Abstract
Pharmacists are uniquely qualified to play essential roles in the clinical implementation of pharmacogenomics. However, specific responsibilities and resources needed for these roles have not been defined. We describe roles for pharmacists that emerged in the clinical implementation of genotype-guided clopidogrel therapy in the University of Florida Health Personalized Medicine Program, summarize preliminary program results, and discuss education, training, and resources needed to support such programs. Planning for University of Florida Health Personalized Medicine Program began in summer 2011 under leadership of a pharmacist, with clinical launch in June 2012 of a clopidogrel-CYP2C19 pilot project aimed at tailoring antiplatelet therapies for patients undergoing percutaneous coronary intervention and stent placement. More than 1000 patients were genotyped in the pilot project in year 1. Essential pharmacist roles and responsibilities that developed and/or emerged required expertise in pharmacy informatics (development of clinical decision support in the electronic medical record), medication safety, medication-use policies and processes, development of group and individual educational strategies, literature analysis, drug information, database management, patient care in targeted areas, logistical issues in genetic testing and follow-up, research and ethical issues, and clinical precepting. In the first 2 years of the program (1 year planning and 1 year postimplementation), a total of 14 different pharmacists were directly and indirectly involved, with effort levels ranging from a few hours per month, to 25–30% effort for the director and associate director, to nearly full-time for residents. Clinical pharmacists are well positioned to implement clinical pharmacogenomics programs, with expertise in pharmacokinetics, pharmacogenomics, informatics, and patient care. Education, training, and practice-based resources are needed to support these roles and to facilitate the development of financially sustainable pharmacist-led clinical pharmacogenomics practice models.
Keywords: pharmacist roles, pharmacogenomics, pharmacogenetics, clinical implementation
Genetic variation can cause differing medication responses in some patients, including supratherapeutic or subtherapeutic drug levels and adverse events.1–7 Since 2007, the Food and Drug Administration (FDA) has recognized the importance of pharmacogenomics and issued black box warnings on several medications, including clopidogrel, abacavir, and carbamazepine; incorporated genotype-guided dosing algorithms into product labeling (warfarin and clobazam); and included pharmacogenomic information in drug labeling for more than 100 medications.8 Incorporation of genetic information into the therapeutic decision-making process has the potential to improve patient adherence, safety, and outcomes.7 This potential has yet to be realized though, as clinical use of pharmacogenomics data to inform drug therapy choices continues to lag behind scientific advancement. However, a growing number of institutions are beginning to develop clinical services in this area.9–18
Pharmacists have provided unique value in the health care system in recent decades through optimizing medication use to improve outcomes and decrease adverse events. During this time, specialized roles for clinical pharmacists have emerged and become the standard of care in a number of practice areas, including pharmacokinetics, critical care, anticoagulation, medication therapy management, and others.19–22 It is our experience that novel opportunities are emerging for pharmacists to provide clinical value in the area of pharmacogenomics as a new standard of care. A 2013 national survey of pharmacy practice settings confirmed that pharmacists already provide similar services in United States hospitals, including formally advising on dosage adjustment (98%), drug information (93%), and pharmacokinetics (92%).23 In a draft policy position statement, the American Society of Health-Systems Pharmacists further reinforced that distinctive knowledge, skills, and abilities make pharmacists “uniquely positioned to lead interdisciplinary efforts to develop processes for ordering, interpreting, and reporting pharmacogenomic test results and for guiding optimal drug selection and drug dosing based on those results, as well as efforts to implement and improve those processes.”24
The need for clinical pharmacist input in pharmacogenomics implementation is being increasingly recognized by other health care professionals. In a recent editorial accompanying an implementation theme issue of the American Journal of Medical Genetics, a physician editorialist noted that “pharmacogenomics may reside more comfortably in the purview of pharmacists rather than physicians, at least as far as programmatic development and leadership are concerned.”25 Similarly, a 2013 Pharmacogenomics commentary authored by genetic counselors proposed a partnership model between pharmacists and genetic counselors. Commentary authors highlighted pharmacists’ unique knowledge and skills as being complementary to other health care professions, noting that a team-based approach could “enable the comprehensive delivery of services essential to the appropriate use of [pharmacogenetic] testing.”26
However, because of the emerging nature of this specialty area, the roles and responsibilities required for pharmacists to support clinical pharmacogenomics implementation have yet to be defined. Likewise, little information exists on required pharmacist education and training and/ or necessary institutional or organizational resources to support such roles. In the current article, we describe pharmacists’ roles that emerged during 1 year after implementing CYP2C19 genotype-guided clopidogrel therapy within the University of Florida (UF) Health Personalized Medicine Program (PMP). We also discuss professional needs for education, training, and practice-based resources in pharmacogenomics, and potential strategies for developing financially sustainable pharmacogenomics service models.
