Abstract
BACKGROUND
Epidemiologic data has shown that metformin confers a survival advantage in patients with cardiovascular disease. Although the underlying cardioprotective mechanism is unclear, it appears to be independent of metformin’s insulin-sensitizing effect. The purpose of this study is to evaluate the effect of metformin on the apoptosis pathway in the ischemic and non-ischemic cardiac tissue in a swine model of metabolic syndrome.
MATERIALS AND METHODS
Ossabaw miniswine were fed either a regular diet (OC, n=8), a high-cholesterol diet (OHC, n=9), or a high-cholesterol diet supplemented with metformin (OHCM n=8). After three weeks, all animals underwent placement of an ameroid constrictor to the left circumflex coronary artery to induce chronic ischemia. Seven weeks after ameroid placement, animals underwent cardiac harvest.
RESULTS
In the chronically ischemic myocardium, metformin significantly up-regulates pro-survival proteins: ERK, NFκB, pENOS and P38. Metformin also significantly inhibits/down-regulates pro-apoptosis proteins: FOXO3 and caspase3. Metformin decreased the percent apoptotic cells in the ischemic and non-ischemic myocardium. There was no difference in arteriolar density, capillary density, intramyocardial fibrosis or collagen deposition in the ischemic or non-ischemic myocardium.
CONCLUSIONS
Metformin selectively alters the apoptosis pathway by inhibiting FoxO3 and decreasing the active form of caspase 3, cleaved caspase 3. Metformin also up-regulates mitogen-activated kinase proteins p38 and ERK1/2, which are considered cardioprotective during ischemic preconditioning. Perhaps the altered activation of the apoptosis pathway in ischemic myocardium is one mechanism by which metformin is cardioprotective.
Keywords: Myocardial Ischemia, Metformin, Metabolic Syndrome, Apoptosis, Cardioprotection
Introduction
Metformin is a widely prescribed anti-hyperglycemic drug for the treatment of type 2 diabetes. Epidemiologic studies have shown that metformin reduces all cause and cardiovascular mortality in treated diabetics1, 2. Despite similar glycemic control, obese patients with type 2 diabetes treated with metformin monotherapy had greater reduction in mortality compared to insulin or sulfonylureas1. The same observational studies have also shown that diabetics with a history of prior myocardial infarction who were treated with metformin had a lower mortality than similar patients treated with sulfonylureas. These findings are significant since patients with type 2 diabetes are at an increased risk for developing coronary artery disease and suffer worse outcomes following a myocardial infarction, angioplasty, or coronary artery bypass grafting3-5.
Although the mechanism is not entirely well understood, metformin has direct cardioprotective properties independent of its glucose lowering effect. Animal studies have investigated the effects of metformin on myocardial ischemia-reperfusion injury, and have found that metformin administration reduces infarct size, limits cardiac hypertrophy, preserves myocardial function and attenuates myocardial remodeling6. In order to further elucidate metformin’s cardioprotective mechanism, we developed a clinically relevant animal model of metabolic syndrome and chronic myocardial ischemia to evaluate the effect of metformin on the apoptosis and cell survival pathways.
Materials and Methods
ANIMAL MODEL
Twenty-four intact male Ossabaw miniswine (Purdue Ossabaw Facility, Indiana University, Indianapolis, IN) were split into three groups according to diet at 6 weeks of age. Male swine were selected in order to minimize the sex-hormone induced variability on ischemic heart disease and metabolic syndrome. The control group was fed 500g/day of regular chow (OC, n=8). The high-cholesterol animals were fed 500g/day of high-cholesterol chow consisting of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow (Sinclair Research, Columbia, MO) (OHC, n=8). High cholesterol metformin animals were also fed high-cholesterol chow (OHCM, n=8). After 9 weeks of diet initiation, all animals underwent surgical placement of an ameroid constrictor to induce chronic myocardial ischemia (see surgical interventions). Postoperatively, the OHCM group was supplemented with 500mg metformin orally twice daily, and all animals continued on their respective diets. Seven weeks after ameroid constrictor placement, all animals underwent euthanasia and cardiac tissue harvest. All animals were observed to ensure complete consumption of food and supplement, had unlimited access to water, and were housed in a warm non-stressful environment for the duration of the experiment.
SURGICAL INTERVENTIONS
Anesthesia
Anesthesia was induced with an intramuscular injection of telazol (4.4 mg/kg). Animals were endotracheally intubated, mechanically ventilated at 12 – 20 breaths per minute, and general anesthesia was maintained with a gas mixture of oxygen at 1.5 – 2 liters/min and isoflurane at 0.75 – 3.0% concentration.
