Abstract
Cathepsin G (CatG), a serine protease present in mast cells and neutrophils, can produce angiotensin-II (Ang-II) and degrade elastin. Here we demonstrate increased CatG expression in smooth muscle cells (SMCs), endothelial cells (ECs), macrophages, and T cells from human atherosclerotic lesions. In low-density lipoprotein (LDL) receptor-deficient (Ldlr−/−) mice, the absence of CatG reduces arterial wall elastin degradation and attenuates early atherosclerosis when mice consume a Western diet for 3 months. When mice consume this diet for 6 months, however, CatG deficiency exacerbates atherosclerosis in aortic arch without affecting lesion inflammatory cell content or extracellular matrix accumulation, but raises plasma total cholesterol and LDL levels without affecting high-density lipoprotein (HDL) or triglyceride levels. Patients with atherosclerosis also have significantly reduced plasma CatG levels that correlate inversely with total cholesterol (r= −0.535, P<0.0001) and LDL cholesterol (r= −0.559, P<0.0001), but not with HDL cholesterol (P=0.901) or triglycerides (P=0.186). Such inverse correlations with total cholesterol (r= −0.504, P<0.0001) and LDL cholesterol (r= −0.502, P<0.0001) remain significant after adjusting for lipid lowering treatments among this patient population. Human CatG degrades purified human LDL, but not HDL. This study suggests that CatG promotes early atherogenesis through its elastinolytic activity, but suppresses late progression of atherosclerosis by degrading LDL without affecting HDL or triglycerides.
Keywords: cathepsin G, atherosclerosis, low-density lipoprotein, elastin, angiotensin-II
1. Introduction
Cathepsin G (CatG), a serine protease, localizes in mast cells and neutrophils (1–3]. The best known function of CatG in a cardiovascular context is its ability to activate the renin-angiotensin system: CatG produces angiotensin II (Ang-II) from angiotensin I (Ang-I) and angiotensinogen [2, 4, 5]. Considerable data implicate Ang-II in the pathogenesis of atherosclerosis [3, 6]. Ang-II affects vascular structure and function. In atherosclerotic lesions, Ang-II induces smooth muscle cell (SMC) growth and migration, activates macrophages, increases platelet aggregation, and causes endothelial dysfunction. It also promotes apoptosis, increases oxidative stress, promotes leukocyte adhesion and migration, stimulates thrombosis, and has proinflammatory effects [7, 8]. In addition to producing Ang-II, CatG also activates matrix metalloproteinase (MMP)-1, −2, −3, and −9 zymogens [9–12] or enhances their expression [13]. MMPs participate in atherogenesis [14, 15]. CatG is also an elastase [16, 17] and collagenase activator [18]. We have recently found that CatG degrades type I collagen [17], the dominant collagen type in human aortic wall. Therefore, CatG may contribute to atherogenesis directly via its elastinolytic and collagenolytic activities or indirectly via its capability of activating collagenases or MMPs, and producing Ang-II.
While the normal human aortas contain negligible CatG, its expression in human atherosclerotic lesions increases by 2-fold as determined by immunoblot analysis, and CatG immunolocalizes to leukocytes and mast cells in the tunica media and adventitia [19]. Increased CatG in aortas may degrade VE-cadherin and fibronectin, thereby enhancing the expression and activation of MMPs [13] and the interaction of blood-borne leukocytes with the luminal endothelium [20]. A recent study using Apoe−/− mice demonstrated that CatG insufficiency (Apoe−/−Ctsg+/−) significantly reduced lesion collagen and SMC content, and apoptotic cell number [21], supporting a role of CatG in experimental atherosclerosis.
This study used CatG and low-density lipoprotein receptor (LDLr) double-deficient mice (Ldlr−/−Ctsg−/−) and examined blood from patients with atherosclerosis to probe further potential roles of CatG in atherosclerosis. The findings implicate CatG in elastin degradation in early atherogenesis, and in LDL catabolism at a later stage. Patients with atherosclerosis have a significant inverse association between plasma LDL and CatG.
