997;272:H2369–H2376. doi: 10.1152/ajpheart.1997.272.5.H2369. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL, Snyder SH. A novel neuronal messenger molecule in brain: The free radical, nitric oxide. Ann. Neurol. 1992;32:297–311. doi: 10.1002/ana.410320302. [DOI] [PubMed] [Google Scholar]
- Do KQ, Benz B, Grima G, Gutteck-Amsler U, Kluge I, Salt TE. Nitric oxide precursor arginine and S-nitrosoglutathione in synaptic and glial function. Neurochem. Int. 1996;29:213–224. doi: 10.1016/0197-0186(96)00002-2. [DOI] [PubMed] [Google Scholar]
- Dreteler GH, Wouters W, Saxena PR, Ramage AG. Pressor effects following microinjection of 5-HT1A receptor agonists into the raphe obscurus of the anesthetized rat. Brit. J. Pharmacol. 1991;102:317–322. doi: 10.1111/j.1476-5381.1991.tb12172.x. 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan CT, Herrick-Davis K, Teitler M. Creation of a constitutively activated state of the 5- hydroxytryptamine2A receptor by site-directed mutagenesis: inverse agonist activity of antipsychotic drugs. J. Pharmacol. Exp. Ther. 1998;286:85–90. [PubMed] [Google Scholar]
- Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT2A and 5-HT2C receptors. Synapse. 2000;35:144–150. doi: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Kemp PA, Bennet T. Effects of NG-nitro-L-arginine methylester on vasodilator responses to adrenaline or BRL 38227 in conscious rats. Brit. J. Pharmacol. 1990;101:632–639. doi: 10.1111/j.1476-5381.1991.tb12496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glusa E, Pertz HH. Further evidence that 5-HT-induced relaxation of pig pulmonary artery is mediated by endothelial 5-HT2B receptors. Brit. J. Pharmacol. 2000;130:692–698. doi: 10.1038/sj.bjp.0703341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood JR, Shaffer RA, Fastenow C, Fink GD, Brody MJ. Regional blood flow measurement with pulsed Doppler flowmeter in conscious rat. Am. J. Physiol. 1981;241:H273–H278. doi: 10.1152/ajpheart.1981.241.2.H273. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M. The role of serotonin in respiratory function and dysfunction. Respir. Physiol. Neurobiol. 2010;174:76–88. doi: 10.1016/j.resp.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WE, Phillips MI. Regional study of cerebral ventricle sites to angiotensin II. Brain Res. 1976;110:313–330. doi: 10.1016/0006-8993(76)90405-4. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Ignarro LJ. Nitric Oxide: A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- Inoue A, Bunag RD. Sympathetic inhibition and vasopressin mediation during centrally induced responses to serotonin in rats. J. Cardiovasc. Pharmacol. 1989;13:902–907. doi: 10.1097/00005344-198906000-00013. [DOI] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. Pharmacological characterisation of the agonist radioligand binding site of 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2004;370:114–123. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- Knowles ID, Ramage AG. Evidence for a role for central 5-HT2B as well as 5-HT2A receptors in cardiovascular regulation in anaesthetized rats. Br. J. Pharmacol. 1999;128:530–542. doi: 10.1038/sj.bjp.0702822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooy NW, Lewis SJ. Nitrotyrosine attenuates the hemodynamic effects of adrenoceptor agonists in vivo: relevance to the pathophysiology of peroxynitrite. Eur. J. Pharmacol. 1996;310:155–161. doi: 10.1016/0014-2999(96)00376-7. [DOI] [PubMed] [Google Scholar]
- Kristie MK, Djurkovic D. Analysis of the cardiovascular responses to central administration of 5-hydroxytryptamine in rats. Neuropharmacology. 1980;19:455–463. doi: 10.1016/0028-3908(80)90053-2. 1980. [DOI] [PubMed] [Google Scholar]
- Kristie MK, Djurkovic D. Comparison of the cardiovascular responses to intracerebroventricular administration of tryptamine, 5-hydroxytryptamine, tryptophan and 5-hydroxytryptophan. Arch. Intern. Physiol. Biochem. 1981;89:385–391. doi: 10.3109/13813458109069488. 1981. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Hashmi-Hill MP, Owen JR, Sandock K, Robertson TP, Bates JN. ACE inhibition restores the vasodilator potency of the endothelium-derived relaxing factor, L-S-nitrosocysteine, in Spontaneously Hypertensive rats. Vasc. Pharmacol. 2006;44:491–507. doi: 10.1016/j.vph.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ. Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Singel DJ, Stamler JS. Nitric oxide in the central nervous system. Prog. Brain Res. 1994;103:359–364. doi: 10.1016/s0079-6123(08)61149-8. [DOI] [PubMed] [Google Scholar]