Clinical Pharmacogenomics Program Description
The UF Health PMP is a pharmacist-led translational clinical practice initiative funded in part by the National Institutes of Health and the Clinical and Translational Science Institute at the University of Florida. The goal of this program is to optimize patient care through the use of genetic information. Program initiatives are guided by the following principles: (1) preemptive genotyping is conducted on a customized genotyping array in the institution; (2) regulatory oversight is provided by an internal pharmacist-led multidisciplinary committee; and (3) clinical decision support (CDS) is implemented within the electronic medical record (EMR; Epic) to help clinicians interpret and use genotype-guided therapies.14
Within this process, CDS is defined as the provision of clearly interpreted genetic test results with patient-specific genotype-guided drug therapy recommendations (e.g., alternate therapy, dose change) to prescribers, either electronically or by personal follow-up. Electronic CDS strategies have included ordering instructions, passive alerts, and active alerts (i.e., “Best Practice Advisories,” or BPAs in Epic). To provide multidisciplinary input in developing and operationalizing evidence-based CDS strategies, a personalized medicine subcommittee was established as a working committee made up of a variety of expert members such as pharmacists, pharmacy resident(s), physicians, information technology specialists, genetics experts, health-system administration support staff, pathology department personnel, and molecular genetics laboratory experts. In making recommendations, the personalized medicine subcommittee analyzes evidence and considers the clinical need for a drug–gene pharmacogenetic initiative, how genetic testing will be facilitated, optimal communication strategies for prescribers and clinicians, and whether drug restrictions are needed.
The initial implementation of UF Health PMP was CYP2C19 genotype–guided antiplatelet therapy in patients undergoing cardiac catheterization and percutaneous coronary intervention (PCI). Clopidogrel is a prodrug that requires bioactivation mediated by CYP2C19; evidence supports decreased clopidogrel activation in patients carrying a CYP2C19 loss-of-function allele.27 In patients undergoing PCI, although conflicting evidence exists, data support an increased risk of adverse cardiovascular events and stent thrombosis in individuals with a variant CYP2C19 genotype.27, 28
Planning for a clopidogrel-CYP2C19 pilot program began at UF Health PMP in summer 2011, with clinical launch of the program in June 2012 in the cardiac catheterization laboratory. The goal of the pilot was to test use of genetic information to tailor antiplatelet therapies for patients undergoing PCI and stent placement. Genotype and clinical results for year 1 of this pilot and a complete description of the program structure have been published previously.14
Pharmacists’ Roles in a Clinical Pharmacogenomics Program
Without question, an essential starting place for a new clinical pharmacogenomics program is with pharmacists knowledgeable about clinical applications of genotype-guided therapy, relevant drug–gene pairs, and pharmacogenetic laboratory testing. Within our program, the PMP program director (JAJ) served as this individual. Prior to the clinical launch in 2012, the program director assembled the initial interdisciplinary team, introduced the concept of clinical implementation of pharmacogenomics, collaborated with other experts to design the customized genotyping array,29 and helped design the CDS and point-of-care prescriber alerts. The initial program leader possessed a mix of clinical practice and pharmacogenomics research experience that allowed this individual to serve as an important catalyst in leading the necessary transition of pharmacogenomics science to practice.10, 13, 18
This clinical practice experience was especially important before implementation to interface with the cardiology department, engage interventional cardiologists, and anticipate the program’s clinical impact and needs on implementation. Clinical pharmacy support was essential through the planning and year-1 implementation processes (Figure 1). Key areas requiring specific pharmacy support, expertise, and leadership that have emerged within our program are highlighted next.
Figure 1.
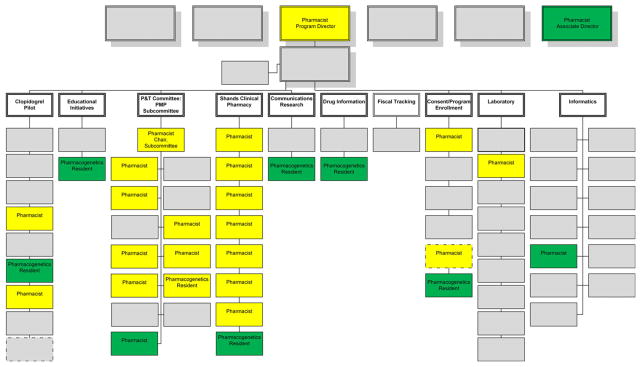
Pharmacists’ roles in the clinical implementation of pharmacogenomics in the University of Florida (UF) Health personalized medicine program (PMP). This organization chart depicts categories of individuals involved in the initial launch of the UF Health PMP clinical pharmacogenomics implementation. Gray boxes, nonpharmacist roles (e.g., physicians, administrators, laboratory personnel); yellow boxes, pharmacist roles at program launch; green boxes, pharmacist roles that were added by end of year 1 postimplementation.
Medication-Use Policies and Processes
Pharmacists with expertise in clinical practice and administration (RAH, JAJ) led the process for planning the health system infrastructure and approval requirements for PMP clinical implementations under the purview of the Pharmacy and Therapeutics (P&T) Committee. After consultation with the P&T Committee chair and relevant stakeholders, a proposal to create a Personalized Medicine Subcommittee was submitted to P&T in January 2012. The objective of the Personalized Medicine Subcommittee was to review the evidence base and make recommendations to the P&T Committee when evidence was sufficient to recommend moving actionable pharmacogenetics and genetic findings to clinical implementation. Initial subcommittee members included the P&T Committee chair; clinical pharmacy practitioners specializing in medication safety, informatics, drug information and pharmacogenomics; representatives from the UF Center for Pharmacogenomics and UF Pathology Department; and ad hoc clinician members. The Personalized Medicine Subcommittee structure was modeled on that of existing P&T subcommittees within UF Health and was structured most like the current formulary subcommittee.