2. Materials and methods
2.1. Human atherosclerotic lesion immunohistology and immunoblot analysis
Human atherosclerotic plaques were obtained at endarterectomy (n=12), and non-atherosclerotic carotid arteries from heart transplant donors (n=10), according to protocols pre-approved by the Human Investigative Review Committee of Brigham and Women’s Hospital. Human atherosclerosis serial cryostat sections (6 µm) were stained for CatG (1:200, Calbiochem, San Diego, CA), CD68 (macrophages, 1:700, Dako, Carpinteria, CA). For localization of CatG to cell types rabbit anti-CatG (1:50) antibody mixed with mouse anti-CD68 (1:50, Dako), or anti-CD31 (endothelial cells [ECs], 1:30, Dako), or -α-actin (SMCs, 1:30, Enzo Diagnostics Inc., Farmingdale, NY), or -CD4 (T cells, 1:20, BD Biosciences, San Jose, CA) antibodies. Consequently, sections incubated with mixture of anti-rabbit Alexa 533 (red, 1:500) and anti-mouse Alexa 488 (green, 1:300, both from Invitrogen, Grand Island, NY). Nuclei stained with Dapi (NucBlue® Fixed Cell ReadyProbes® Reagent, Molecular Probes, Eugene, OR) and slides were coverslip with fluorescent mounting medium (Dako). Human carotid atherosclerotic lesions (n=7) and non-atherosclerotic carotid arteries (n=6) were also lysed in a protein lysis buffer containing 10 mM Tris.HCl, (pH 8.0), 1 mM EDTA, 0.5 mM EGTA, 1% Triton, 0.1% Sodium deoxycholate, 0.1% SDS, and 140 mM NaCl. Equal protein from each sample was separated on a 12% SDS-PAGE for immunoblot analysis with rabbit anti-human CatG polyclonal antibody (1:1000, Calbiochem). Mouse anti-actin monoclonal antibody (1:2000, Santa Cruz Biotechnology, Inc., Dallas, TX) was used to ensure equal protein loading.
2.2. Mouse experimental atherosclerosis
We crossbred Ctsg−/− mice (C57BL/6/129/SvJ) [22] with atherosclerosis-prone Ldlr−/− mice (C57BL/6, N11, The Jackson Laboratory, Bar Harbor, ME) to generate Ldlr+/−Ctsg+/− breeding pairs to produce Ldlr−/−Ctsg+/− mice and their littermate Ldlr−/−Ctsg+/+ control mice. All mice used in this study were male. To induce atherosclerosis, 6-week-old mice from each group consumed a Western diet (C12108, Research Diets Inc.) for 3 months or 6 months. At each time point, blood pressures were measured and plasma collected. Lesion characterizations for mouse atherosclerosis, including thoracic and abdominal aorta oil-red O staining, aortic arch lesion intima and media areas, lesion macrophages (Mac-3, 1:900, BD Biosciences), T cells (CD4, 1:90, BD Biosciences), MHC class II-positive cells (MHC class-II, 1:250, BD Biosciences), SMCs (α-actin, 1:750, Sigma), collagen (picrosirius red birefringence), elastin (Verhoeff-van Gieson), arch lipid deposition (0.5% oil-red O), and apoptosis (TUNEL, EMD Millipore, Billerica, MA) were performed as described previously [23]. The 3-mm long aortic arch from the brachiocephalic artery to the ascending artery perpendicular line toward the descending artery was used for atherosclerotic lesion analysis, as previously described [24]. We captured images digitally; and measured the stained area using computer-assisted image quantification (Image-Pro Plus software, Media Cybernetics), and immunopositive cells were counted manually. All mouse experiments were performed, and data were analyzed in a blinded fashion, by at least three observers. All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health, and were approved by the Harvard Medical School Standing Committee on Animals (protocol # 03759).
2.3. Mouse plasma ELISA
Blood samples were collected from Ldlr−/−Ctsg−/− mice and their littermate Ldlr−/−Ctsg+/+ control mice at harvest by retro-orbital venous plexus puncture. Plasma total cholesterol, triglyceride, and HDL were determined using ELISA kits according to the manufacturer’s instructions (Pointe Scientific, Inc., Canton, MI). LDL cholesterol was calculated as follows: serum LDL cholesterol concentration (mg/dl) = total cholesterol – HDL cholesterol – (triglycerides/5). Mouse plasma Ang-II and angiotensin-converting enzyme (ACE) levels were determined using the Ang-II (USCN Life Science Inc., Houston, TX) and ACE (R&D Systems, Minneapolis, MN) ELISA kits, respectively, according to the manufacturers’ instructions. Both albumin and alanine aminotransferase (ALT) levels were determined from plasma samples from mice that consumed a Western diet for 3 or 6 months to assess whether CatG deficiency affected mouse liver functions (Mouse Metabolic Phenotyping Center, Yale University School of Medicine, New Haven, CT).
