Abstract
Neuroinflammation is a characteristic feature of the Alzheimer’s disease (AD) brain. Significant inflammatory markers such as activated microglia and cytokines can be found surrounding the extracellular senile plaques predominantly composed of amyloid-β protein (Aβ). Several innate immune pathways, including Toll-like receptors (TLRs) and the NLRP3 inflammasome, have been implicated in AD inflammation. Aβ plays a primary role in activating these pathways which likely contributes to the progressive neurodegeneration in AD. In order to better understand the complexities of this interaction we investigated the inflammatory response of primary microglia to Aβ(1–42) protofibrils. Aβ(1–42) protofibrils triggered a time- and MyD88-dependent process that produced tumor necrosis factor alpha (TNFα) and interleukin-1β (IL-1β) mRNA, and intracellular pro and mature forms of IL-1β protein. The accumulation of both IL-1β forms indicated that Aβ(1–42) protofibrils were able to prime and activate the NLRP3 inflammasome. Surprisingly, Aβ-induced accumulation of intracellular mature IL-1β did not translate into greater IL-1β secretion. Instead, we found that Aβ elicited a quantized burst of secreted IL-1β and this process occurred even prior to Aβ priming of the microglia suggesting a basal level of either pro or mature IL-1β in the cultured primary microglia. The IL-1β secretion burst was rapid but not sustained, yet could be re-evoked with additional Aβ stimulation. The findings from this study demonstrated multiple sites of IL-1β regulation by Aβ(1–42) protofibrils including TLR/MyD88-mediated priming, NLRP3 inflammasome activation, and modulation of the IL-1β secretory process. These results underscore the wide-ranging effects of Aβ on the innate immune response.
Keywords: Amyloid-beta protein, aggregation, protofibrils, microglia, inflammation, Toll-like receptors, MyD88, NLRP3 inflammasome, Interleukin 1-beta
Introduction
Alzheimer’s disease (AD) is the most prevalent form of late-life dementia and has been defined by the presence of two pathological hallmarks, neurofibrillary tangles (NFTs) and senile (amyloid) plaques. The NFTs are intracellular lesions of insoluble, highly stable filamentous aggregates of the microtubule-associated protein, tau, whereas the senile plaques are extracellular lesions of insoluble, amyloid fibrils that are polymers of the amyloid-β protein (Aβ) [1, 2]. However, Aβ is the protein that has been found to be most clearly associated to the cause of AD, while tau protein has shown to be most closely related to the clinical manifestations of AD [2]. The complexity of AD pathology reaches beyond NFTs and amyloid plaques. Recent findings have shown that the toxicity of Aβ may be due to soluble oligomeric intermediates found in the AD brain and cerebrospinal fluid rather than insoluble fibrils [3, 4]. These soluble oligomeric Aβ forms appear to contribute to AD onset by causing neuronal and/or synaptic dysfunction [3, 5].
There has been an array of different aggregate morphologies of Aβ found within the AD brain [6]. The core of the Aβ plaques is primarily composed of the 42-amino acid Aβ fragment, Aβ(1–42). However Aβ(1–40) has also been found colocalized with the Aβ(1–42) [7]. In vitro, Aβ monomer undergoes self-assembly by non-covalent interactions to form polydisperse mixtures of soluble oligomers [4] and protofibrils [8, 9]. These soluble aggregates go through a conformational transition from predominately random coil to increasing amounts of β-sheet structure and ultimately produce insoluble fibrils [10, 11]. As a precursor to mature fibrils, protofibrils have been fairly well characterized and have been shown to alter the normal physiology of cultured neurons [12], disrupt ion channels [13], block long-term potentiation (LTP), inhibit synapse remodeling and impair memory consolidation [14].
A prominent component of AD pathology is neuroinflammation found in the affected areas of the AD brain. Inflammatory markers, such as activated microglia and proinflammatory cytokines, are most often observed surrounding the Aβ lesions within the AD brain [15]. Although the exact sequence of events leading to AD is still unclear, it is thought that Aβ accumulation initiates a pathological cascade of events leading to neuronal dysfunction and ultimately dementia. One part of this pathological cascade is an immune cell-mediated inflammatory response, involving proliferation and activation of microglia and astrocytes. The increase in inflammation-associated proteins and oxidative stress by-products is more than just a biomarker of AD (Golde et al. 2002). Persistent inflammation can lead to a chronic inflammatory state, suggested to be an underlying mechanism of the progressive neurodegeneration in AD [16, 17].
The innate immune system protects an organism by detection of invading pathogens via pattern recognition receptors (PRRs). Both microglia and astrocytes express Toll-like receptors (TLRs), which are a family of PRRs that recognize pathogen-associated molecular patterns (PAMPs) within specific molecules produced by bacteria, fungi, and viruses [18]. TLR engagement, with the exception of TLR3, leads to the recruitment of the adaptor protein myeloid differentiation protein 88 (MyD88), activation of the transcription factor nuclear factor-κB (NF-κB), and expression of a variety of genes involved in the immune response, such as tumor necrosis factor-α (TNFα) and interleukin-1β (IL-1β) [18]. Multiple TLRs and accessory proteins play a role in the proinflammatory response evoked by Aβ in monocyte/macrophages and microglia including CD14, TLR4, and TLR2 [19–23] and TLR4 and TLR6 in concert with CD36 [24]. In addition to TLRs, a multireceptor complex comprised of scavenger receptor class B (SR-B), CD36, α6β1-integrin and the integrin-associated protein CD47 has also been shown to interact with Aβ and initiate a proinflammatory response [25].
Extracellular recognition of PAMPs by TLRs and downstream signaling events can trigger oligomerization of cytoplasmic Nod-like receptors (NLRs) to form a multisubunit inflammasome complex [26]. Different NLR family members vary in their N-terminal protein-protein interaction region. For example, NLRP3 contains a pyrin domain which is responsible for complexation with ASC, the adaptor molecule termed apoptosis-associated speck-like protein containing a caspase-recruitment domain (CARD) [27]. An important aspect of TLR signaling is production of IL-1β mRNA and the pro-form of IL-1β protein (pro-IL-1β). This event is considered “priming” of the inflammasome and represents Signal 1 of a 2-stage process [28]. Many molecules, including the classical TLR4 agonist lipopolysaccharide (LPS), stimulate priming of the inflammasome (Signal 1) but not activation. Inflammasome assembly via a CARD-CARD interaction brings caspase-1 to the complex and activation of the complex triggers caspase-1-catalyzed proteolytic cleavage of pro-IL-1β to mature IL-1β [29]. A second signal is required for the inflammasome activation step and subsequent production of mature IL-1β. Signal 2 may be provided by a growing number of endogenous human molecules, referred to as danger-associated molecular patterns (DAMPs). These molecules include certain pore-forming toxins, ATP, K+ efflux, and crystalline particles such as silica and uric acid crystals [28].
Increasing data has demonstrated a significant role for the NLRP3 inflammasome in AD (reviewed in [30]. Double transgenic mice that overexpress human APP with familial AD mutations and have a deficiency in the NLRP3 inflammasome showed a reduction in AD pathology and were protected from the ensuing cognitive defects [31]. Earlier in vitro studies demonstrated that fibrillar Aβ stimulated NLRP3 inflammasome activation and IL-1β production in microglia and this process was shown to involve phagocytosis, lysosomal damage, and release of cathepsin B [32]. Another report confirmed fibrillar Aβ-stimulated inflammasome activation but demonstrated that pre-priming of the inflammasome was required [33]. However, it was recently shown that Aβ may provide both priming and activation signals [34].
We have recently demonstrated that Aβ(1–42) protofibrils, but not Aβ(1–42) fibrils, are robust activators of microglia [35] and that these soluble fibrillar precursors can trigger proinflammatory events via Toll-like receptors (TLRs) [20]. In the current study we investigated the role of MyD88 in both Aβ(1–42) protofibril-induced cytokine production and NLRP3 inflammasome activation. During this investigation, we identified multiple points of regulation by Aβ protofibrils in both pathways including new insights into the IL-1β secretion process.
Experimental Procedures
Preparation of Aβ Peptides
Aβ(1–42) was obtained from W. M. Keck Biotechnology Resource Laboratory (Yale School of Medicine, New Haven, CT) in lyophilized form and stored at −20° C. Aβ(1–42) peptides were dissolved in 100% hexafluoroisopropanol (HFIP) (Sigma-Aldrich, St. Louis) at 1 mM, separated into aliquots in sterile microcentrifuge tubes, and evaporated uncovered at room temperature overnight in a fume hood. The following day the aliquots were vacuum-centrifuged to remove any residual HFIP and stored in dessicant at −20° C.
Size Exclusion Chromatography
Aβ protofibrils were isolated as previously described [35]. Briefly, lyophilized Aβ was dissolved in 50 mM NaOH to yield a 2.5 mM Aβ solution followed by dilution to 250 µM Aβ in prefiltered artificial cerebrospinal fluid (aCSF, 15 mM NaHCO3, 1 mM Na2HPO4, 130 mM NaCl, 3 mM KCl, pH 7.8). The solution was then centrifuged at 18,000g for 10 min and the supernatant was fractionated on a Tricorn Superdex 75 10/300 GL column (GE Healthcare) using an AKTA FPLC system (GE Healthcare). Prior to injection of Aβ, the Superdex 75 column was coated with sterile bovine serum albumin (Sigma) to prevent any non-specific binding of Aβ to the column matrix. Following a 1 mL loading of the sample, Aβ was eluted at 0.5 mL min−1 in aCSF and 0.5 mL fractions were collected and immediately placed on ice. Aβ concentrations were determined in line by UV absorbance using an extinction coefficient of 1450 cm−1 M−1 at 280 nm.