Ameroid Constrictor Placement
Animals were given a single dose of antibiotic prophylaxis, intravenous enrofloxacin 5mg/kg, and general anesthesia was induced and maintained. Animals were prepped and draped in the usual sterile fashion. The heart was exposed through a left mini-thoracotomy. The left atrial appendage was retracted and the proximal left circumflex artery was dissected at the take off of the left main coronary artery. The ameroid constrictor was placed around the left circumflex artery (Research Instruments NW, Escondito, CA). The pericardium was loosely re-approximated followed by a layered closure of the surgical incision. Post-operative pain was controlled with a single dose of intramuscular Buprenorphine (0.03 mg/kg) and 72 hour Fentanyl patch (4 g/kg). All animals received 325mg of aspirin daily for thromboembolic prophylaxis starting 1 day pre-operatively and continuing for a total of 5 days. All animals continued perioperative antibiotics: enrofloxacin 68mg orally daily for 5 days.
Cardiac Harvest
Under general anesthesia, the heart was exposed via a median sternotomy and animals were euthanized by exsanguination. Of note, prior to euthanasia and harvest, myocardial perfusion was measured by injecting isotope-labeled microspheres at rest and with demand pacing at 150 beats per minute. We previously reported that there was no difference in myocardial perfusion in the normally perfused or chronically ischemic myocardium.7
Full thickness sections from the chronically ischemic left ventricle immediately adjacent to the left circumflex artery, distal to the ameroid constrictor were collected. Also, full thickness sections from the normally perfused left ventricle immediately adjacent to the left anterior descending artery were collected for analysis. The previously reported myocardial perfusion analysis confirmed that the ischemic territory had the lowest microsphere counts and the normally perfused normal ventricle had the highest microsphere counts.7
The Institutional Animal Care and Use Committee of the Rhode Island Hospital approved all experiments. Animals were cared for in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” (NIH publication no. 5377-3 1996).
IMMUNOHISTOCHEMICAL STAINING FOR ANGIOGENESIS
Frozen myocardium was sectioned (10-m-thickness) and fixed in 10% formalin for 10 minutes. Sections were blocked with 1% bovine serum albumin in phosphate buffered saline for 1 hour at room temperature and incubated with antibodies against porcine endothelial marker CD-31 (R&D Systems, Minneapolis, MN) and smooth muscle actin (Sigma Aldrich, St. Louis MO), followed by the appropriate alexa-fluor conjugated antibody (Jackson ImmunoResearch, West Grove, PA) for 45 minutes. Slides were then mounted with Vectashield with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Images were captured at 20X magnification with a Nikon E800 Eclipse microscope (Nikon, Tokyo, Japan) at the same exposure in three random fields. Capillaries were defined as structures between 5-25 m2 in cross-sectional area and arterioles were defined by co-localization of smooth muscle actin (red) and CD-31 (green) staining. Arteriolar density and capillary density was measured using Image J software in a blinded fashion. The percent capillary and arteriolar density for each animal was averaged from the three randomly selected myocardial tissue sections.
PROTEIN EXPRESSION
Forty micrograms of the Radio-Immunoprecipitation Assay (Boston BioProducts, Ashland, MA) soluble fraction of myocardial lysates were fractionated by Sodium dodecyl sulfate polyacrylamide gel electrophoresis 3-8% Tris-Acetate gel (NuPage Novex Mini Gel, Invitrogen, Carlsbad, CA) for molecular weight targets >100 kilodaltons and 4-12% Bis-Tris gels for molecular weight targets <100 kilodaltons (NuPage Novex Mini Gel, Invitrogen). The protein was then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA) and incubated overnight at 4°C with primary antibodies at dilutions recommended by the manufacturer against phosphorylated Forkhead Box 03a (pFOXO3a Ser253), FOXO3a, phosphorylated extracellular-signal-regulated kinases (pERK Thr202/Tyr204), ERK, p-p38 (Thr180/Tyr182), p38, cleaved caspase3, caspase3, phosphorylated endothelial nitric oxide synthase (pENOS Ser 1177), vascular endothelial-cadherin (VE-Cadherin), tumor necrosis factor alpha (TNFα), nuclear factor κB (NFκB), heat shock protein 90 (HSP90) (all from Cell Signaling, Danvers, MA). Membranes were incubated with the appropriate horseradish peroxidase-linked secondary antibody for one hour at room temperature (Jackson ImmunoResearch, West Grove, PA). Immune complexes were visualized with enhanced chemiluminescense and images were captured with a digital camera system (G-Box, Syngene, Cambridge, England). Band densitometry was quantified as arbitrary light units using Image J software (National Institutes of Health, Bethesda, MD). All membranes were probed with GAPDH (Cell Signaling) to correct for loading error.
TUNEL Staining
Frozen myocardium from the non-ischemic and ischemic territory was sectioned (10-m-thickness). Apoptotic cells were identified using the commercially available ApopTag detection kit (Millipore) according to the manufacturer’s instructions. Images were captured at 20X magnification using Aperio ScanScope (Vista, CA) in three random fields from each animal. The percentage of TUNEL-positive cardiomyocytes was measured using Image J software in a blinded fashion.