Literature Evaluation and Application of Evidence-Based Medicine
The need for literature evaluation and interpretation in the P&T process was anticipated. In fact, the director of the UF Health Drug Information Center (RAH) was enlisted as a co-preceptor for the PGY2 pharmacogenetics residency. However, the importance and scope of this role have continued to grow. The pharmacist’s role is particularly important in clinical pharmacogenomics because new data can quickly impact and require changes during implementation. For example, during the first year, PMP opted to remove the option for triple-dose clopidogrel in the CDS for intermediate metabolizers (carriers of one loss-of-function CYP2C19 allele) based on emerging literature. This required PMP to coordinate literature review for presentation to the P&T Committee, implement subsequent changes to the electronic health record, and provide clinician and staff education. A second change to the initial implementation was required to alter the interpretation of unknown or indeterminate genotype (CYP2C19 *2/*17) to intermediate metabolizers. Near the end of year 1, an additional clinical pharmacy faculty member (KWW) was added to the PMP to coordinate retrieval of emerging pharmacogenomic literature; facilitate its evaluation, interpretation, and application; and provide needed clinical education for ongoing initiatives.
Pharmacy Informatics
Although we envisioned a significant role for electronic CDS in our program, we did not foresee the need for a pharmacist with informatics training to spearhead this component. Fortunately, the UF Health Shands Pharmacy Department was equipped with such an individual (BJS) to assist in our program, and this pharmacist has been key in developing point-of-care alerts and other electronic support. The importance of a well-developed informatics component has been demonstrated in other established programs as well.30
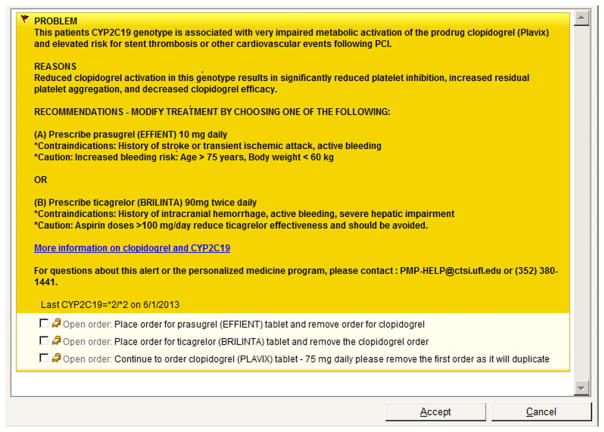
For the informatics component of the clopidogrel implementation, physicians were alerted to patients with an actionable genotype in Epic via a BPA when a clopidogrel order was entered for patients who were CYP2C19 intermediate or poor metabolizers. The BPA text included the patient’s genotype, a description of the clinical problem resulting from the genetic variation, the mechanism of the clinical effects, alternative treatment recommendations, and a hyperlink to access more detailed information sources (Figure 2). BPA language was developed and vetted through the Personalized Medicine Subcommittee. Additionally, we determined that an alert directed to a clinical pharmacist in the form of an “in-basket” message in Epic was needed to facilitate postprocedure follow-up on all patients with a reported genotype. This supplemental messaging to clinical pharmacists (JA and the PGY2 resident) was indispensable, especially for patients who were already taking clopidogrel on admission when an actionable genotype was reported and for those variant-allele carriers who were discharged with clopidogrel before their genetic information became available.
Figure 2.
Sample best practice advisory for a CYP2C19 poor metabolizer (*2/*2).
Direct Patient Care
Although the need for clinical pharmacist support in patient care areas after the clinical launch was expected, our understanding of the magnitude and specificity of this need evolved during implementation in both the inpatient and outpatient areas. Specifically for the clopidogrel implementation, the rapid turnaround of the target patient cohort (outpatient left heart catheterization procedure and less than 24-hour inpatient observation for uncomplicated percutaneous coronary intervention) and the practitioner learning curve necessary to adopt new processes demanded support in addition to electronic CDS alerts. To this end, a clinical pharmacy specialist dedicated to the care of cardiology and cardiothoracic surgery patients (JA) was enlisted to coordinate follow-up with cardiology interventionalists and support staff in reviewing “actionable” patients’ antiplatelet regimens for drug therapy changes.
When the “in-basket” messages described earlier indicated the presence of an actionable genotype, the clinical pharmacist reviewed the patient’s information and, if a therapy change was warranted, notified the patient’s cardiologist. This pharmacist also researched other drug-related issues that may affect the optimal therapy choice (e.g., contraindications or precautions to alternative agents, drug cost, insurance coverage, etc.) and communicated these to the provider in person or via telephone, e-mail, or EMR messaging. Prescribers communicated test results and any drug therapy changes directly to patients at a follow-up visit; patient information and/or additional clinical support was provided by the UF Health PMP pharmacists on an as-needed basis via patient information leaflet or e-mail address/page number within the CDS alert.
This novel type of CDS tied to pharmacist follow-up ensured that patients were monitored longitudinally, even after discharge. Similarly, the presence of collaborative inpatient clinical pharmacy support continues to allow for detailed continuity of care when these patients are readmitted to noncardiology services that were not familiar with the patient’s genetic history and/or PMP initiatives.