Primary Microglia Isolation
Primary murine microglia were obtained from wild-type (WT) C57BL/6 (Harlan Laboratories) or MyD88−/− mice (gift from Dr. Tammy Kielian, University of Nebraska Medical Center). Microglia were isolated as previously described [35, 36] from 3–4 day old mouse pups. Briefly, brains were isolated under sterile conditions, minced, and trypsinized. The brain tissue was then resuspended in complete DMEM containing 10% fetal bovine serum (FBS), 4 mM L-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin and 0.25 µg/mL amphotericin-B, OPI medium supplement (oxalocetate, pyruvate, insulin), and 0.5 ng/ml recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF). The cell suspension was filtered, centrifuged, resuspended in complete medium and seeded into 150 cm2 flasks. Cells were cultured at 37 °C in 5% CO2 until confluent (1–2 weeks) and microglia were selectively harvested from the adherent astrocyte layer by overnight shaking of the flask at 37 °C in 5% CO2 and collection of the medium. The flasks were replenished with fresh medium, and incubated further to obtain additional microglia. Typically, this procedure was repeated 3–4 times for one flask without removal of the astrocyte layer.
Cell Stimulation Assay
For cellular studies, WT and MyD88−/− primary murine microglia were collected as described above by overnight shaking, collection of the cells, and seeding in a sterile 96-well cell culture plate for 24 h at a density of 5 × 105 cells/ml in growth medium with serum and GM-CSF (0.1 mL per well). Prior to cell treatment, medium was replaced with 0.1 mL medium lacking FBS and GM-CSF. Cells were then treated with Aβ (15 µM) or TLR stimuli ultra-pure lipopolysaccharide (LPS, 10 ng/ml or 100 ng/ml, InvivoGen), N-Palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-[R]-cysteinyl-[S]-seryl-[S]-lysyl-[S]-lysyl-[S]-lysyl-[S]-lysine (Pam3CSK4, 100 ng/ml, InvivoGen), or (S,R)-(2,3-bispalmitoyloxypropyl)-Cys-Gly-Asp-Pro-Lys-His-Pro-Lys-Ser-Phe (FSL-1, 100 ng/ml, InvivoGen). The cells were incubated at 37 °C for the indicated times in 5% CO2, the medium was collected and stored at −20 °C for subsequent analysis by enzyme-linked immunosorbent assay (ELISA) and the cells were lysed for either intracellular ELISA analysis or mRNA analysis. The background cellular response was assessed using the particular buffer vehicle for the Aβ. Inhibition of secreted IL-1β by the caspase-1 inhibitor z-YVAD-fmk (EMD Millipore) was carried out by dissolution of the inhibitor in 100% DMSO. Microglia were pretreated for 30 min with final concentrations of 0, 5, 10, and 20 µM z-YVAD-fmk containing 0.05% DMSO followed by treatment with 15 µM Aβ(1–42) protofibrils for 6 hr at 37°C and 5% CO2. The conditioned medium was then collected and analyzed by ELISA for secreted IL-1β .
ELISA
Levels of murine TNFα and IL-1β were determined by ELISA as previously detailed [20]. Briefly, 96-well plates were coated overnight with monoclonal anti-mouse TNFα or IL-1β capture antibody (R&D Systems), washed with phosphate-buffered saline (PBS) containing 0.05% Tween-20 and blocked with PBS containing 1% bovine serum albumin (BSA), 5% sucrose and 0.05% NaN3 following by a wash step. Successive treatments with washing in between were done with samples or standards, biotinylated polyclonal anti-mouse TNFα or IL-1β detection antibody (R&D Systems) in 20 mM Tris with 150 mM NaCl and 0.1% BSA, streptavidin-horseradish peroxidase (HRP) conjugate, and equal volumes of HRP substrates 3,3',5,5'-tetramethylbenzidine and hydrogen peroxide. The reaction was stopped by the addition of 1% H2SO4 solution. The optical density of each sample was analyzed at 450 nm with a reference reading at 630 nm using a SpectraMax 340 absorbance plate reader (Molecular Devices, Union City, CA). The concentration of TNFα and IL-1β in the experimental samples was calculated from a mouse TNFα or IL-1β standard curve of 15–16000 pg/ml. When necessary, samples were diluted to fall within the standard curve. TNFα and IL-1β concentrations for absorbance values below the lowest 15 pg/ml standard were determined by extrapolation of the standard curve regression line. Intracellular pro-IL-1β was determined using a mouse IL-1β pro-form ELISA set (eBioscience 88–8014). The procedure was done according the manufacturer guidelines and is similar to the TNFα and IL-1β described above. The concentration of pro-IL-1β in the experimental samples was calculated from a mouse pro-IL-1β standard curve of 15–2000 pg/ml. All cytokine levels are reported as pg/mL and are based on the output from 104 cells in each well. For intracellular measurements with combined wells used for extract preparation, the cytokine concentrations have been normalized to reflect 104 cells per well.
Determination of mRNA levels
Following treatment of microglia and removal of the medium, total RNA was obtained using a GeneJET RNA purification kit (Fermentas). The procedure was done according to manufacturer guidelines. RNA was collected from five replicate samples for each condition to increase the amount of mRNA in each condition. Total RNA concentration was determined using 260/280 nm absorbance ratio and the highest amount of total RNA available up to 1 µg was concentrated. Genomic DNA was removed in the presence of an RNase inhibitor and each sample was stored on ice. Synthesis of cDNA was done according to manufacturer guidelines (RevertAid Kit, Fermentas) and cDNA samples were stored at −20 °C. Selected sequences were amplified using real-time quantitative (RT-q) PCR (C1000 Thermocycler, BioRad, Hercules, CA) and the SYBR Green mode of detection. Primers (Invitrogen) (TNFα, TTCCCAAATGGCCTCCCTCTCATC, forward, TCCTCCACTTGGTGGTTTGCTAC, reverse; IL-1β, CCTGTGTAATGAAAGACGGCACAC, forward, ATTGATTGGGATCCACACTCTCC, reverse) and cDNA samples were subjected to a three-step cycling protocol using Bio-Rad CFX Manager software. mRNA levels were calculated and reported in terms of relative quantity (RQ). RQ was determined from the equation RQ = E^(Cq, control – Cq, stimulated), where Cq is the quantification cycle value obtained from the fluorescence amplification plot and E is efficiency with a value of 2. In numerous experiments β-actin was used as an internal control and did not vary significantly between samples regardless of microglia stimulation state.
Western Blot
Extracts of treated primary microglia were prepared by removal of the conditioned medium and lysis with RIPA buffer (20 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% deoxycholic acid, and 0.1% SDS) supplemented with protease inhibitor cocktail (Sigma). Five replicate wells were combined for each condition. Collected lysates were centrifuged at 9,000g to remove cell debris and the supernatants were mixed 1:1 with Laemmli SDS sample buffer (Bio-Rad) containing 5% β-mercaptoethanol. Samples were denatured (95°C, 5 min) and separated on 15% Tris-HCl gels (Ready Gel, Bio-Rad) under denaturing conditions (25 mM Tris, 192 mM glycine, 0.1% SDS at pH 7.8) using a Mini Protean 3 Cell (Bio-Rad).
Gels were transferred to PVDF membrane (Milipore) in transfer buffer containing 25 mM Tris base, 192 mM glycine, and 10% methanol at pH 8.3 using a Tank VEP-2 electroblotting system (Owl Separation Systems). Following protein transfer, the membrane was blocked for 1 hr at 25 °C with PBS containing 5 % nonfat dry milk and 0.1% Tween 20) and probed with a 1:500 dilution of 3ZD anti-IL-1β monoclonal antibody (National Cancer Institute) in PBS with 1 % milk and 0.1 % Tween 20 overnight at 4 °C and a 1:1000 dilution of goat anti-mouse IgG-HRP secondary antibody (R & D systems) in the same buffer for 1 hr at 25 °C. Protein detection was accomplished using ECL Western Blotting Substrate Detection reagents (Pierce) and exposure to film (Kodak). The membrane was stripped with Restore Western Blot Stripping Buffer (Thermo Scientific) and re-probed with α-tubulin (TU-02) (Santa Cruz Biotechnology) as a loading control.
Statistical Analysis
Statistical analysis was performed to determine the confidence limit at which two measurements were statistically different. In most cases one-way analysis of variance (ANOVA) was used although some data were treated with univariate ANOVA. Statistical differences with a p-value greater than 0.05 were considered insignificant.
Results
MyD88-dependent microglia stimulation and priming of the NLRP3 inflammasome by Aβ(1–42) protofibrils
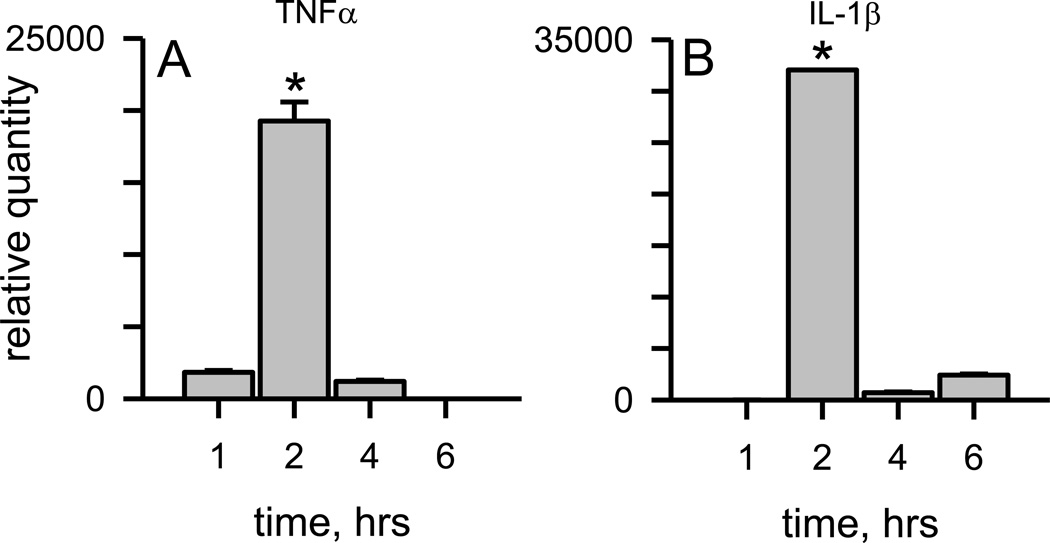
Aβ(1–42) protofibrils were prepared as previously described in a modified artificial cerebrospinal fluid (aCSF) buffering system and isolated by SEC [37]. This preparation produced a Superdex 75 void-volume fraction enriched in curvilinear Aβ protofibrillar structures less than 100 nm in length with a range of hydrodynamic radii from 10–40 nm (mean 21 nm) [37]. We have previously shown that soluble protofibrils prepared in either F-12 cell culture medium or aCSF bound and significantly enhanced thioflavin T (ThT) fluorescence emission at 480 nm whereas isolated Aβ(1–42) monomers eluted in the included volume did not display any ThT fluorescence [35, 37]. In the current study, primary murine microglia were treated with SEC-isolated Aβ(1–42) protofibrils for different incubation times and mRNA levels for proinflammatory cytokines, TNFα and IL-1β, were quantified as described in the Methods. Protofibrils significantly elevated TNFα mRNA by 2 hrs followed by a rapid drop of the transcript at the 4 and 6 hr time points (Fig. 1A). Aβ(1–42) protofibrils also induced significant levels of IL-1β mRNA in primary murine microglia. The time course was similar to TNFα in that the majority of stimulated IL-1β mRNA occurred at 2 hrs (Fig. 1B).