HISTOLOGY
Frozen myocardium from the ischemic territory was sectioned (10-m-thickness) and fixed in 10% formalin. Trichrome and Picrosirius Red staining was performed on frozen tissue sections by the Pathology and Histology Core Facility at Rhode Island Hospital. Images of the picrosirius stained tissue were digitally captured using Aperio at 20X magnification and analyzed using Image J to calculate the transmural area fraction that stained red. The reported transmural collagen fraction = collagen area/transmural area. Images of the trichrome stained tissue was digitally captured using Aperio Scanscope at 20X magnification and analyzed using Image J software to calculate the intramyocardial fibrosis. The reported intramyocardial fibrosis represents the percent of myocardium replaced by fibrotic tissue.
DATA ANALYSIS
All results are reported as mean ± standard error of the mean. A one-way ANOVA was used to compare the means among groups followed by a post-hoc Bonferroni test to compare the means between groups using GraphPad Prism 5.0 Software (GraphPad Software Inc., San Diego, CA). Protein expression is reported as fold change compared to OC.
Results
Angiogenesis in Ischemic Myocardium
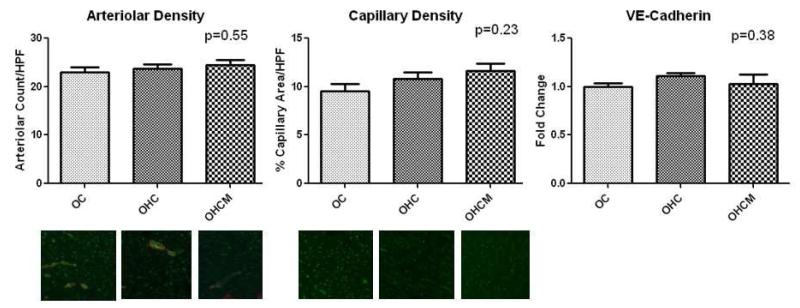
There was no difference in percent capillary density/HPF in the OC, OHC and OHCM groups in the chronically ischemic territory (9.5±0.72, 10.8±0.74, 11.6±0.78 respectively p=0.34). There was no difference in the percent arteriolar density/HPF in the OC, OHC and OHCM groups (22.9±1.18, 23.7±0.93, 24.5±0.93 respectively p=0.55). There was no difference in the expression of angiogenesis marker VE-cadherin in the OC, OHC and OHCM groups (1±0.04, 1.11±0.03, 1.03±0.10 expressed as fold change compared to OC p=0.38) (Figure 1).
Figure 1.
Angiogenesis in the chronically ischemic myocardium. Immunohistochemical staining for SMA (red) depicting arterioles and CD-31 (green) depicting capillaries. Fold change compared to OC.
Angiogenesis in Non-Ischemic Myocardium
There was no difference in percent capillary density/HPF in the OC, OHC and OHCM groups in the normally perfused, non-ischemic myocardium (12.4±0.78, 11.2±0.70, 14.1±0.93 respectively p=0.08). There was no difference in the percent arteriolar density/HPF in the OC, OHC and OHCM groups (26.8±0.97, 27.7±0.91, 27.0±0.88 respectively p=0.80). There was no difference in the expression of angiogenesis marker VE-cadherin in the OC, OHC and OHCM groups (1±0.10, 1.07±0.09, 1.21±0.08 expressed as fold change compared to OC p=0.23) (Figure 2).
Figure 2.
Angiogenesis in the normally perfused, non-ischemic myocardium. Immunohistochemical staining for SMA (red) depicting arterioles and CD-31 (green) depicting capillaries. Fold change compared to OC.
Protein Expression in Ischemic Myocardium
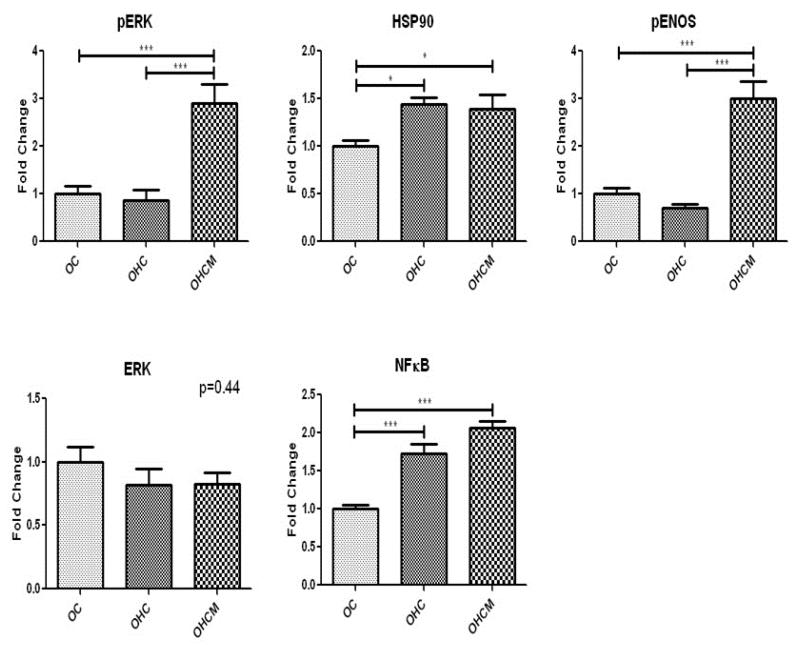
Western blot analysis of survival protein expression in the ischemic myocardium demonstrated marked up-regulation of pERK and pENOS in the OHCM group compared to OC and OHC. There was also up-regulation of NFKB and HSP90 in the OHC and OHCM groups (Figure 3).