Response to Medication Safety Needs
We also found that it was essential for a clinical pharmacist (PGY2 resident) to closely follow medication safety trends both on a national level and within the institution. In some cases, the use of genotype-guided therapy may help to avoid adverse events in individuals particularly susceptible because of their genetic makeup.31–34 For example, the FDA issued a drug safety communication in August 2012 regarding the effects of genetic variations in CYP2D6 on codeine toxicity in children post tonsillectomy and/or adenoidectomy (http://www.fda.gov/Drugs/DrugSafety/ucm313631.htm). In response to this alert, the pediatrics service approached PMP for help in developing a strategy for the safe use of codeine. PMP worked with the clinical pharmacy (RAH and others) to rapidly deliver targeted education to providers in patient care services with high codeine utilization, with subsequent removal of codeine from all standing order-sets, followed by removal from the inpatient formulary until CYP2D6 genotyping was available to guide its safe use. Clinical implementation of CYP2D6 genotyping to guide codeine (and tramadol) use in targeted populations is under way.
Research and Ethical Issues
Although PMP is a clinical program, this dynamic area is one of emerging science. As such, some PMP initiatives are associated with patient care and/or implementation sciences research. The leadership and input of pharmacists (JAJ, RMC, and others) well versed with research, institutional review board requirements, and potential ethical issues associated with implementing clinical pharmacogenetics were essential in program development and implementation and remain important roles to date.
Design and Implementation of Professional Education Programs
The pharmacist leading a pharmacogenomics service becomes a leader in facilitating practice change throughout the institution. In this manner, there was a large need for education and support of prescribers and staff to ensure the newly available genetic test was ordered and implemented per protocol. In our case, despite prelaunch in-services and education for providers and nursing staff, the CYP2C19 genetic test was ordered, collected, and documented in only 63% of PCI patients in the first months after initiating the test. To address this, a clinical pharmacist (PGY2 resident) began overseeing education, data management, and logistical concerns surrounding the genetic testing. Additionally, this individual led clinician group discussions, participated in patient care activities in targeted clinical services, conducted professional seminars and in-services, and created written patient and provider educational materials. By the end of year 1, there was a 98% rate of test order, collection, and documentation, which we perceive to be largely due to these educational efforts.14
PGY2 Pharmacogenetics Resident Support
The PGY2 specialty residency began in year 1 of the program launch in July 2012, with the first resident graduating in June 2013. Anticipated resident responsibilities were primarily in the areas of literature evaluation and development and presentation of evidence summaries to the Personalized Medicine Subcommittee. However opportunities for resident involvement increased in year 1 as the program expanded.
During this time, the resident served as the liaison between PMP and clinical pharmacy and in many cases between PMP and the P&T Committee. The resident was involved in all steps of clinical implementation, with the exception of genotyping and billing. In addition to the educational activities described earlier, the resident assisted the clinical pharmacist with addressing Epic “in-basket” messages, documenting and tracking genotype information, providing follow-up with cardiologists and support staff regarding “actionable” patients, and proposing alterations in therapy. The resident also provided patient care services in areas targeted for future implementations to better understand patient care flow and processes. This role continues to be very helpful for PMP planning to ensure that new clinical processes related to pharmacogenetic testing are feasible, provide a benefit to patients and providers, do not hinder patient care efficiency, or burden providers.
The resident also assisted with key operational aspects of implementation. Along with the program manager, the resident addressed on a daily basis logistical issues associated with the genetic test ordering, sample collection and transport to pathology, and timely reporting of results. Information gained led to strategic revisions in testing workflow, such as addition of a preselected CYP2C19 test to the “pre-cath” order-set and a switch to collecting samples in the patients’ preprocedure visit in the cardiology clinic. The resident’s participation in staffing activities for the pharmacy satellites of UF Health Shands Hospital was also instrumental. This role ensured the resident’s proficient use of Epic to assist with design and content development for CDS and in accessing aggregate data and reports to track implementation metrics.
Clinical Teaching and Precepting
Our understanding of the need for practice-based, experiential training of student pharmacists and postgraduate trainees in this area has also developed since program initiation. Currently, a clinical pharmacy faculty member serves as the residency program director and primary preceptor for the PGY2 pharmacogenetics residency and preceptor for an elective advanced pharmacy practice experience in personalized and evidence-based medicine (KWW). The advanced pharmacy practice experience (APPE) students provide support to clinical pharmacogenetics initiatives through participation in patient care activities, literature mining and evidence evaluation, and dissemination of pharmacogenetic information.
Preliminary Program Results
During year 1, 1097 patients undergoing left heart catheterization, including 291 PCI patients, were genotyped in the clopidogrel-CYP2C19 initiative, with 26.8% (80/291) of PCI patients identified as carriers of at least one loss-of-function allele. Of these patients, 70% (56/80) were switched to alternate antiplatelet therapy at the pharmacist’s recommendation.14 During this time period, the Personalized Medicine Subcommittee reviewed several CYP2C19 substrates including voriconazole, omeprazole and other proton-pump inhibitors, clobazam, and phenytoin for potential clinical implementation along with CYP2D6 substrates such as codeine, tramadol, and oxycodone. Two revised recommendations were also presented to and approved by the P&T Committee for the clopidogrel-CYP2C19 implementation: one to alter the alternative therapy recommendations for intermediate metabolizers (carriers of one loss-of-function allele) and another to change the interpretation of patients with an unknown or indeterminate genotype (CYP2C19 *2/*17) to intermediate metabolizers.