Figure 1.
Aβ(1 −42) protofibrils formed and isolated in aCSF are significant stimulators of TNFα and IL-1β transcription. SEC-isolated Aβ(1–42) protofibrils in aCSF were incubated with WT primary microglia at a final concentration of 1 5 µM for 1, 2, 4, and 6 hrs in serum-free medium. At each time point the cells were lysed for total RNA extraction. TNFα (Panel A) and IL-1β (Panel A) mRNA levels were measured by qPCR as described in the Methods. The term relative quantity is described in the Methods and represents the mRNA comparison between microglia treated with Aβ(1–42) protofibrils and those treated with buffer control. β-actin was used as an internal control in separate experiments and did not vary significantly between samples. Data bars represent the mean ± std error of n=8 qPCR replicates. TNFα and IL-1β mRNA levels at 2 hours were significantly different than any other time point (*p<0.001).
A role for several TLRs has been demonstrated in the recognition of extracellular Aβ and initiation of proinflammatory events in monocyte/macrophages and microglia [19–24]. One of the cytoplasmic mediators of signal transduction linking TLR stimulation and NFκB activation is the adaptor protein MyD88 [18]. In order to determine the contribution of TLR-MyD88 signaling in Aβ protofibril-induced cytokine mRNA upregulation, microglia isolated from either WT or MyD88−/− mice were compared for their responsiveness to Aβ(1–42) protofibrils. Wild type microglia again showed marked increases in both TNFα and IL-1β mRNA in response to treatment with Aβ(1–42) protofibrils (Fig. 2). However, this response was dramatically reduced in MyD88−/− microglia compared to WT microglia. Both TNFα and IL-1β mRNA levels were close to that of buffer control in the MyD88−/− microglia (Fig. 2).
Figure 2.
TNFα and IL-1β mRNA production is reduced in the MyD88−/− microglia in response to protofibrils. SEC-isolated Aβ(1–42) protofibrils were incubated with WT primary microglia and MyD88−/− (KO) microglia at a final concentration of 15 µM for 2 hrs in serum-free medium. After 2 hrs the total RNA was collected and TNFα (Panel A) and IL-1β (Panel B) mRNA levels were quantified by qPCR as described in Fig. 1 legend. Data bars represent the mean ± std error of n=8 qPCR replicates. Protofibril-treated microglia were compared to aCSF-treated microglia to determine relative quantity. When comparing WT and MyD88−/− microglia, experiments were always done on the same day. Statistical analysis confirmed a significant difference (*p<0.001) between the WT and MyD88−/− microglial TNFα and IL-1β mRNA response.
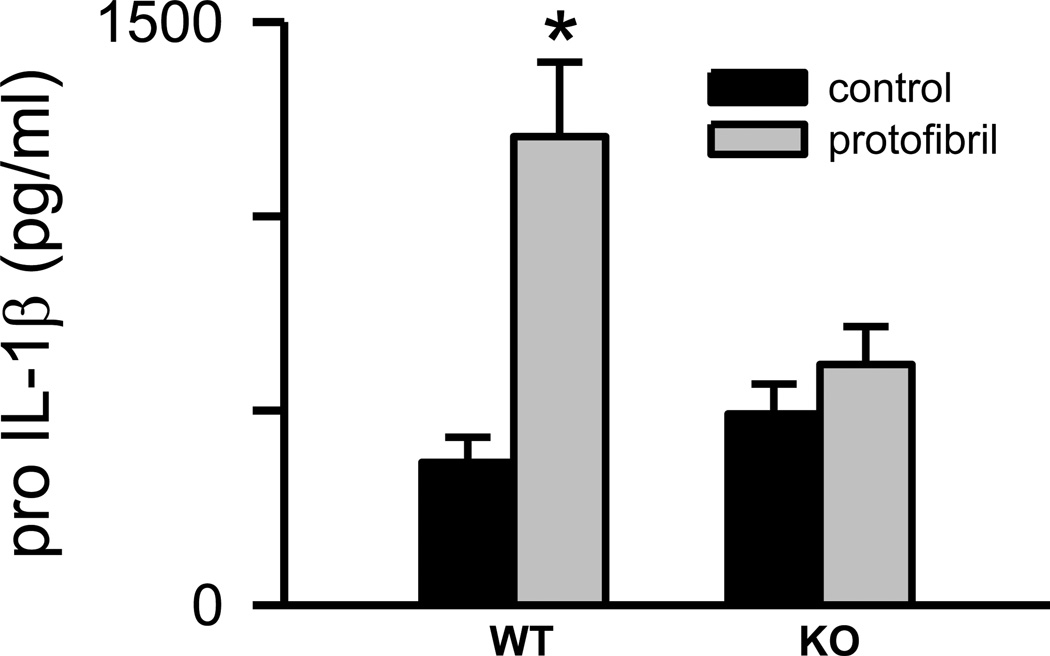
The mRNA findings revealed a TLR/MyD88-dependent transcriptional activation process triggered by Aβ(1–42) protofibrils. This pathway was explored in further detail by measuring intracellular levels of pro-IL-1β using a pro-form specific ELISA. The specificity was confirmed by the observation that serial dilutions of mature IL-1β protein standard were not detected by the pro-IL-1β-specific antibodies. Only pro- IL-1β and not mature IL-1β standard curves were able to be constructed (Supplementary Figure 1). Analysis of microglial extracts following removal of the conditioned medium and washing of the cells yielded several observations. First, the presence of existing stores of pro-IL-1β in unstimulated WT and MyD88−/− microglia was confirmed at 6 hr (Fig 3) which indicated some level of underlying basal NLRP3 inflammasome priming in the isolated primary microglia. This may be inadvertently caused by the isolation and culturing process or purposefully established as an intracellular reservoir enabling a rapid microglial response to stimuli. Secondly, a significant increase in pro-IL-1β levels compared to control treatment occurred after treatment of the WT microglia with Aβ(1–42) protofibrils for 6 hr (p<0.005) (Fig 3) when a one-way ANOVA was used to compare control and stimulated in WT microglia . This same analysis did not show a significant difference in MyD88−/− microglia (p>0.05) (Fig 3). Further analysis of the time points by univariate ANOVA considering both treatment and the presence of MyD88 showed a lesser, but still significant, difference (p<0.05) between the WT and MyD88−/− microglial response. Pro-IL-1β production was somewhat variable and the difficulty in quantitatively measuring pro-IL-1β stimulated by Aβ(1–42) protofibrils may be partially explained by the subsequent observation that protofibrils also activate the NLRP3 inflammasome, thus creating a dynamic pool of pro-IL-1β with a balance between production and conversion to mature IL-1β.
Figure 3.
Aβ(1–42) protofibrils stimulate intracellular pro-IL-1β production in microglia. SEC-isolated Aβ(1–42) protofibrils in aCSF (gray bars) or aCSF buffer alone (black bars) were incubated with WT and MyD88−/− (KO) primary microglia at a final concentration of 15 µM for 6 hrs in serum-free medium. After incubation the conditioned medium was removed, each cell well was treated with 100 µL of lysis buffer and the extract was collected to measure intracellular pro-IL-1β protein by ELISA. Data bars represent the average ± std error of n=5 replicates. One-way ANOVA looking at WT and KO microglia individually showed a significant difference between control and protofibril-stimulated WT microglia (p<0.005) with no statistical difference in the KO microglia. Univariate ANOVA considering both treatment and the presence of MyD88 showed a lesser, but still significant, difference (*p<0.05) between the WT and KO microglial response.