Figure 3.
Pro-survival protein expression in chronically ischemic myocardium. Fold change compared to OC. *p<0.05 **p<0.01 ***p<0.001
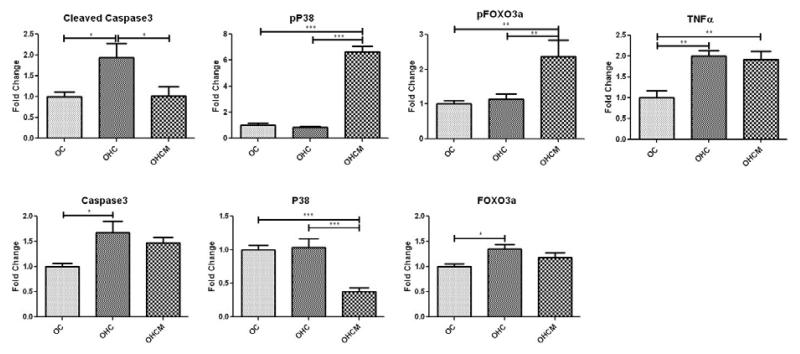
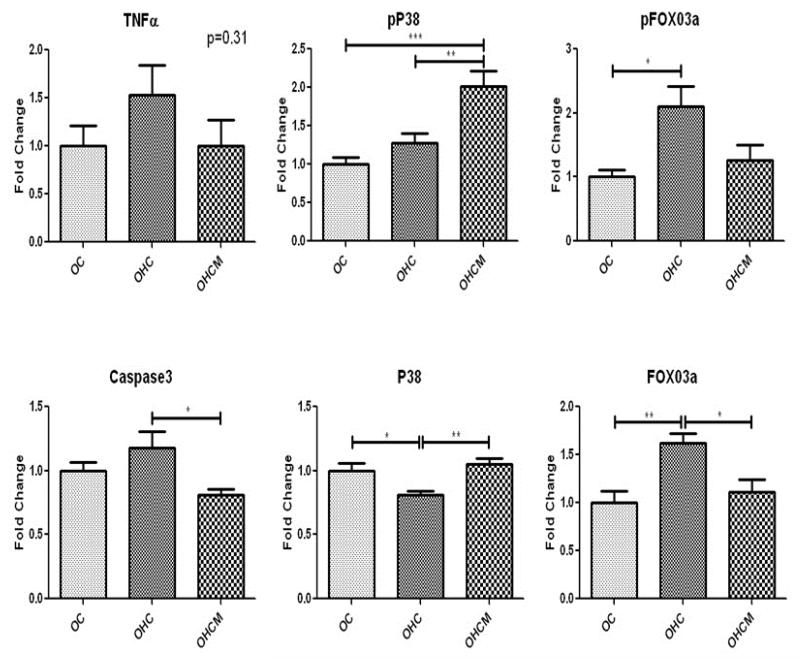
Western blot analysis of apoptosis protein expression in the ischemic myocardium demonstrated upregulation of pFOX03a and pP38, and down regulation of P38 in the OHCM group. There was also upregulation of proteins cleaved caspase 3, caspase3 and FOX03a in the OHC group. There was upregulation of TNFα in the OHC and OHCM groups (Figure 4).
Figure 4.