Discussion
This innovative program has been implemented with 14 pharmacists directly or indirectly contributing to key clinical and support roles throughout. Under the leadership of a pharmacist, the PMP expanded in less than 1 year to include an additional pharmacist (associate director) and a program coordinator. Currently in its third year, this program continues to support an active clopidogrel-CYP2C19 service and has expanded to include development and/or support of thiopurine methyltransferase (TPMT) testing in pediatric hematology/ oncology patients and in individuals with auto-immune disorders, IFNL3 (IL28B) testing for use of pegylated interferon–based treatment regimens in patients with hepatitis C, and a CYP2D6 pilot project in development with outpatient family medicine clinics.32, 34, 35 Our experiences with these services have reinforced the essential and emerging roles of clinical pharmacists in making the science of pharmacogenomics a reality.
However, there are important unmet needs in the profession for the development and delivery of pharmacist education and training, provision of practical clinical implementation resources, and creation and dissemination of sustainable business models to support pharmacists’ expanded roles. Regarding education, initial steps have been taken to meet these needs within the profession, but a number of challenges remain.36, 37 Although pharmacists generally acknowledge the importance of pharmacogenomics to the profession, research has shown that the majority of pharmacists feel unprepared to accurately apply these data to drug-therapy selection, dosing, or monitoring.38 A short continuing education course may not be sufficient to prepare practitioners for this role. In one study, a traditional, 1-hour, case-based pharmacogenomics educational program led to only a 7% difference in participants’ pretest and posttest scores (46% average pretest score vs 53% average posttest score; p=0.0003).39 Additionally, our program model is only one method of using pharmacogenomic data in patient care. Pharmacists can play a variety of potential roles in this area, including a front-line clinical pharmacogeneticist in a hospital setting, a pain management or oncology specialist helping to further guide drug dosing in these populations, and a community pharmacist counseling a patient who received test results from a direct-to-consumer genetic testing company. To overcome these challenges, a variety of pharmacogenomics education and training offerings that incorporate practice-based and “train-the-trainer” strategies will likely be needed. These may include standardized didactic and experiential curricular offerings for student pharmacists, postgraduate residency training, and continuing education and/or certificate courses that incorporate hands-on experiential training for practitioners.
Colleges and schools of pharmacy are increasingly responding to these needs through the development of innovative educational strategies to ensure graduates are prepared to apply pharmacogenomics to patient-centered care.39–41 Pharmacogenomics competencies are included in Accreditation Council for Pharmacy Education standards, and some pharmacy organizations have jointly adopted professional pharmacogenomic competencies for pharmacists.36, 37 This need is not unique to the profession of pharmacy as nursing, medicine, and other health care professional educators are also addressing genomic medicine education and practitioner needs.42, 43 Within UF Health PMP, we have adopted an interdisciplinary interactive educational model that incorporates personal genotype evaluation into a health-science center student elective course to facilitate personal understanding and adoption of pharmacogenomics. Early data support the benefit of similar educational initiatives that enable participants to use their own genetic data in completing course assignments.44 Our course will be offered to UF Health Science Center students in fall 2014, and we plan to expand to online professional, interdisciplinary, graduate level, and/or “train-the-trainer” initiatives thereafter in efforts to help meet these varied educational needs.
Clinical and experiential pharmacogenomics education and training programs and resultant practice opportunities are also emerging. Although our residency is currently one of only two such active clinical training programs in the United States, our experiences in this area are reinforced by the growth of practice opportunities we see emerging in the profession. After completion of her residency, the inaugural UF Health resident (AOO) accepted a position at Icahn School of Medicine at Mount Sinai and The Mount Sinai Hospital with three primary appointments: (1) Charles Bronfman Institute for Personalized Medicine staff; (2) clinical pharmacogenomics coordinator in the Department of Pharmacy; and (3) research assistant professor in the Division of General Internal Medicine. Her current responsibilities include spearheading clinical implementations of genotype-guided therapy integrated into the electronic medical record system and creating novel educational content and experiences for pharmacists, pharmacy residents, and student pharmacists at the site. Specialized postgraduate training in pharmacogenetics afforded the resident the opportunity to experience first-hand all components of a clinical implementation startup and to develop knowledge and skillsets to lead future implementations. Current residents hope to purse similar clinical pharmacogenetics positions on completion of their training. Similar to other programs that have successfully developed pharmacogenomics-focused APPEs, we have seen considerable student response to a Personalized and Evidence-Based Medicine APPE elective that has been offered since March 2014 at UF Health PMP.41
The importance of pharmacist leadership in this area is increasingly being highlighted through development of organizational guidance and practice-based resources. Clinical pharmacogenetics is recognized by the American Society of Health-Systems Pharmacists (ASHP) Foundation Pharmacy Practice Model Initiative (http://www.ashpmedia.org/ppmi/), an ASHP statement summarizing the pharmacist’s clinical role in this area is in publication,24 and an online ASHP resource center provides practitioner support (http://www.ashp.org/menu/PracticePolicy/ResourceCenters/Emerging-Sciences/Pharmacogenomics.aspx). The American Pharmacists Association has also recognized the pharmacist’s unique role in incorporating pharmacogenomics into the practice of medication therapy management.45 The American College of Clinical Pharmacy and American Association of Colleges of Pharmacy host pharmaocogenetics/pharmacogenomics practitioner and educator groups. The National Institutes of Health also recently incorporated pharmacist competencies in pharmacogenomics and pharmacists’ training and education resources into its Genetics/Genomics Competency Center for Education (http://www.g-2-c-2.org/).