Intracellular mature IL-1β production induced by Aβ(1–42) protofibrils in primary microglia
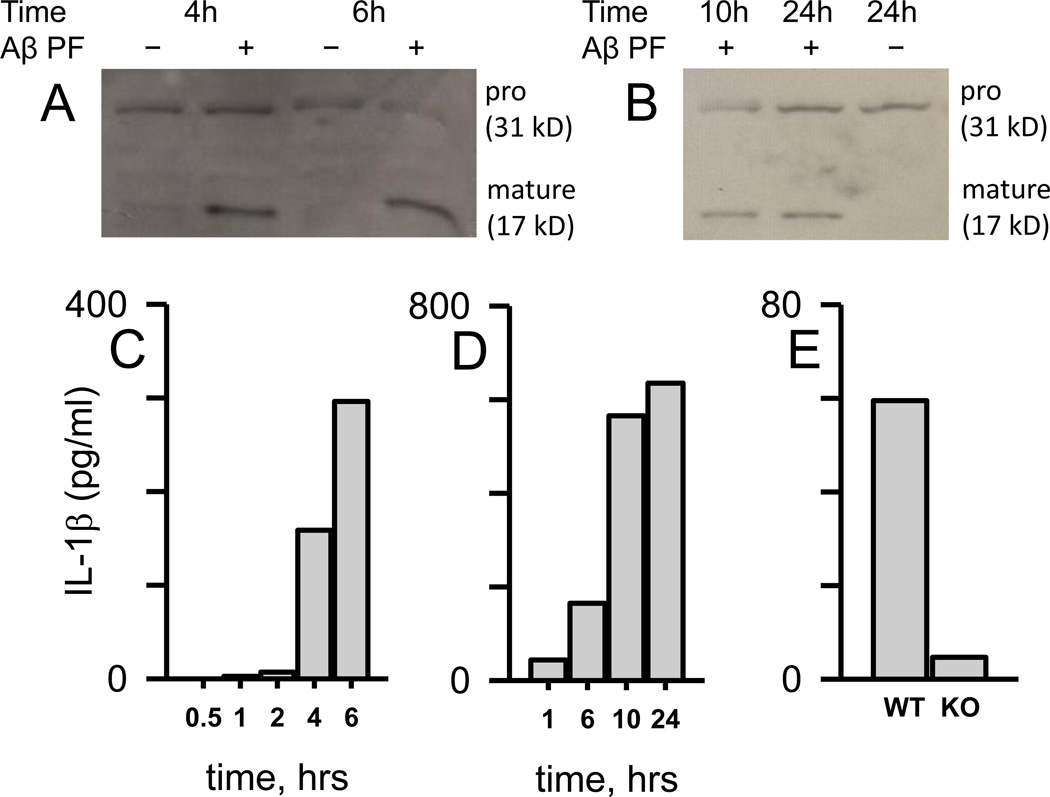
Recent evidence has demonstrated a role for Aβ in the activation of the NLRP3 inflammasome [31–34]. The result of NLRP3 complex assembly and activation is caspase-1 mediated proteolytic cleavage of pro-IL-1β (31 kD) to the mature form of the protein (17 kD). In order to study intracellular inflammasome processing of pro-IL-1β to mature IL-1β by Aβ(1–42) protofibrils in microglia, immunoblots were combined with ELISA measurements for evaluation of both IL-1β species. Primary murine microglia were treated with isolated Aβ(1–42) protofibrils and at increasing incubation times, the cells were lysed to probe intracellular IL-1β levels. Immunoblot analysis using 3ZD antibody, which recognizes both pro and mature forms of IL-1β, showed a consistent pool of pro-IL-1β throughout the time course for both unstimulated and Aβ-stimulated microglia (Fig 4A). Significant levels of intracellular mature IL-1β were not observed until the 4 and 6 hr Aβ-stimulated time points. ELISA-based IL-1β measurements, using a different set of antibodies (DuoSet, R&D Systems), were also conducted on the same samples. The same time-dependent production of intracellular mature IL-1β observed by immunoblot was also detected by IL-1β ELISA with the most substantial increases at 4 and 6 hr (Fig 4C). The IL-1β ELISA antibodies likely detect both pro and mature IL-1β forms. However, the large change in IL-1β levels as measured by ELISA (Fig 4C) corresponded with the clearly observable increase in mature IL-1β in the immunoblot (Fig 4A) suggesting that marked increase was the mature protein. Intracellular cell extracts were prepared from multiple wells (n=5) using a smaller volume of lysis buffer to increase detection by immunoblot. This method resulted in relatively high intracellular mature IL-1β concentrations when measured by ELISA. Therefore, these levels were normalized to reflect the number of wells (104 cells per well) and the volume of the extract. A longer time course was conducted that extended the incubation time of the Aβ(1–42) protofibrils with primary microglia to 24 hr (Fig 4B, D). Immunoblot analysis again showed the pro and mature form of IL-1β (Fig 4B) and both immunoblot and ELISA measurements (Fig 4D) further demonstrated that intracellular IL-1β continued to increase after stimulation of the microglia with Aβ(1–42) protofibrils.
Figure 4.
Aβ(1–42) protofibrils stimulate time- and MyD88-dependent intracellular mature IL-1β accumulation in microglia. Panel A, C. WT primary microglia were treated with SEC-isolated Aβ(1–42) protofibrils (15 µM) in aCSF (+) or just aCSF buffer (-) and incubated for 0.5, 1, 2, 4, and 6 hrs in serum-free medium at 37 °C. At each time point the cells were lysed and extracts prepared for intracellular IL-1β Western blot (Panel A) and ELISA (Panel C) analysis. A separate but similar experiment was done over a longer time course with the same analysis (Panel B Western blot, Panel D ELISA). Each intracellular cell extract sample for all panels was obtained from a combination of five replicate wells in a 96-well plate treated with 20 µL total of lysis buffer, thus the band or data bar is representative of a 5-well extract pool. Panel E. Intracellular mature IL-1β was measured by ELISA in extracts prepared as described above after treatment of WT and MyD88−/− primary microglia (KO) for 6 hrs with Aβ(1–42) protofibrils (15 µM) as described in Panel A and C. Corresponding control treatments with a volume of aCSF equal to that carrying the Aβ were also analyzed for IL-1β. These control levels, which averaged 10% of the Aβ-stimulated levels, were subtracted from the overall Aβ response. The measured intracellular IL-1β levels in C-E (pg/ml), which were much higher, have been normalized to reflect the response from pooling of multiple wells containing 104 microglial cells per well and in a lesser volume.
The time course for the marked build-up of intracellular mature IL-1β fit a model whereby Aβ triggers the TLR/MyD88 pathway leading to transcription of IL-1β mRNA, synthesis of pro-IL-1β , and conversion of pro to mature IL-1β . These data show that the last step is also triggered by Aβ(1–42) protofibrils. Subsequent experiments testing this model compared intracellular mature IL-1β levels between WT and MyD88−/− microglia after treatment with Aβ(1–42) protofibrils. While WT microglia produced significant levels of intracellular IL-1β , MyD88−/− microglia were severely diminished in their ability to produce intracellular mature IL-1β (Fig 4E). At 6 hr, MyD88−/− microglia IL-1β levels were only 8% of WT microglia (Fig 4E) and no IL-1β above control was observed at 24 or 48 hr after treatment of MyD88−/− microglia with Aβ(1–42) protofibrils (data not shown).
Time course of Aβ(1–42) protofibril-induced microglial TNFα and IL-1β protein secretion
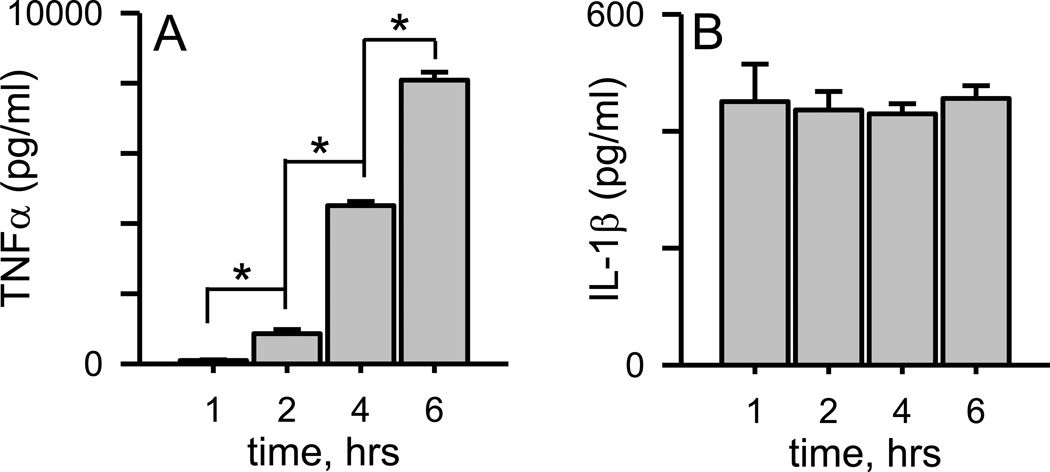
Primary murine microglia were treated with SEC-isolated Aβ(1–42) protofibrils for different incubation times and secreted protein levels for proinflammatory cytokines TNFα and IL-1β were quantified in the conditioned medium as described in the Methods. Protofibrils induced a steady increase in secreted TNFα protein during the 6 hr incubation (Fig. 5A). Much of the TNFα protein secretion occurred after the majority of the TNFα mRNA had been transcribed (Fig 1A). Control treatments with aCSF buffer alone were just under 3% of the protofibril-stimulated levels. The time course for Aβ(1–42) protofibril-induced IL-1β secretion was much different than for TNFα. Unexpectedly, maximal levels of secreted mature IL-1β protein were observed at 1 hr and this level was maintained through the 6 hr collection time point. This observation was surprising since maximal IL-1β protein secretion by Aβ(1–42) protofibrils occurred prior to induction of maximum IL-1β mRNA transcription (Fig 1B). The findings revealed several things, (1) the apparent presence of a small pre-existing level of pro-IL-1β in the isolated primary microglia, (2) that Aβ-activation of the NLRP3 inflammasome occurs much more rapidly than Aβ stimulation of the TLR/MyD88 pathway and cytokine transcription/translation, and (3) the build-up of intracellular mature IL-1β does not necessarily translate into increased IL-1β secretion. Importantly, the data again demonstrated that Aβ(1–42) protofibrils did not need a secondary molecule, or an additional stimulus, to produce mature IL-1β protein as is the case with LPS (Signal 1) and ATP (Signal 2) [18]. Additional time-dependent experiments found that maximal levels of secreted IL-1β protein could be observed by 15 min (the earliest time point tested) and there was no residual secreted IL-1β remaining from the overnight plating of the microglia (data not shown). From these experiments, it was apparent that the microglia rapidly secreted a quantum of mature IL-1β after Aβ(1–42) protofibril stimulus and the time point at which the conditioned medium was collected was immaterial. This same pattern of rapid IL-1β secretion was observed repeatedly. In fact, during the experiments presented in Figure 4A–D, secreted IL-1β was also measured in the conditioned medium collected prior to cell lysate preparation. It did not matter whether the samples were collected at 0.25, 1, 2, 4, 6 or 24 hrs, a similar quantum of secreted mature IL-1β was found in the medium (Supplementary Figure 2) despite the significant increase in intracellular mature IL-1β shown in Figure 4C and D. The measurement of both secreted and intracellular mature IL-1β from the same cells provided even stronger evidence that the rapid IL-1β secretion stimulated by Aβ(1–42) protofibrils was temporally distinct from, and not fully dependent on, Aβ-triggered intracellular mature IL-1β accumulation .