2.4. Blood pressure measurement
Mouse blood pressures were measured at different time points while consuming a Western diet. To measure blood pressures from live mice, we used the CODA standard non-invasive blood pressure system — the tail-cuff method, according to the manufacturer’s instructions (Kent Scientific Corporation, Torrington, CT). Briefly, mice were trained for 3–5 times before the experiments to confirm that they became accustomed to the tail-cuff procedure. A single investigator recorded blood pressures in a quiet environment without disturbance. At least 30 measurements were obtained from each mouse to determine the mean values of systolic and diastolic blood pressures and heart rate.
2.5. Human patient population and plasma CatG ELISA
A total of 232 patients from Wuhan Union Hospital, Wuhan, China, were enrolled in the study due to symptoms of chest pain or electrocardiogram abnormalities including ST-T abnormalities, and were scheduled to undergo coronary angiography. The local Hospital Review Committee approved the human study protocol, and all patients gave informed consent. 171 of the 232 subjects were diagnosed with coronary heart disease (CHD) with one or more major coronary arteries having ≥50% stenosis; 61 subjects had <50% or no luminal narrowing of the coronary artery, and were selected as non-CHD controls. Among the 171 patients with CHD, 59 were diagnosed with acute myocardial infarction (AMI) due to increased levels of creatinine kinase-MB (twofold higher than the upper reference limit) or troponin-I (fivefold higher than the upper reference limit), ischemic symptoms, or ST-T abnormalities by electrocardiography indicative of ischemia and/or infarction; 67 were diagnosed with unstable angina pectoris (UAP) due to the progression of ischemic symptoms less than 3 months before admission to the hospital; and 45 were diagnosed with stable angina pectoris (SAP) based on predictable exertional chest discomfort more than 3 months before enrollment. Patients who were treated with anti-inflammatory drugs; who had connective tissue disease, thromboembolism, disseminated intravascular coagulation, advanced liver disease, renal failure, malignant disease, or other inflammatory diseases (such as septicemia or pneumonia); who had other heart diseases such as rheumatic heart disease, valvular heart disease, congenital heart disease; who had atrial fibrillation or had a pacemaker, were excluded. Among all (232) selected patients, 6/61 (9.8%) non-CHD, 22/45 (49%) SAP, 37/67 (55%) UAP, and 37/59 (63%) AMI patients received lipid-lowering treatment with statins. Human plasma CatG levels were determined using commercial ELISA kits according to manufacturer’s recommendation (Alpco, Salem, NH).
2.6. Isolation and modification of LDL and HDL3
Human LDL (d=1.019–1.050 g/ml) and human HDL3 (d=1.125–1.210 g/mL) were isolated from plasma of healthy volunteers (from the Finnish Red Cross Bloos Service, Helsinki, Finland) by sequential ultracentrifugation in the presence of 3 mM EDTA [25, 26]. Lipoprotein amounts are expressed in terms of their protein concentrations. Lipoproteins (1 mg/ml) were incubated for 18 hours at 37 °C in phosphate-buffered saline with or without 2 mU/ml of human neutrophil CatG (Calbiochem), after which they underwent FPLC analysis using a Superose™ 6 column in an Äkta chromatography system (GE Healthcare) and by monitoring absorbance at 280 nm.
2.7. Statistical analysis
Human plasma results are expressed as percentages or median (minimum, maximum). Differences in frequencies were compared using the chi-square test. Normal Gaussian distribution of the data was verified by the Kolmogorov-Smirnov goodness-of-fit test. Whenever the data were not normally distributed, the Kruskal-Wallis H test was used, followed by post hoc analysis (Mann-Whitney U test). To analyze the correlation of serum CatG and blood lipids, we used Pearson’s correlation test. To adjust for the impact of statin treatment on the association between plasma CatG and lipid profiles, we performed partial correlation test with the statin treatment as the controlling variable. All mouse data were expressed as mean ± SEM. Due to our small sample sizes and often skewed data distributions, we performed a pairwise non-parametric Mann-Whitney test followed by Bonferroni corrections to examine the statistical significances. SPSS 16.0 was used for analysis.