As with other emerging practice roles, realization of this vision requires more than just education and resources. It is essential that cost-effective and sustainable models for pharmacogenetic testing and the pharmacist’s role within these models are developed and documented. Cost-related data for pharmacogenetic tests are accumulating, with cost-utility analyses of tests (including pharmacogenetic and genetic risk prediction tests) demonstrating health improvements, although at higher costs, as measured by cost per quality-adjusted life years.46 Published analyses of test reimbursement data point to increased likelihood of reimbursement with reactive (vs preemptive) pharmacogenetic tests that have evidence supporting accuracy and clinical utility, are accompanied by FDA-approved drug labeling, and associated with established analyte-specific Current Procedural Terminology codes.46–49
We addressed these questions in our program to gain additional perspectives based on our experiences. Pharmacogenetic testing for CYP2C19 in targeted patients was supported by research funds through end of year 1 postlaunch (June 2013), after which clinical billing was implemented. It quickly became clear that for reimbursement purposes it was necessary to move away from a preemptive model to test only those patients with a PCI. In PCI patients during year 1, the number needed to genotype to identify a patient in whom an alternative treatment would be recommended was 3.64 (i.e., approximately four PCI patients would need to be genotyped to result in one recommended change in therapy). During the first month of clinical billing, a total of seven different third party payors, including Medicare, reimbursed for CYP2C19 testing, with an 85% reimbursement rate for out-patient claims.14
Although research funding provides partial salary support of key leadership personnel and one pharmacy resident in this program, the majority of pharmacists involved at any level of our program are not supported by research funds. Rather, our service has been developed as a clinical program offered along with other clinical services (e.g., pharmacokinetics, drug information) in the health system. Clinical pharmacogenetics activities have been incorporated into the pharmacists’ normal dispensing workflow and are supported by CDS in the electronic health record, with brief provider and pharmacist training before launching a new implementation. As such, it is our experience that many components of a pharmacogenetics service can be integrated relatively seamlessly into the existing clinical pharmacy service infrastructure in a health system. However, we also acknowledge that there remains an important need for at least partial institutional support of a pharmacy clinician who is responsible for developing and supporting a new pharmacogenetics implementation. As most institutions will not have access to an experienced pharmacogeneticist, we advocate for engagement of pharmacists involved in drug information, therapeutic drug monitoring, medication-use systems, patient safety initiatives, and/or clinical education as early institutional adopters and potential leaders in this field.
Finally, it is our experience that multidisciplinary engagement and support are necessary in developing effective and sustainable pharmacist-led clinical pharmacogenomics practice models. As we have continued to grow our pilot initiative and expand to other drug–gene pairs, ongoing communication with targeted nonpharmacist clinicians and staff, pathology/laboratory personnel, health-system administrators, and informatics support has remained essential to program sustainability. This need is met within the PMP through periodic in-person meetings between program leadership and key health-system stakeholders and educational or inservice opportunities to discuss new workflow or laboratory ordering procedures for practitioners, nurses, and staff.
Conclusion
Use of genotype information to guide drug decisions in clinical practice is an exciting, emerging area in which clinical pharmacists can play a role. The profession needs to be prepared to embrace the opportunities that the increasing availability of genetic information on patients will present and should work collectively to position the profession in a manner that clinical pharmacists are the recognized experts on the health care team for clinical use of pharmacogenomics.
In our experience, pharmacists should be instrumental in leadership, development, and implementation of clinical pharmacogenomics initiatives. One year after implementation of one such program, important roles have emerged for pharmacists that span beyond the anticipated and traditional direct patient care activities of clinical pharmacists. Development and dissemination of effective education and training initiatives, clinical practice-based resources, and financially sustainable pharmacist-led practice models are needed to provide evidence and further define pharmacists’ roles and potential value to the health care system in this area.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health: UL1 TR000064, U01 HL105198, U01 GM074492, and U01 HG007269. We also acknowledge and thank the University of Florida and UF Health Shands Hospital for in-kind support of the Personalized Medicine Program and the large number of faculty and staff who made the pilot genomic medicine implementation a success.
Dr. Owusu-Obeng (AOO) was the inaugural postgraduate year 2 (PGY2) pharmacogenomics resident in the University of Florida Health Personalized Medicine Program.
The authors would like to thank Amanda Elsey, program manager of the UF Health PMP, for her contributions toward the creation of the figures included in the manuscript and her outstanding management of the UF Health Personalized Medicine Program. Special thanks are given to Joanna Isidro for her help in the program as well.