Figure 5.
Aβ(1–42) protofibrils stimulate rapid IL-1β secretion but a slower time-dependent TNFα secretion. SEC-isolated Aβ(1–42) protofibrils in aCSF were incubated with WT primary microglia at a final concentration of 15 µM for 1, 2, 4, and 6 hrs in serum-free medium. At each time point the conditioned microglial medium was collected for TNFα and IL-1β protein determination by ELISA. Panel A. TNFα protein levels were determined from the supernatant of individually treated wells. Data bars represent the mean ± std error of n=5 replicates at each time point. Control treatments with an equal volume of aCSF produced very low TNFα levels compared to protofibrils ranging from 36–290 pg/ml (<4% of the Aβ-stimulated response) at the different time points and were subtracted from Aβ-stimulated samples. TNFα levels at each successive time point are statistically different than the preceding time point (p<0.001). Panel B. IL-1β protein levels were measured in the same manner as for TNFα. Control treatments with an equal volume of aCSF produced IL-1β levels of 1 pg/ml and were subtracted from Aβ-stimulated samples. The length of incubation time had no statistical difference on the amount of secreted IL-1β (p>0.05).
MyD88 impacts Aβ(1–42) protofibril-induced microglial TNFα and IL-1β protein secretion differently
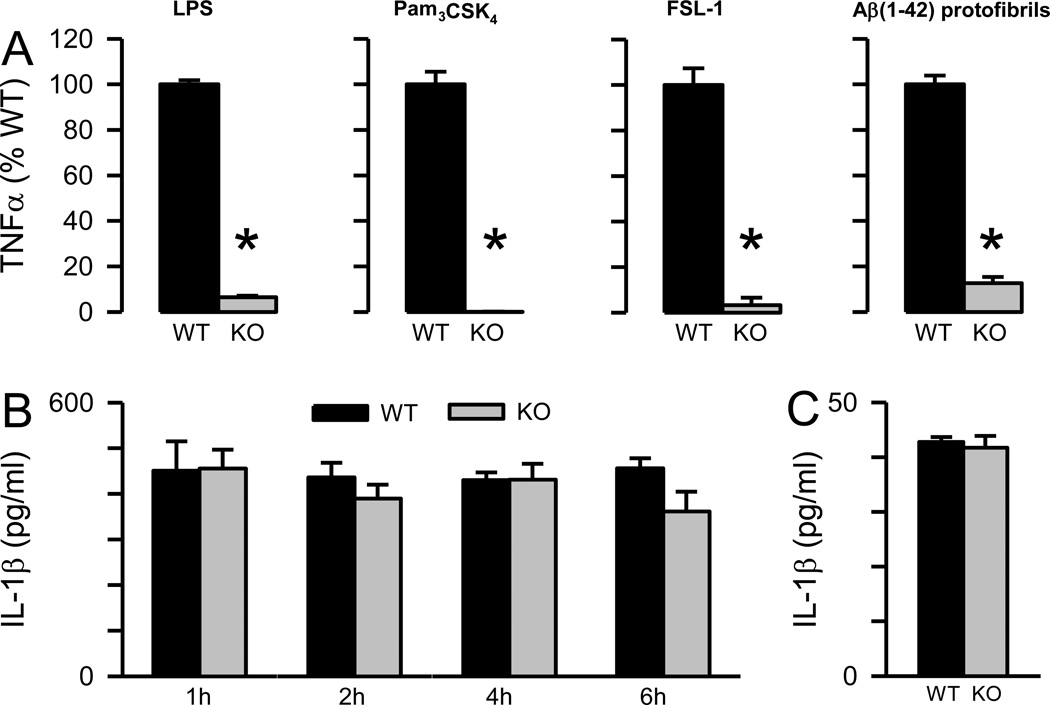
TNFα protein secretion was compared between WT and MyD88−/− microglia after stimulation with three known TLR ligands, LPS (TLR4), PAM3CSK4 (TLR2/1) and FSL-1 (TLR2/6). TNFα levels were markedly reduced in MyD88−/− microglia in response to LPS (92% reduction) but not completely abolished confirming both MyD88-dependent and independent pathways (Fig 6A). However, the absence of MyD88 completely eliminated TNFα production in response to PAM3CSK4 or FSL-1 (Fig 6A). The results for the TLR ligands presented in Figure 6 were obtained using 100 ng/ml of each ligand although additional experiments at 10 ng/ml ligand concentration gave similar results (data not shown). The microglial TNFα response to Aβ(1–42) protofibrils was significantly lowered in the absence of MyD88 (88% reduction) indicating that the adaptor protein was a major mediator of Aβ(1–42) protofibril-induced TNFα production. The remaining response suggested additional pathways for mediating TNFα secretion that were independent of MyD88.
Figure 6.
Aβ(1–42) protofibril-induced TNFα secretion, but not rapid IL-1β secretion, is dependent on MyD88. Panel A. TLR4 ligand LPS, TLR1/2 ligand Pam3CSK4, TLR2/6 ligand FSL-1, and SEC-isolated Aβ(1–42) protofibrils were incubated with WT and MyD88−/− (KO) primary microglia at a final concentration of 100 ng/ml for all TLR ligands and 15 µM for Aβ for 6 hrs in serum-free medium. Secreted TNFα was measured by ELISA in the conditioned medium. Data bars represent the average ± std error of n=9 replicates (triplicates from 3 separate experiments) for LPS, Pam3CSK4, and FSL-1, and n=6 replicates (triplicates from 2 separate experiments) for Aβ(1–42) protofibrils. Data is presented as % WT response. Actual TNFα levels were 21667, 4584, 2888, and 12652 pg/ml for LPS, Pam3CSK4, FSL-1, and Aβ(1–42) protofibrils respectively. Control treatments with an equal volume of H2O or aCSF were less than 3% of the TNFα response and were subtracted from TLR ligand- or Aβ-stimulated samples. Statistical analysis showed a significant difference (p<0.005) between the WT and MyD88−/− results for all four treatments. Panel B. SEC-isolated Aβ(1–42) protofibrils were incubated with WT primary microglia and MyD88−/− (KO) microglia at a final concentration of 15 µM for 1, 2, 4, and 6 hrs in serum-free medium. Secreted IL-1β was measured by ELISA in the conditioned medium. Data bars represent the average ± std error of n=5 replicates. Control treatments with an equal volume of aCSF produced 1 pg/ml IL-1β at all time points for both the WT microglia and the MyD88−/− microglia and were subtracted from Aβ-stimulated samples. Statistical analysis showed no significant difference between the WT and MyD88−/− results at any time point (p>0.05). Panel C. Secreted IL-1β was measured by ELISA after treatment of WT and MyD88−/− primary microglia (KO) with Aβ(1–42) protofibrils (15 µM) for 6 hrs in serum-free medium. Conditioned medium was collected and secreted IL-1β was measured by ELISA. The secreted data bars are the average ± std error for n=5 replicates. Control treatments with an equal volume of aCSF were subtracted from Aβ-stimulated samples and averaged 0.4%. No statistical difference was observed between the WT and MyD88−/− response (p>0.05).
The levels of secreted mature IL-1β protein were evaluated in WT microglia medium after treatment with stimuli. Neither LPS, PAM3CSK4, nor FSL-1 alone induced mature IL-1β protein secretion in WT microglia (data not shown) confirming that these TLR ligands do not activate the NLRP3 inflammasome without an additional stimulus. Aβ(1–42) protofibrils, however, induced similar levels of secreted IL-1β protein in both WT and MyD88−/− microglia conditioned medium collected after 1, 2, 4, or 6 hrs of stimulation (Fig 6B). The observation that the absence of MyD88 did not decrease protofibril-induced IL-1β protein secretion yet dramatically reduced IL-1β mRNA (Fig 2) was the second line of evidence suggesting a potential intracellular reservoir of pro- or mature IL-1β produced prior to stimulation of the microglia with Aβ(1–42) protofibrils.