Apoptosis protein expression in chronically ischemic myocardium. Fold Change compared to OC. *p<0.05 **p<0.01 ***p<0.001
Protein Expression in Non-Ischemic Myocardium
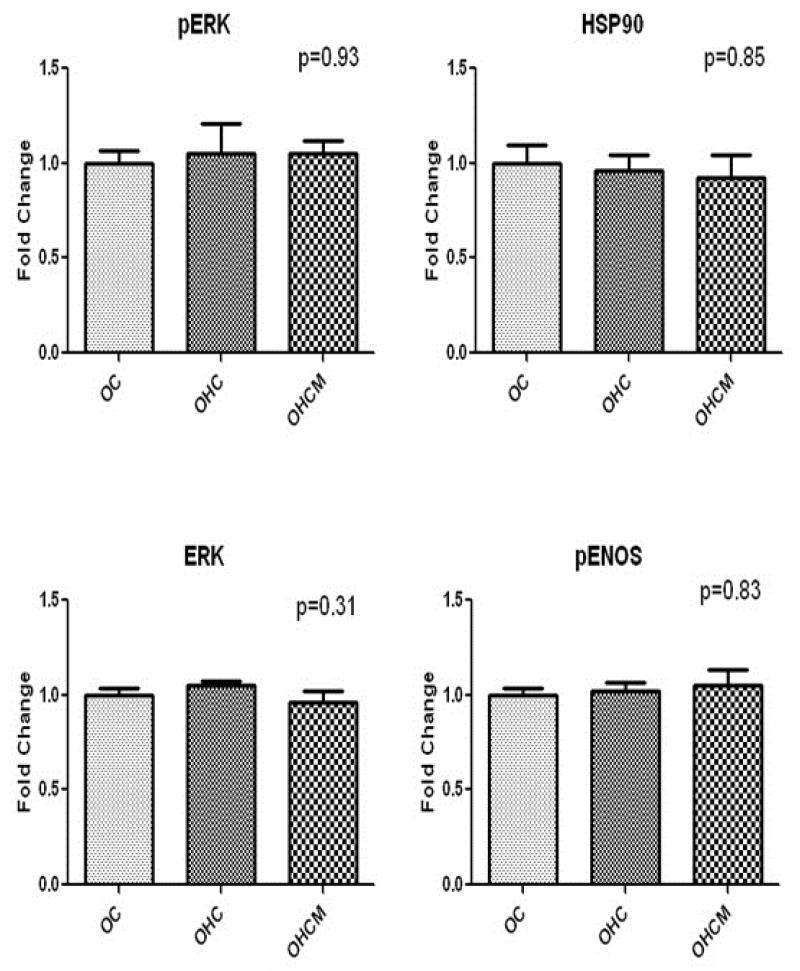
In the normally perfused, non-ischemic myocardium, there was no difference in expression of survival proteins pERK (1.0±0.07, 1.05±0.16, 1.05±0.07 p=0.93), ERK (1.0±0.04, 1.05±0.02, 0.96±0.06 p=0.31), HSP90 (1.0±0.10, 0.96±0.8, 0.92±0.12 p=0.85), or pENOS (1.0±0.03, 1.02±0.04, 1.05±0.09 p=0.83) in the OC, OHC and OHCM groups respectively.
Western blot analysis of apoptosis protein expression in the normally perfused non-ischemic myocardium demonstrated no difference in TNFα expression in the OC, OHC and OHCM groups. There was an increase in pP38 and decrease in caspase 3 expression in the OHCM group. There was also up-regulation of FOX03a and pFOX03a expression and decrease in p38 expresison in the OHC group (Figure 5).
Figure 5.
Apoptosis protein expression in non-ischemic, normally perfused myocardium. Fold change compared to OC. *p<0.05 **p<0.01 ***p<0.001
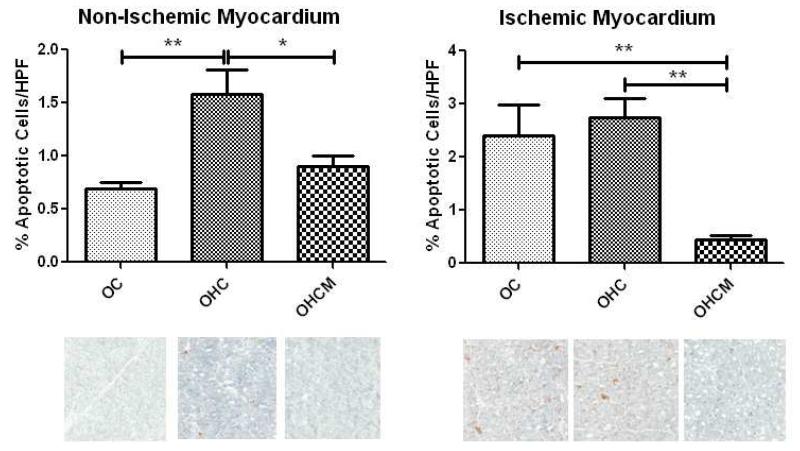
TUNEL Staining for Apoptosis
In the non-ischemic territory, there was a decrease in cell death in the OHCM group compared to OHC (0.9%/HPF±0.10 and 1.6%±0.23 per HPF respectively). Similarly, in the ischemic territory, there was a decrease in cell death in the OHCM group compared to OC and OHC (0.5%/HPF±0.07, 2.4%±0.58, 2.7%±0.36 per HPF respectively) (Figure 6).