References
- 1.Motulsky AG, Qi M. Pharmacogenetics, pharmacogenomics and ecogenetics. J Zhejiang Univ Sci B. 2006;7:169–70. doi: 10.1631/jzus.2006.B0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamer UM, Musshoff F, Kobilay M, Madea B, Hoeft A, Stuber F. Concentrations of tramadol and O-desmethyltramadol enantiomers in different CYP2D6 genotypes. Clin Pharmacol Ther. 2007;82:41–7. doi: 10.1038/sj.clpt.6100152. [DOI] [PubMed] [Google Scholar]
- 3.Poulsen L, Arendt-Nielsen L, Brosen K, Sindrup SH. The hypoalgesic effect of tramadol in relation to CYP2D6. Clin Pharmacol Ther. 1996;60:636–44. doi: 10.1016/S0009-9236(96)90211-8. [DOI] [PubMed] [Google Scholar]
- 4.Kiyotani K, Mushiroda T, Imamura CK, et al. Dose-adjustment study of tamoxifen based on CYP2D6 genotypes in Japanese breast cancer patients. Breast Cancer Res Treat. 2012;131:137–45. doi: 10.1007/s10549-011-1777-7. [DOI] [PubMed] [Google Scholar]
- 5.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–62. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Kim BH, Nam WS, et al. Effect of CYP2C19 polymorphism on the pharmacokinetics of voriconazole after single and multiple doses in healthy volunteers. J Clin Pharmacol. 2012;52:195–203. doi: 10.1177/0091270010395510. [DOI] [PubMed] [Google Scholar]
- 7.Evans WE, McLeod HL. Pharmacogenomics–drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538–49. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- 8.Table of Pharmacogenomic Biomarkers in Drug Labels. U.S. Food and Drug Administration; 2013. [Accessed June 4, 2014]. [updated May 22, 2014]. Available from http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm. [Google Scholar]
- 9.Gottesman O, Scott SA, Ellis SB, et al. The CLIPMERGE PGx Program: clinical implementation of personalized medicine through electronic health records and genomics-pharmacogenomics. Clin Pharmacol Ther. 2013;94:214–7. doi: 10.1038/clpt.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JA, Elsey AR, Clare-Salzler MJ, Nessl D, Conlon M, Nelson DR. Institutional profile: University of Florida and Shands Hospital Personalized Medicine Program: clinical implementation of pharmacogenetics. Pharmacogenomics. 2013;14:723–6. doi: 10.2217/pgs.13.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donnell PH, Bush A, Spitz J, et al. The 1200 patients project: creating a new medical model system for clinical implementation of pharmacogenomics. Clin Pharmacol Ther. 2012;92:446–9. doi: 10.1038/clpt.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulley JM, Denny JC, Peterson JF, et al. Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clin Pharmacol Ther. 2012;92:87–95. doi: 10.1038/clpt.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crews KR, Cross SJ, McCormick JN, et al. Development and implementation of a pharmacist-managed clinical pharmacogenetics service. Am J Health Syst Pharm. 2011;68:143–50. doi: 10.2146/ajhp100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weitzel KW, Elsey AR, Langaee TY, et al. Clinical pharmacogenetics implementation: approaches, success, and challenges. Am J Med Genet C Semin Med Genet. 2014;166:56–67. doi: 10.1002/ajmg.c.31390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman JM, Haidar CE, Wilkinson MR, et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014;166:45–55. doi: 10.1002/ajmg.c.31391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shuldiner AR, Palmer K, Pakyz RE, et al. Implementation of pharmacogenetics: the University of Maryland personalized anti-platelet pharmacogenetics program. Am J Med Genet C Semin Med Genet. 2014;166:76–84. doi: 10.1002/ajmg.c.31396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bielinski SJ, Olson JE, Pathak J, et al. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin Proc. 2014;89:25–33. doi: 10.1016/j.mayocp.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nutescu EA, Drozda K, Bress AP, et al. Feasibility of implementing a comprehensive warfarin pharmacogenetics service. Pharmacotherapy. 2013;33:1156–64. doi: 10.1002/phar.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudis MI, Brandl KM. Position paper on critical care pharmacy services. Society of Critical Care Medicine and American College of Clinical Pharmacy Task Force on Critical Care Pharmacy Services. Crit Care Med. 2000;28:3746–50. doi: 10.1097/00003246-200011000-00037. [DOI] [PubMed] [Google Scholar]
- 20.Bishop MA, Streiff MB, Ensor CR, Tedford RJ, Russell SD, Ross PA. Pharmacist-managed international normalized ratio patient self-testing is associated with increased time in therapeutic range in patients with left ventricular assist devices at an academic medical center. ASAIO J. 2014;60:193–8. doi: 10.1097/MAT.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 21.Kogut SJ, Goldstein E, Charbonneau C, Jackson A, Patry G. Improving medication management after a hospitalization with pharmacist home visits and electronic personal health records: an observational study. Drug Healthc Patient Saf. 2014;6:1–6. doi: 10.2147/DHPS.S56574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratanajamit C, Kaewpibal P, Setthawacharavanich S, Faroongsarng D. Effect of pharmacist participation in the health care team on therapeutic drug monitoring utilization for antiepileptic drugs. J Med Assoc Thai. 2009;92:1500–7. [PubMed] [Google Scholar]
- 23.Pedersen CA, Schneider PJ, Scheckelhoff DJ. ASHP national survey of pharmacy practice in hospital settings: prescribing and transcribing-2013. Am J Health Syst Pharm. 2014;71:924–42. doi: 10.2146/ajhp140032. [DOI] [PubMed] [Google Scholar]
- 24.American Society of Health-Systems Pharmacists. DRAFT ASHP statement on the pharmacist’s role in clinical pharmacogenomics. Bethesda, MD: American Society of Health-Systems Pharmacists; [Accessed June 4, 2014]. Available from http://www.ashp.org/DocLibrary/BestPractices/DraftDocs/DrftStPharmacogenomics.aspx. [Google Scholar]