Mechanisms of Aβ(1–42) protofibril-induced IL-1β secretion
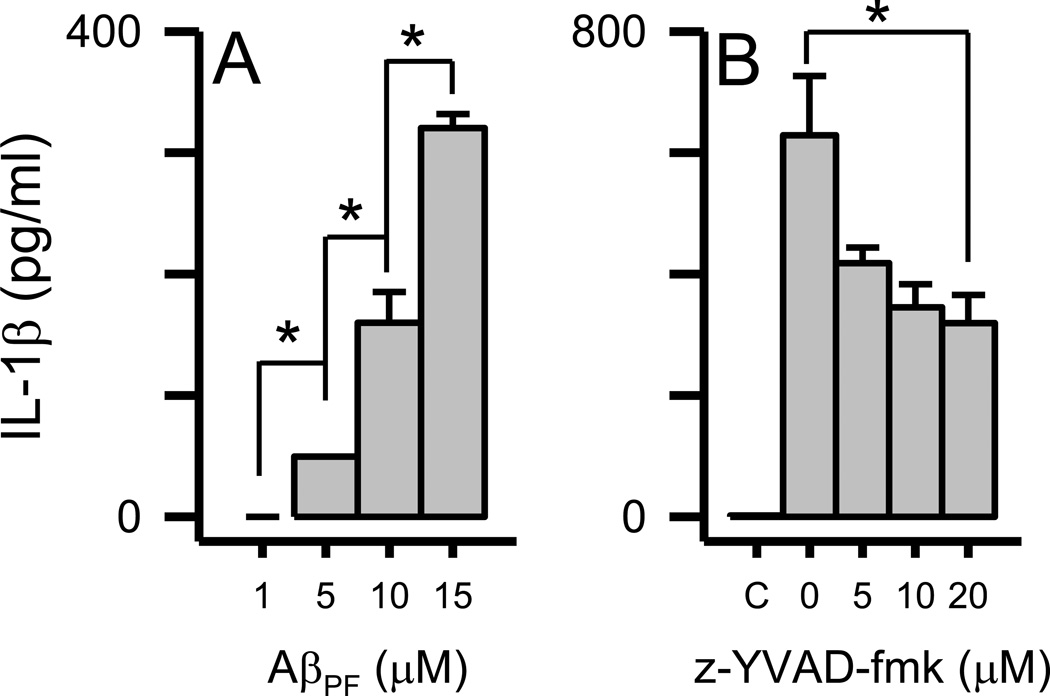
Two possible explanations for the disconnect between TLR/MyD88-mediated IL-1β mRNA production and mature IL-1β protein secretion were (1) rapid Aβ(1–42) protofibril-triggered microglial secretion of existing mature IL-1β reservoirs, or (2) rapid activation of the NLRP3 inflammasome by Aβ(1–42) protofibrils followed by caspase-1-mediated cleavage of existing pro-IL-1β reservoirs and secretion of mature IL-1β . Both of these scenarios would presumably occur prior to TLR/MyD88-mediated IL-1β transcription and translation to pro-IL-1β protein. In order to investigate these hypotheses, we examined the rapid secretion process in more detail. First, stimulation of microglia with a concentration range of Aβ(1–42) protofibrils showed a dose-dependent effect on secreted IL-1β (Fig 7A) and indicated that the level of secreted mature IL-1β initially triggered by Aβ(1–42) protofibrils correlated with the magnitude (concentration) of the stimulus. Secondly, the amount of secreted IL-1β triggered by Aβ protofibril stimulation was lowered in a dose dependent manner by the caspase-1 inhibitor z-YVAD-fmk (Fig 7B). This finding supported the case that the rapidly secreted IL-1β originated from existing reservoirs of pro-IL-1β.
Figure 7.
Dose-dependent IL-1β secretion in primary microglia induced by Aβ(1–42) protofibrils. Panel A. Primary microglia were treated with SEC-isolated Aβ(1–42) protofibrils (1, 5, 10, and 15 µM) and incubated for 6 hrs. Secreted IL-1β levels were measured in the conditioned medium by ELISA. Data bars represent the average ± std error of n=3 replicates for each condition. Statistical differences (p<0.05) in secreted IL-1β elicited by each Aβ protofibril concentration are denoted with an asterisk. Panel B. Primary microglia were pretreated as described in the Methods with increasing concentrations of the caspase-1 inhibitor z-YVAD-fmk or 0.5% DMSO vehicle followed by 15 µM Aβ(1–42) protofibrils. A control treatment (C) that contained 0.5% DMSO but neither z-YVAD-fmk or Aβ was also included. Secreted IL-1β levels were measured in the conditioned medium by ELISA. Data bars represent the average ± std error of n=3 replicates for each condition. Statistical differences (p<0.05) in secreted IL-1β elicited by Aβ protofibrils in the absence or presence of the inhibitor are denoted with an asterisk.
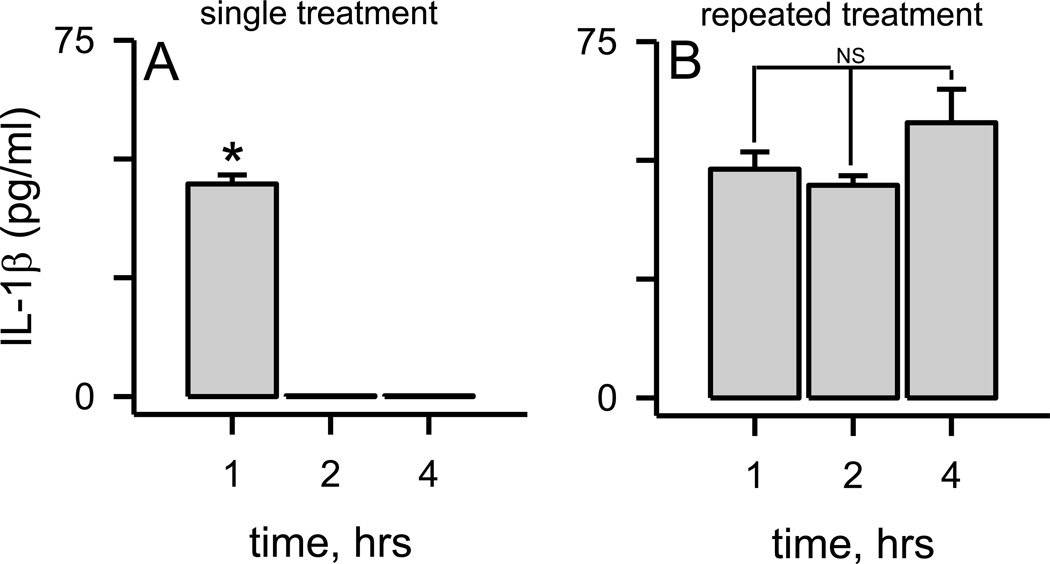
In all of the previous experiments in this report, and in most cases in the literature, the microglia were typically treated once with the Aβ(1–42) protofibril stimulus and then secreted IL-1β was analyzed at increasing incubation times. In order to test whether microglia elicited a sustained response to the initial Aβ stimulus, experiments were conducted where the cell medium was collected, replaced, and collected again at increasing times after microglial exposure to Aβ(1–42) protofibrils. Using this procedure, secreted IL-1β was only observed after the first collection of the conditioned medium (Fig 8A) indicating that the protofibril-induced secretion occurred right after stimulation and was not sustained. Repeated stimulation of the same microglia with Aβ(1–42) protofibrils produced similar quantum of secreted IL-1β each time (Fig 8B). This implied that the microglial IL-1β secretion machinery was not desensitized, or depleted, by Aβ protofibrils. These same findings were obtained in MyD88−/− microglia (Supplementary Figure 3), again demonstrating MyD88-independent reservoirs of pro or mature IL-1β.
Figure 8.
Aβ protofibril-induced rapid IL-1β secretion is not sustained but is repeatable. Panel A. Primary microglia were exposed to SEC-isolated Aβ(1–42) protofibrils (15 µM) and allowed to incubate at 37 °C for 1 hr. The conditioned medium was then removed, fresh medium applied, and collected at 2 hr. This process was repeated again for the 4 hr analysis. No further IL-1β was secreted compared to the 1 hr time point (p<0.001). Panel B. Experiments were conducted as described in Panel A, although Aβ(1–42) protofibrils (15 µM) were added with the fresh medium at 1 and 2 hrs. Data bars represent the average ± std error of n=5 replicates. No statistical difference (NS) was noted in the amount of secreted IL-1β elicited by each successive Aβ(1–42) profibril treatment (p>0.05).
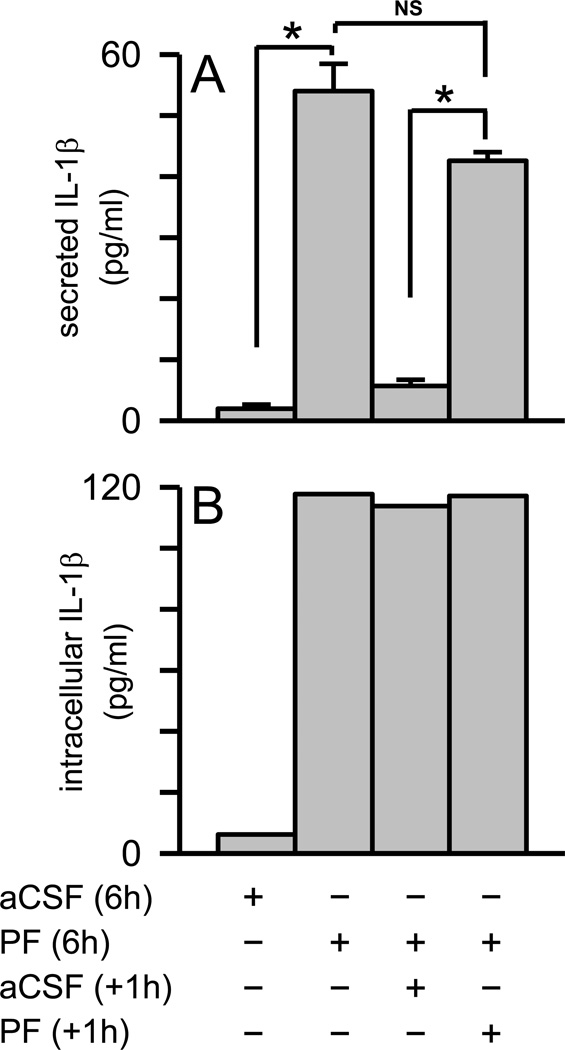
It was noted in Figure 4 and Supplementary Figure 2 that the Aβ(1–42) protofibril-induced accumulation of intracellular IL-1β at longer time points (6, 10, 24 hrs) did not result in a corresponding increase in secreted mature IL-1β protein. These results suggested that an additional Aβ stimulus may be needed to trigger release of the accumulated intracellular mature IL-1β. In order to test this idea, primary microglia were treated with Aβ(1–42) protofibrils for 6 hr to allow intracellular mature IL-1β accumulation, and then treated a second time. However, a substantial increase in secreted mature IL-1β was not observed (Fig 9A) even though intracellular IL-1β markedly increased during the initial 6 h exposure of Aβ(1–42) protofibrils to the microglia (Fig 9B). As in Figure 8, the second Aβ(1–42) protofibril stimulus provoked the same quantum of secreted IL-1β as the initial stimulus despite the substantial increase in intracellular IL-1β . It was evident from the cumulative data that Aβ(1–42) protofibrils stimulated both a time- and MyD88-dependent accumulation of intracellular mature IL-1β and secreted TNFα in microglia. However, the stimulation of pro-IL-1β and the buildup of mature IL-1β inside the cell did not translate into greater levels of secreted IL-1β . In fact, the Aβ(1–42) protofibrils evoked a rapid quantized secretion of IL-1β prior to upregulation of the intracellular pathway and this secretion process was limited, or perhaps regulated, in its response. These findings provide further detail into the mechanisms by which Aβ(1–42) protofibrils prime and activate the NLRP3 inflammasome.