Figure 6.
TUNEL staining for apoptosis in the non-ischemic and ischemic myocardium. *p<0.05 **p<0.01
Histology
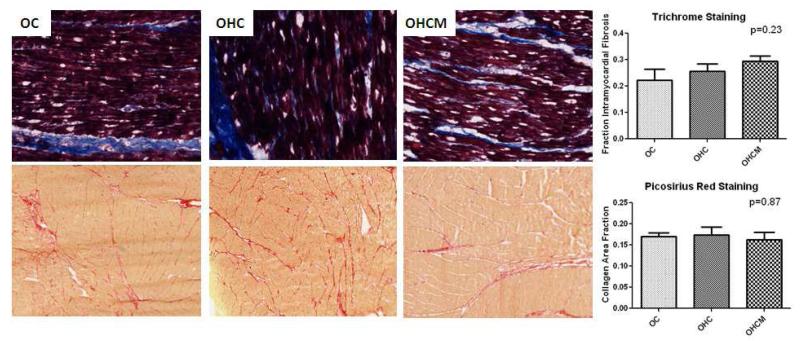
There was no difference in intramyocardial fibrosis as measured by trichrome staining in the OC, OHC or OHCM groups in the ischemic myocardium (0.22±0.04, 0.26±0.03, 0.29±0.02, p=0.23). There was no difference in intramyocardial fibrosis in the OC, OHC or OHCM group in the non-ischemic myocardium (0.03±0.003, 0.03±0.005, 0.03±0.003 p=0.96). There was no difference in transmural collagen deposition as measured by picrosirius red staining in the OC, OHC or OHCM groups in the ischemic myocardium (0.17±0.01, 0.17±0.02, 0.16±0.02 p=0.87). Also, there was no difference in transmural collagen deposition in the OC, OHC or OHCM groups in the non-ischemic myocardium (0.10±0.01, 0.11±0.01, 0.10±0.01 p=0.42) (Figure 7).
Figure 7.
Trichrome and Picrosirius Red staining for intramyocardial fibrosis and transmural collagen deposition in ischemic and non-ischemic myocardium.
Discussion
Our group previously reported that chronic hypercaloric/hypercholesterolemic diet supplementation results in several components of metabolic syndrome including weight gain, glucose intolerance, dyslipidemia and hypertension.7 In this animal model, we reported that metformin supplementation improved reversed diet induced hypertension and glucose intolerance, but did not affect global or regional myocardial contractility or perfusion.7 We also reported that metformin supplementation improved insulin signaling in ischemic myocardium.8 This study demonstrates that metformin administration in the setting of chronic myocardial ischemia selectively mitigates the apoptosis pathway and promotes survival protein expression in the hearts of swine with metabolic syndrome.
Metformin has cardioprotective properties that are independent of its anti-hyperglycemic effects. One reported mechanism by which metformin is cardioprotective is by activating AKT, which promotes cell survival during myocardial ischemia9. AKT inhibits apoptosis by phosphorylation of FOXO3, which results in exclusion from the nucleus and prevents FOX03 from transcribing pro-apoptotic target genes10. In this study, there was an increase in pFOX03 in the OHCM group and an increase in FOX03 in the OHC group in the ischemic myocardium. This suggests that metformin treatment inhibits FOXO3. This is a novel finding, which may represent another mechanism by which metformin is cardioprotective by inhibiting apoptosis in ischemic myocardium. Interestingly, in the non-ischemic myocardium, there was increased FOXO3 and pFOXO3 expression in the OHC group. The up-regulation of pFOXO3 may be due to increased FOXO3 expression, which results in a shift in the stoichiometric balance favoring FOXO3 phosphorylation.
Metformin mediated AKT activation also protects cardiomyocytes from ischemia induced mitochondrial permeability transition pore opening, and subsequent cytochrome c release, and activation of the pro-apoptosis caspase cascade11. Heat shock protein 90 (HSP90) prevents cytochrome c mediated activation of caspases12. HSP90 is typically activated in response to cell stress, and inhibits the apoptosis cascade. In this study, HSP90 was up-regulated in both OHC and OHCM groups in the ischemic myocardium and unchanged in the non-ischemic myocardium. HSP90 was likely up-regulated in response to chronic ischemia.
Although HSP90 expression was increased in both OHC and OHCM groups, expression of its downstream target, caspase3, was different. In the ischemic territory, there was a significant up-regulation of caspase3 and its active counterpart, cleaved caspase3 in OHC group, and down-regulation of cleaved caspase3 in the OHCM group. In the non-ischemic myocardium, metformin down-regulated caspase3 expression. This suggests that metformin selectively down-regulates and inhibits caspase3 expression, thereby protecting the cardiomyocytes from apoptosis signal transduction. This result is consistent with the TUNEL staining, which demonstrated that metformin supplementation decreased cell death in both the ischemic and non-ischemic myocardium.