- 25.Williams MS. Genomic medicine implementation: learning by example. Am J Med Genet C Semin Med Genet. 2014;166:8–14. doi: 10.1002/ajmg.c.31394. [DOI] [PubMed] [Google Scholar]
- 26.Mills R, Haga SB. The clinical delivery of pharmacogenetic testing services: a proposed partnership between genetic counselors and pharmacists. Pharmaocogenomics. 2013;14:957–68. doi: 10.2217/pgs.13.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott SA, Sangkuhl K, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–23. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson JA, Roden DM, Lesko LJ, Ashley E, Klein TE, Shuldiner AR. Clopidogrel: a case for indication-specific pharmacogenetics. Clin Pharmacol Ther. 2012;91:774–6. doi: 10.1038/clpt.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JA, Burkley BM, Langaee TY, Clare-Salzler MJ, Klein TE, Altman RB. Implementing personalized medicine: development of a cost-effective customized pharmacogenetics genotyping array. Clin Pharmacol Ther. 2012;92:437–9. doi: 10.1038/clpt.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hicks JK, Crews KR, Hoffman JM, et al. A clinician-driven automated system for integration of pharmacogenetic interpretations into an electronic medical record. Clin Pharmacol Ther. 2012;92:563–6. doi: 10.1038/clpt.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Relling MV, Gardner EE, Sandborn WJ, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89:387–91. doi: 10.1038/clpt.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Relling MV, Gardner EE, Sandborn WJ, et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther. 2013;93:324–5. doi: 10.1038/clpt.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crews KR, Gaedigk A, Dunnenberger HM, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin Pharmacol Ther. 2012;91:321–6. doi: 10.1038/clpt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crews KR, Gaedigk A, Dunnenberger HM, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for Cytochrome P450 2D6 Genotype and Codeine Therapy: 2014 Update. Clin Pharmacol Ther. 2014;95:376–82. doi: 10.1038/clpt.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muir AJ, Gong L, Johnson SG, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for IFNL3 (IL28B) genotype and PEG interferon-α-based regimens. Clin Pharmacol Ther. 2014;95:141–6. doi: 10.1038/clpt.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genetics and Genomics Competency Center for Education, National Human Genome Research Institute. Pharmacist Pharmacogenomic Competencies and Outcomes. Bethesda, MD: [Accessed June 4, 2014]. Available from http://www.g-2-c-2.org/attachments/pdf/Pharmacist-Comp.pdf. [Google Scholar]
- 37.Accreditation Council for Pharmacy Education. [Accessed June 4, 2014];Accreditation standards and guidelines for the professional program in pharmacy leading to the Doctor of Pharmacy degree. 2011 Feb 14; Available from https://www.acpe-accredit.org/deans/standards.asp.
- 38.McCullough KB, Formea CM, Berg KD, et al. Assessment of the pharmacogenomics educational needs of pharmacists. Am J Pharm Educ. 2011;75:51. doi: 10.5688/ajpe75351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Formea CM, Nicholson WT, McCullough KB, et al. Development and evaluation of a pharmacogenomics educational program for pharmacists. Am J Pharm Educ. 2013;77:Article 10. doi: 10.5688/ajpe77110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farrell CL, Pedigo NG, Messersmith AR. Application of genomic principles to pharmacotherapy of cancer. Am J Pharm Educ. 2014;78:55. doi: 10.5688/ajpe78355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drozda K, Labinov Y, Jiang R, et al. A pharmacogenetics service experience for pharmacy students, residents, and fellows. Am J Pharm Educ. 2013;77:175. doi: 10.5688/ajpe778175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demmer LA, Waggoner DJ. Professional medical education and genomics. Annu Rev Genomics Hum Genet. 2014;15:3.1–3.10. doi: 10.1146/annurev-genom-090413-025522. [DOI] [PubMed] [Google Scholar]
- 43.Feero WG, Green ED. Genomics education for health care professionals in the 21st century. J Am Med Assoc. 2011;306:989–90. doi: 10.1001/jama.2011.1245. [DOI] [PubMed] [Google Scholar]
- 44.Salari K, Karczewski KJ, Hudgins L, Ormond KE. Evidence that personal genome testing enhances student learning in a course on genomics and personalized medicine. PLoS ONE. 2013;8:e68853. doi: 10.1371/journal.pone.0068853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owen JA, Assoc AP. Integrating pharmacogenomics into pharmacy practice via medication therapy management. J Am Pharm Assoc. 2011;51:E64–74. doi: 10.1331/JAPhA.2011.11543. [DOI] [PubMed] [Google Scholar]
- 46.Phillips KA, Sakowski JA, Trosman J, Douglas MP, Liang SY, Neumann P. The economic value of personalized medicine tests: what we know and what we need to know. Genet Med. 2014;16:251–7. doi: 10.1038/gim.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong WB, Carlson JJ, Thariani R, Veenstra DL. Cost effectiveness of pharmacogenomics: a critical and systematic review. Pharmacoeconomics. 2010;28:1001–13. doi: 10.2165/11537410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Cohen J, Wilson A, Manzolillo K. Clinical and economic challenges facing pharmacogenomics. Pharmacogenomics J. 2013;13:378–88. doi: 10.1038/tpj.2011.63. [DOI] [PubMed] [Google Scholar]
- 49.Cohen JP, Felix AE. Personalized medicine’s bottleneck: diagnostic test evidence and reimbursement. J Pers Med. 2014;4:163–75. doi: 10.3390/jpm4020163. [DOI] [PMC free article] [PubMed] [Google Scholar]