Figure 9.
A second stimulus of microglia with Aβ protofibrils secretion does not release accumulated intracellular IL-1β . Primary microglia were exposed to SEC-isolated Aβ(1–42) protofibrils (15 µM) or aCSF buffer alone and allowed to incubate at 37 °C for 6 hrs. The conditioned medium was then removed and one intracellular extract was prepared for each condition (combination of five wells in 20 µL of lysis buffer). The measured intracellular IL-1β response in pg/mL, which was much higher, was normalized based on the number of wells and lysis buffer volume. For the remaining cells, fresh medium was applied along with a second protofibril (n=5 wells or replicates) or buffer treatment (n=5 replicates). After an additional 1 hr incubation, the conditioned medium from each well was removed separately and one cell extract was again prepared for each condition. Secreted (Panel A) and intracellular (panel B) IL-1β was determined by ELISA. IL-1β data bars represent the average ± std error of n=5 replicates for secreted and one intracellular extract for each condition. Statistically significant differences between treatments are denoted with an asterisk (p<0.001). No statistical difference (NS) was found between the microglial IL-1β response when treated with Aβ(1–42) protofibrils for 6 hr and again for 1 hr (p>0.05).
Discussion
Numerous studies have identified aggregated Aβ as a danger-associated molecular pattern (DAMP). DAMPs have qualities similar to PAMPs but are endogenous, rather than external, pathogens. The unique structural characteristics acquired as Aβ monomers self-assemble into oligomers and ultimately fibrils give the protein proinflammatory properties. We have recently shown that Aβ(1–42) protofibrils were far superior to isolated Aβ(1–42) fibrils in their ability to activate microglia and they displayed no toxicity to the microglia as measured by XTT cell viability assay [35].
TLRs, the primary recognition molecules of the innate immune system, are major mediators of Aβ-triggered inflammation in monocyte/macrophage and microglial cells. Previous studies have demonstrated a role for TLR4 and TLR2 [19–23] and TLR4 and TLR6 [24] in cytokine production triggered by aggregated Aβ. The intracellular TLR adaptor protein, MyD88, has also been implicated in microglial inflammatory responses induced by Aβ [38].
The most widely-accepted 2-signal model [28] linking TLRs and the NLRP3 inflammasome describes a “priming” of the immune cell wherein TLR engagement, MyD88 signaling, and transcriptional activation result in mRNA and protein production. For IL-1β, the pro-form of the protein would be the priming product. The priming process is followed by a second signal (e.g. ATP, K+ efflux) which triggers inflammasome activation and cleavage of pro-IL-1β to IL-1β. The number of innate immune pathways sensitive to Aβ stimulation has expanded over the last five years and several reports have now characterized the interplay between Aβ and the NLRP3 inflammasome [31–34]. Some of these Aβ/NLRP3 studies utilized LPS as a TLR/MyD88 priming agent to produce pro-IL-1β and prime the inflammasome [32]. However, it has become apparent that Aβ alone can stimulate both priming [24] and activation of the inflammasome. Our current report clearly shows that this is the case and that Aβ can provide both signals necessary to elicit mature IL-1β production and secretion. There are likely other mechanistic steps in microglia that link mature IL-1β formation and release from the cell. A recent report delineated between intracellular and secreted mature IL-1β in microglia stimulated with LPS [39]. While this report and others show that LPS alone does not induce IL-1β secretion, Savage et al. found that LPS alone did trigger intracellular mature IL-1β production. This suggests that there may be additional sites for regulation for IL-1β metabolism or trafficking including the secretory process.
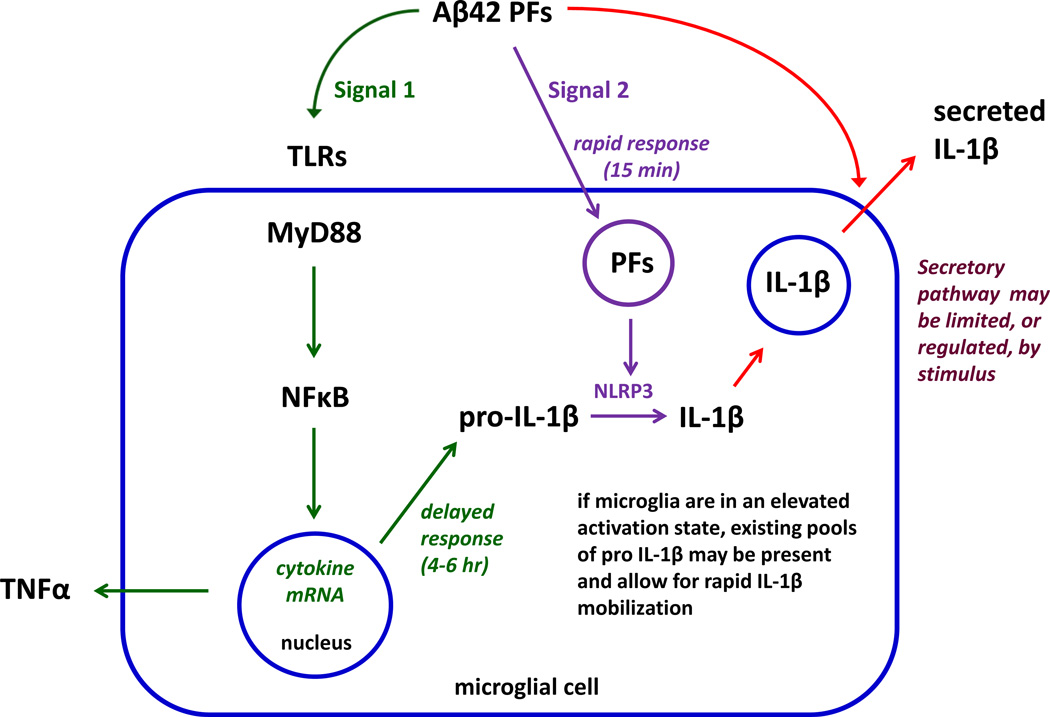
We have applied the current general model linking TLRs and the NLRP3 inflammasome to the more specific situation of Aβ protofibrils and their effect on multiple innate immune pathways. The expanded, but still partial, model brings together the observations presented from the current study (Figure 10). In this model, Aβ(1–42) protofibrils can trigger two signals with an additional potential effect on the secretory process. Activation of the TLR/MyD88 pathway represents Signal 1 and Aβ(1–42) protofibrils stimulated a dramatic upregulation of IL-1β and TNFα mRNA and an increase in pro-IL-1β which was highly dependent on the TLR/MyD88 pathway. Aβ(1–42) protofibrils also clearly provided Signal 2 which involves activation of the NLRP3 inflammasome and conversion of pro-IL-1β to mature IL-1β. Aβ(1–42) protofibrils stimulated time-dependent production of intracellular mature IL-1β that was notably reduced in MyD88−/− microglia likely due to diminished priming of the inflammasome. Activation of the inflammasome by fibrillar Aβ has been demonstrated previously and several reports have implicated a phagocytosis/lysosomal-leakage mechanism by which the activation occurs [32–34]. This mechanism has been incorporated into the Figure 10 model.
Figure 10.
Proposed model of Aβ(1–42) protofibril stimulation of the TLR/MyD88 and NLRP3 inflammasome pathways.
Although TLR/MyD88 engagement is generally accepted as Signal 1, the first response we observed to Aβ protofibrils was a rapid secretion of mature IL-1β (<15 min). This novel rapid secretion process was not dependent on Aβ protofibril-induced, MyD88-mediated, priming of the cells which occurred over a longer time period. Through the course of our investigation we observed that the isolated primary microglia in culture exhibited markers of low-level activation with measurable levels of pro-IL-1β by Western blot and ELISA. The presence of existing pro-IL-1β reservoirs is not uncommon in primary microglial cultures and has been observed in several studies by Western blot [33, 40, 41]. This basal level of inflammasome priming in unstimulated microglia may provide reservoirs of pro-IL-1β poised for rapid caspase-1 cleavage and secretion. These pools of pro-IL-1β may not exist in vivo unless triggered by a separate event that may prime the microglia for a rapid IL-1β secretory response to Aβ.
The Aβ(1–42) protofibril-triggered rapid IL-1β secretion, which has not been demonstrated previously, was very reproducible and occurred as a quantum of IL-1β . The secretion burst could be repeatedly triggered by additional protofibril stimulation and the magnitude of IL-1β secretion was dependent on Aβ protofibril concentration. The Aβ concentrations required to induce IL-1β secretion were in the low micromolar range (5–15 µM). This Aβ concentration range is similar to what we have previously observed for TNFα secretion [35] and to other reports describing microglial activation by Aβ [24, 32–34]. While these concentrations are higher than the low nanomolar range for normal circulating Aβ [42, 43], it has been shown that total Aβ is ~100–200-fold higher in AD brain homogenates compared to controls [44]. Furthermore, it is possible that local Aβ concentrations in areas of accumulation may be much higher. A recent review by Lopez-Castejon and Brough discussed five major mechanisms for IL-1β secretion [45]. These include (1) exocytosis of IL-1β-containing secretory lysosomes, (2) release of IL-1β from shed plasma membrane microvesicles, (3) fusion of multivesicular bodies with the plasma membrane and subsequent release of IL-1β-containing exosomes, (4) export of IL-1β through the plasma membrane using specific membrane transporters, and (5) release of IL-1β upon cell lysis. The last mechanism is not likely since Aβ(1–42) protofibrils are not toxic to microglia, IL-1β secretion was not sustained, and IL-1β secretion could be re-evoked by Aβ from the same cells (Fig 8 & Fig 9). A possible scenario is that Aβ(1–42) protofibrils can trigger rapid IL-1β secretion from microglia that have already attained some level of activation and either directly modulate the mature IL-1β secretory process or stimulate inflammasome-mediated pro-IL-1β cleavage and mature IL-1β secretion.