Metformin administration activates AMP-Kinase (AMPK) by altering the intracellular AMP to ATP ratio. Activated AMPK phosphorylates and activates endothelial nitric oxide synthase (eNOS)13. In this study, metformin markedly up-regulated pENOS expression in the ischemic myocardium, however there was no difference in pENOS expression in the normal ventricle. The ischemic insult combined with metformin’s known AMPK activation is likely the reason why pENOS expression was increased in the OHCM group in the ischemic territory. pENOS expression increases NO bioavailability, increases vasodilation, inhibits oxidative stress and apoptosis, which are all cardioprotecitve in the setting of myocardial ischemia14. Davis et al demonstrated that metformin increases HSP90 and that AMPK facilitates the formation of an HSP90-AMPK-eNOS complex, which phosphorylates eNOS, increases eNOS derived NO, and reduces eNOS derived oxygen radical species. Our results are in agreement with previous rodent and in-vitro studies, which demonstrate that metformin increases HSP90 and pENOS expression in response to ischemia.
Whereas the intrinsic apoptosis pathway is activated from stress signals from within the cell, the extrinsic apoptosis pathway is activated by stress ligands such as TNFα, binding death receptors, and propagation of the apoptosis pathway. In this study, both the OHC and OHCM groups had increased TNFα expression in the ischemic myocardium, and similar levels in the normal ventricle. The up-regulation of TNFα in the ischemic myocardium is likely due to the ischemic stimulus.
TNFα can also promote cell survival by activating NFκB, which regulates expression of genes involved in cell survival, inflammation and cell proliferation15. Here, we demonstrated that there was significant up-regulation of NFκB in both the OHC and OHCM groups in the ischemic myocardium, though the highest levels of NFκB expression was in the OHCM group.
TNFα also activates the mitogen-activated protein kinase cascades: ERK and P38. In the ischemic cardiac tissue, there was similar expression of ERK among groups, but substantially up-regulated the activated pERK in OHCM compared to OHC and OC. ERK and pERK expression was the same in the non-ischemic myocardium, likely due to a lack of ischemic stimulus. Similar to AKT, ERK is also a survival kinase and is considered cardioprotective and promotes myocyte survival after the ischemic insult11.
Metformin supplementation significantly down-regulated P38 and dramatically up-regulated activated pP38 in the ischemic myocardium. These results were similar in the non-ischemic myocardium with up-regulation of pP38 in the OHCM group. P38 activity in ischemic myocardial injury is controversial as there is ample evidence to support the conclusion that P38 promotes cell injury and evidence to suggest that P38 promotes cell survival16, 17. Further investigation has reconciled P38’s apparently competing functions. P38 has several isoforms with α and β isoforms predominately expressed in the heart. When P38α is primarily expressed, it promotes apoptosis and when P38β is expressed, it promotes cell survival and hypertrophy. Given metformin’s known cardioprotective properties, perhaps it promotes the P38β isoform to predominate over P38α and optimizes myocardial response to ischemia. Given P38’s dual functionality, further investigation on the effect of metformin on P38 isoform regulation is warranted.
Despite the increase in metformin-mediated pro-survival and anti-apoptosis signaling in ischemic myocardium, it did not result in any improvement in angiogenesis. There was no difference in VE-cadherin expression or capillary or arteriolar density in the ischemic and non-ischemic myocardium. Also, metformin did not effect intramyocardial fibrosis or collagen deposition in the ischemic or non-ischemic myocardium.
In summary, our findings demonstrated that metformin treatment promotes survival protein expression and inhibits the apoptosis pathway in animals with metabolic syndrome and chronic myocardial ischemia. These findings provide further insight for the mechanism by which metformin confers significant cardioprotection against ischemia/reperfusion injury.
Limitations
Several limitations exist in the presentation and analysis of the current data. Though this study demonstrates the effects of metformin supplementation on apoptosis and survival signaling in ischemic myocardium and normally perfused myocardium remote from an ischemic territory, we did not investigate the effects of metformin supplementation in normally perfused hearts devoid of any ischemic insult. While it was beyond the scope of the current study, it would be useful to repeat this study in a sham operative group to further delineate the effects of metformin on the myocardium and its role in cardioprotection.
Acknowledgements
We would like to thank the Rhode Island Hospital Animal Research Facility for their excellent care of the animals in this study.
Funding: Funding for this research was provided by the National Heart, Lung, and Blood Institute (R01HL46716, R01HL69024, and R01HL85647, Dr. Sellke), NIH Training grant 5T32-HL076134 (Dr. Lassaletta), NIH Training grant 5T32-HL094300-03, Drs. Elmadhun, Sabe, Chu).