- Ma S, Abboud FM, Felder RB. Effects of L-arginine-derived nitric oxide synthesis on neuronal activity in nucleus tractus solitarius. Am. J. Physiol. 1995;268:R487–R491. doi: 10.1152/ajpregu.1995.268.2.R487. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147(Suppl 1):S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes R, Johnson AK. Efferent mechanisms mediating renal sodium and water excretion induced by central administration of serotonin. Am. J. Physiol. 1990;259:R1267–R1273. doi: 10.1152/ajpregu.1990.259.6.R1267. [DOI] [PubMed] [Google Scholar]
- Possas OS, Lewis SJ. NO-containing factors mediate hindlimb vasodilation produced by superior laryngeal nerve stimulation. Am. J. Physiol. 1997;273:H234–H243. doi: 10.1152/ajpheart.1997.273.1.H234. [DOI] [PubMed] [Google Scholar]
- Possas O, Johnson AK, Lewis SJ. Role of nitrosyl factors in the hindlimb vasodilation produced by baroreceptor afferent nerve stimulation. Am. J. Physiol. 2006;290:R741–R748. doi: 10.1152/ajpregu.00660.2005. [DOI] [PubMed] [Google Scholar]
- Nosjean A, Guyenet PG. Role of the ventral lateral medulla in the sympatholytic effect of 8-OH-DPAT in rats. Am. J. Physiol. 1991;260:R600–R609. doi: 10.1152/ajpregu.1991.260.3.R600. [DOI] [PubMed] [Google Scholar]
- Saxena PR. Serotonin receptors: subtypes, functional responses and therapeutic relevance. Pharmacol. Ther. 1995;66:339–368. doi: 10.1016/0163-7258(94)00005-n. [DOI] [PubMed] [Google Scholar]
- Share L. Role of vasopressin in cardiovascular regulation. Physiol. Rev. 1988;68:1248–1284. doi: 10.1152/physrev.1988.68.4.1248. [DOI] [PubMed] [Google Scholar]
- Smits JF, Struyker-Boudier HA. Intrahypothalamic serotonin and cardiovascular control in the rat. Brain Res. 1976;111:422–427. doi: 10.1016/0006-8993(76)90788-5. [DOI] [PubMed] [Google Scholar]
- Sporton SCE, Shepheard SL, Jordan D, Ramage AG. Microinjections of 5-HT1A agonists into the dorsal motor vagal nucleus produce a bradycardia in the atenolol-pretreated anesthetized rat. Br. J. Pharmacol. 1991;104:466–470. doi: 10.1111/j.1476-5381.1991.tb12452.x. 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukamoto T, Yamamoto T, Watanabe S, Ueki U. Cardiovascular responses to centrally administered serotonin in conscious normotensive and spontaneously hypertensive rats. Eur. J. Pharmacol. 1984;100:173–179. doi: 10.1016/0014-2999(84)90219-x. [DOI] [PubMed] [Google Scholar]
- Szczepanska-Sadowska E, Cudnoch-Jedrzejewska A, Ufnal M, Zera T. Brain and cardiovascular diseases: common neurogenic background of cardiovascular, metabolic and inflammatory diseases. Physiol. Pharmacol. 2010;61:509–521. [PubMed] [Google Scholar]
- Teran FA, Massey CA, Richerson GB. Serotonin neurons and central respiratory chemoreception: where are we now? Prog. Brain Res. 2014;209:207–233. doi: 10.1016/B978-0-444-63274-6.00011-4. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta B, Singer EA. Hypotensive effects of 8-hydroxy-2-(di-n.propylamino)tetralin and 5-methylurapadil following stereotaxic microinjection into the ventral medulla of the rat. Brit. J. Pharmacol. 1990;99:713–716. doi: 10.1111/j.1476-5381.1990.tb12994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC. Serotonin agonists and antagonists in obstructive sleep apnea: therapeutic potential. Am. J. Respir. Med. 2003;2:21–29. doi: 10.1007/BF03256636. [DOI] [PubMed] [Google Scholar]
- Villalón CM, Centurión D. Cardiovascular responses produced by 5-hydroxytriptamine: a pharmacological update on the receptors/mechanisms involved and therapeutic implications. Naunyn Schmiedebergs Arch. Pharmacol. 2007;376:45–63. doi: 10.1007/s00210-007-0179-1. [DOI] [PubMed] [Google Scholar]
- Virarkar M, Alappat L, Bradford PG, Awad AB. L-arginine and nitric oxide in CNS function and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2013;53:1157–1167. doi: 10.1080/10408398.2011.573885. [DOI] [PubMed] [Google Scholar]
- Wang Y, Golledge J. Neuronal nitric oxide synthase and sympathetic nerve activity in neurovascular and metabolic systems. Curr. Neurovasc. Res. 2013;10:81–89. doi: 10.2174/156720213804805963. [DOI] [PubMed] [Google Scholar]
- Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacol. Rev. 2012;64:359–388. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Nakayama M, Nakano H, Mimura N, Yoshida S. Endothelium-dependent vasorelaxation evoked by desmopressin and involvement of nitric oxide in the rat aorta. Am. J. Physiol. 1993;264:E203–E207. doi: 10.1152/ajpendo.1993.264.2.E203. [DOI] [PubMed] [Google Scholar]
- Yusof APM, Coote JH. Excitatory and inhibitory actions of intrathecally administered 5-hydroxytryptamine on sympathetic nerve activity in the rat. J. Auton. Nerv. Syst. 1988;22:229–236. doi: 10.1016/0165-1838(88)90111-7. [DOI] [PubMed] [Google Scholar]