There are still remaining questions from this investigation. First, the significant time-dependent accumulation of intracellular IL-1β triggered by Aβ protofibrils without a corresponding increase in secreted IL-1β was puzzling. It is possible that the secretion process may be under further regulation or controlled in some manner to limit over-secretion. Alternatively, the protofibrils may have additional unknown effects on the NLRP3 inflammasome or secretory machinery. Second, what is the significance or consequence of Aβ protofibril-triggered TLR/MyD88-dependent intracellular IL-1β production since it does not immediately lead to secretion. Third, what is the role of other Aβ forms in stimulating the TLR/MyD88 and NLRP3 inflammasome pathways and how does the response differ from protofibrils.
Conclusion
The findings presented in this report highlight differences between Aβ(1–42) protofibrils and other TLR ligands and NLRP3 inflammasome activators. Furthermore, these findings demonstrate multiple sites of innate immune regulation by Aβ protofibrils. These sites include the IL-1β secretory process, TLR priming, and NLRP3 inflammasome activation. The multi-pronged interactions of Aβ with the innate immune system and the complexities of the ensuing response are daunting but provide a number of avenues for therapeutic intervention.
Supplementary Material
Highlights.
-
□
Aβ42 protofibrils prime and activate the microglia inflammasome.
-
□
The upregulation of TNFα and IL-1β mRNA by Aβ42 protofibrils is dependent on MyD88.
-
□
A quantum of IL-1β is rapidly secreted in response to Aβ42 protofibrils.
-
□
Aβ protofibrils regulate multiple sites within innate immune pathways.
Acknowledgements
We would like to thank Dr. Tammy Kielian, University of Nebraska Medical Center, for the MyD88−/− mice and helpful discussions.
This work was supported by Award Number R15AG033913 from the National Institute on Aging (MRN).
Abbreviations used
- AD
Alzheimer’s disease
- Aβ
amyloid-β protein
- aCSF
artificial cerebrospinal fluid
- HFIP
hexafluoroisopropanol
- IL-1β
interleukin-1β
- SEC
size exclusion chromatography
- ThT
thioflavin T
- TNFα
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Masters CL, Selkoe DJ. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickson DW. Apoptotic mechanisms in Alzheimer neurofibrillary degeneration: cause or effect? J Clin Invest. 2004;114:23–27. doi: 10.1172/JCI22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 4.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 5.Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, Lee VM. Targeting amyloid-p peptide (Aβ) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Aβ precursor protein (APP) transgenic mice. J Biol Chem. 2006;281:4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- 6.Selkoe DJ. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–638. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- 7.Selkoe DJ. Alzheimer‧s disease: genes, proteins, and therapy, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 8.Harper JD, Wong SS, Lieber CM, Lansbury PT., Jr. Observation of metastable Aβ amyloid protofibrils by atomic force microscopy. Chem. Biol. 1997;4:119–125. doi: 10.1016/s1074-5521(97)90255-6. [DOI] [PubMed] [Google Scholar]
- 9.Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. Amyloid β-protein fibrillogenesis: Detection of a protofibrillar intermediate. J. Biol. Chem. 1997;272:22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- 10.Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB. Amyloid β-protein fibrillogenesis: Structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 11.Harper JD, Wong SS, Lieber CM, Lansbury PT., Jr. Assembly of Aβ amyloid peptides: an in vitro model for a possible early event in Alzheimer's disease. Biochemistry. 1999;38:8972–8980. doi: 10.1021/bi9904149. [DOI] [PubMed] [Google Scholar]
- 12.Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ. Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–8884. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye CP, Selkoe DJ, Hartley DM. Protofibrils of amyloid β-protein inhibit specific K+ currents in neocortical cultures. Neurobiol Dis. 2003;13:177–190. doi: 10.1016/s0969-9961(03)00068-8. [DOI] [PubMed] [Google Scholar]
- 14.O’Nuallain B, Freir DB, Nicoll AJ, Risse E, Ferguson N, Herron CE, Collinge J, Walsh DM. Amyloid β-protein dimers rapidly form stable synaptotoxic protofibrils. J Neurosci. 2010;30:14411–14419. doi: 10.1523/JNEUROSCI.3537-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 16.McGeer EG, McGeer PL. The importance of inflammatory mechanisms in Alzheimer disease. Exp. Gerontol. 1998;33:371–378. doi: 10.1016/s0531-5565(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 17.Golde TE. Inflammation takes on Alzheimer disease. Nat. Med. 2002;8:936–938. doi: 10.1038/nm0902-936. [DOI] [PubMed] [Google Scholar]
- 18.Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, Rivest S, Lentschat A, Gulbins E, Jucker M, Staufenbiel M, Brechtel K, Walter J, Multhaup G, Penke B, Adachi Y, Hartmann T, Beyreuther K. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. Faseb J. 2004;18:203–205. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- 20.Udan ML, Ajit D, Crouse NR, Nichols MR. Toll-like receptors 2 and 4 mediate Aβ(1–42) activation of the innate immune response in a human monocytic cell line. J Neurochem. 2008;104:524–533. doi: 10.1111/j.1471-4159.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 21.Jana M, Palencia CA, Pahan K. Fibrillar amyloid-p peptides activate microglia via TLR2: implications for Alzheimer’s disease. J Immunol. 2008;181:7254–7262. doi: 10.4049/jimmunol.181.10.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and Toll-like receptors 2 and 4 are required for fibrillar Aβ-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rube CE, Walter J, Heneka MT, Hartmann T, Menger MD, Fassbender K. TLR2 Is a primary receptor for Alzheimer's amyloid β peptide to trigger neuroinflammatory activation. J Immunol. 2012;188:1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 24.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, Khoury JE, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar β-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.K Kersse, Bertrand MJ, Lamkanfi M, Vandenabeele P. NOD-like receptors and the innate immune system: coping with danger, damage and death. Cytokine Growth Factor Rev. 2011;22:257–276. doi: 10.1016/j.cytogfr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanamsagar R, Hanke ML, Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012;33:333–342. doi: 10.1016/j.it.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 30.Tan MS, Yu JT, Jiang T, Zhu XC, Tan L. The NLRP3 Inflammasome in Alzheimer’s Disease. Mol Neurobiol. 2013 doi: 10.1007/s12035-013-8475-x. [DOI] [PubMed] [Google Scholar]
- 31.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-p. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Z, Sun L, Hashioka S, Yu S, Schwab C, Okada R, Hayashi Y, McGeer PL, Nakanishi H. Differential pathways for interleukin-1 β production activated by chromogranin A and amyloid β in microglia. Neurobiol Aging. 2013;34:2715–2725. doi: 10.1016/j.neurobiolaging.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;8:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paranjape GS, LK Gouwens, Osborn DC, Nichols MR. Isolated amyloid-β(1–42) protofibrils, but not isolated fibrils, are robust stimulators of microglia. ACS Chem Neurosci. 2012;3:302–311. doi: 10.1021/cn2001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esen N, Kielian T. Effects of low dose GM-CSF on microglial inflammatory profiles to diverse pathogen-associated molecular patterns (PAMPs) J Neuroinflammation. 2007;4:10. doi: 10.1186/1742-2094-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paranjape GS, Terrill SE, Gouwens LK, Ruck BM, Nichols MR. Amyloid-β(1–42) protofibrils formed in modified artificial cerebrospinal fluid bind and activate microglia. J Neuroimmune Pharmacol. 2013;8:312–322. doi: 10.1007/s11481-012-9424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim JE, Kou J, Song M, Pattanayak A, Jin J, Lalonde R, Fukuchi KI. MyD88 deficiency ameliorates β-amyloidosis in an animal model of Alzheimer’s disease. Am J Pathol. 2012;179:1095–1103. doi: 10.1016/j.ajpath.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savage CD, Lopez-Castejon G, Denes A, Brough D. NLRP3-inflammasome activating DAMPs stimulate an inflammatory response in glia in the absence of priming which contributes to brain inflammation after injury. Front Immunol. 2012;3:1–11. doi: 10.3389/fimmu.2012.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.K Terada, Yamada J, Hayashi Y, Wu Z, Uchiyama Y, Peters C, Nakanishi H. Involvement of cathepsin B in the processing and secretion of interleukin-1 β in chromogranin A-stimulated microglia. Glia. 2010;58:114–124. doi: 10.1002/glia.20906. [DOI] [PubMed] [Google Scholar]
- 41.Takenouchi T, Iwamaru Y, Sugama S, Tsukimoto M, Fujita M, Sekigawa A, K Sekiyama, Sato M, Kojima S, Conti B, Hashimoto M, Kitani H. The activation of P2×7 receptor induces cathepsin D-dependent production of a 20-kDa form of IL-1β under acidic extracellular pH in LPS-primed microglial cells. J Neurochem. 2011;117:712–723. doi: 10.1111/j.1471-4159.2011.07240.x. [DOI] [PubMed] [Google Scholar]
- 42.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, McCormack R, Wolfert R, Selkoe DJ, Lieberberg I, Schenk D. Isolation and quantification of soluble Alzheimer’s β-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 43.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gravina SA, Ho L, Eckman CB, Long KE, Otvos L, Jr., Younkin LH, Suzuki N, Younkin SG. Amyloid β protein (Aβ) in Alzheimer’s disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at Aβ40 or Aβ42(43) J. Biol. Chem. 1995;270:7013–7016. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.