Abbreviations
- OC
Ossabaw Control
- OHC
Ossabaw High Cholesterol
- OHCM
Ossabaw High Cholesterol Metformin
- FOX01
Forkhead Box 01
- pFOX01
Phosphorylated Forkhead Box 01
- ERK
Extracellular-signal-regulated kinases
- pERK
Phosphorylated extracellular-signal-regulated kinases
- pENOS
Phosphorylated endothelial nitric oxide synthase
- VE-Cadherin
Vascular endothelial cadherin
- TNFα
Tumor Necrosis Factor α 3707
- NFκB
Nuclear Factor κB 4764
- HSP90
Heat Shock Protein 90 4874
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: Each of the authors listed below have made substantial contributions in each of these three areas: (1) conceiving and designing the study; or collecting the data; or analyzing and interpreting the data; (2) writing the manuscript or providing critical revisions that are important for the intellectual content; and (3) approving the final version of the manuscript. Nassrene Y. Elmadhun; Ashraf A. Sabe; Antonio D. Lassaletta; Louis. M. Chu; Frank W. Sellke MD
Disclosures: None
References
- 1.Uk prospective diabetes study (ukpds) group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (ukpds 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 2.Johnson JA, Majumdar SR, Simpson SH, Toth EL. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care. 2002;25:2244–2248. doi: 10.2337/diacare.25.12.2244. [DOI] [PubMed] [Google Scholar]
- 3.Norhammar A, Lindback J, Ryden L, Wallentin L, Stenestrand U. Improved but still high short- and long-term mortality rates after myocardial infarction in patients with diabetes mellitus: A time-trend report from the swedish register of information and knowledge about swedish heart intensive care admission. Heart. 2007;93:1577–1583. doi: 10.1136/hrt.2006.097956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathew V, Gersh BJ, Williams BA, Laskey WK, Willerson JT, Tilbury RT, Davis BR, Holmes DR., Jr. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: A report from the prevention of restenosis with tranilast and its outcomes (presto) trial. Circulation. 2004;109:476–480. doi: 10.1161/01.CIR.0000109693.64957.20. [DOI] [PubMed] [Google Scholar]
- 5.Alserius T, Hammar N, Nordqvist T, Ivert T. Risk of death or acute myocardial infarction 10 years after coronary artery bypass surgery in relation to type of diabetes. Am Heart J. 2006;152:599–605. doi: 10.1016/j.ahj.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 6.El Messaoudi S, Rongen GA, de Boer RA, Riksen NP. The cardioprotective effects of metformin. Curr Opin Lipidol. 2011;22 doi: 10.1097/MOL.0b013e32834ae1a7. [DOI] [PubMed] [Google Scholar]
- 7.Lassaletta AD, Chu LM, Robich MP, Elmadhun NY, Feng J, Burgess TA, Laham RJ, Sturek M, Sellke FW. Overfed ossabaw swine with early stage metabolic syndrome have normal coronary collateral development in response to chronic ischemia. Basic research in cardiology. 2012;107:243. doi: 10.1007/s00395-012-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmadhun NY, Lassaletta AD, Chu LM, Sellke FW. Metformin alters the insulin signaling pathway in ischemic cardiac tissue in a swine model of metabolic syndrome. The Journal of thoracic and cardiovascular surgery. 2013;145:258–265. doi: 10.1016/j.jtcvs.2012.09.028. discussion 265-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 10.Maiese K, Chong ZZ, Shang YC, Hou J. A “foxo” in sight: Targeting foxo proteins from conception to cancer. Med Res Rev. 2009;29:395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perrelli MG, Pagliaro P, Penna C. Ischemia/reperfusion injury and cardioprotective mechanisms: Role of mitochondria and reactive oxygen species. World J Cardiol. 2011;3:186–200. doi: 10.4330/wjc.v3.i6.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, Weichselbaum R, Nalin C, Alnemri ES, Kufe D, Kharbanda S. Negative regulation of cytochrome c-mediated oligomerization of apaf-1 and activation of procaspase-9 by heat shock protein 90. Embo J. 2000;19:4310–4322. doi: 10.1093/emboj/19.16.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via ampk-enos-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 14.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the amp-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119:651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagy N, Shiroto K, Malik G, Huang CK, Gaestel M, Abdellatif M, Tosaki A, Maulik N, Das DK. Ischemic preconditioning involves dual cardio-protective axes with p38mapk as upstream target. J Mol Cell Cardiol. 2007;42:981–990. doi: 10.1016/j.yjmcc.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Steenbergen C. The role of p38 mitogen-activated protein kinase in myocardial ischemia/reperfusion injury; relationship to ischemic preconditioning. Basic Res Cardiol. 2002;97:276–285. doi: 10.1007/s00395-002-0364-9. [DOI] [PubMed] [Google Scholar]