3. Results
3.1. CatG expression in human atherosclerotic lesions
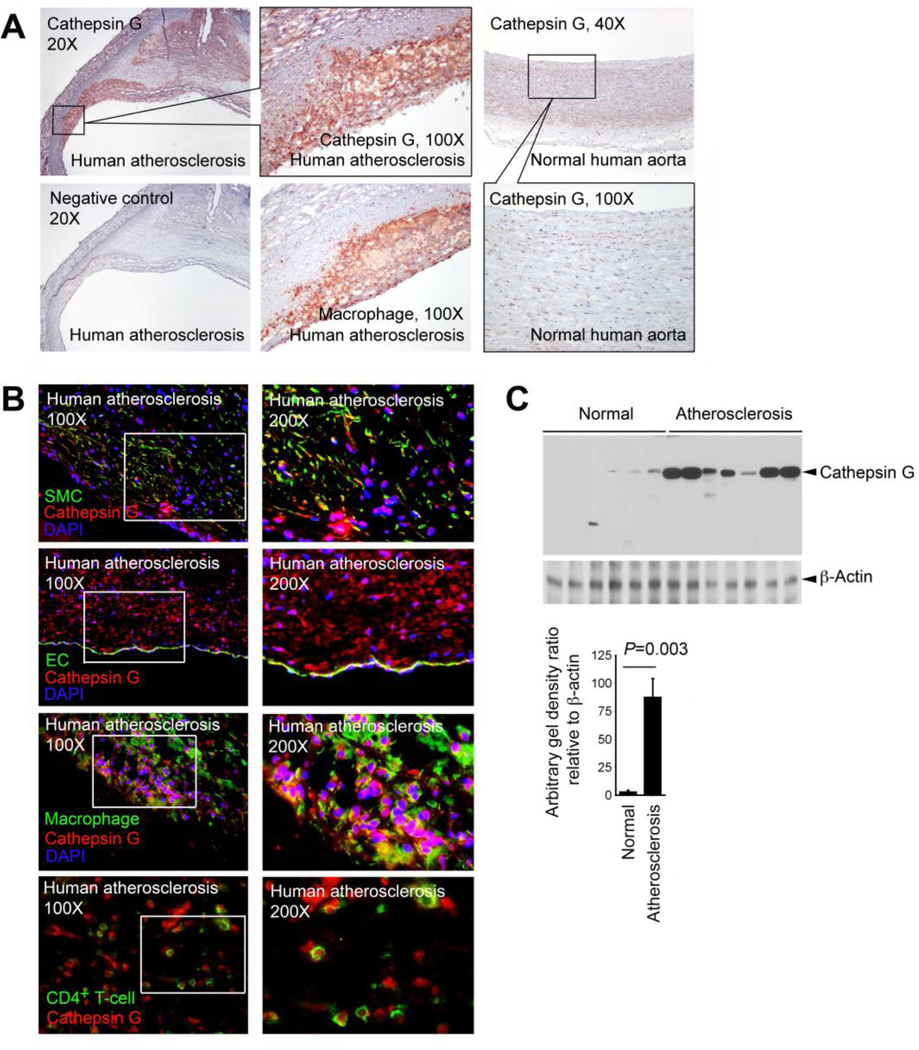
Prior studies demonstrated CatG expression in mast cells and neutrophils in human atherosclerotic lesions [19, 27]. Immunostaining of parallel sections from human carotid atherosclerotic lesions with a rabbit anti-human CatG polyclonal antibody demonstrated CatG expression in macrophage-rich regions, whereas human normal carotid arteries displayed much less immunoreactive CatG (Fig. 1A). Immunofluorescent double staining demonstrated that not only macrophages, but also α-actin-positive SMCs, CD31-positive ECs, and CD4+ T cells all contained CatG protein (Fig. 1B). Therefore, cells other than mast cells and neutrophils may contribute to the elevated CatG in human atherosclerotic lesions. CatG localizes in intracellular organelles [28], at the cell surface [29], and also binds to the nucleic acids in the nuclei [30]. Immunofluorescent double staining detected both intracellular and nuclear CatG (co-localized with DAPI [4′,6′-diamidino-2-phenylindole hydrochloride] staining) in SMCs, ECs, and macrophages in human atherosclerotic lesions (Fig. 1B). CatG expression in human atherosclerotic lesions was semi-quantified with immunoblot analysis, followed by densitometric analysis using ImageJ. Human carotid atherosclerotic lesions contained significantly more CatG protein than did non-atherosclerotic carotid arteries (Fig. 1C).
Fig. 1.
CatG expression in human atherosclerotic lesions. A. Immunostaining of human atherosclerotic lesion parallel frozen sections with rabbit anti-human CatG and mouse anti-human CD68 (macrophage) antibodies on serial frozen sections from representative human atherosclerotic lesion and normal human carotid artery. Staining with rabbit IgG showed no staining (negative control). Inserts with higher magnifications are shown. B. Immunofluorescent double staining localized CatG expression in SMCs (α-actin), ECs (CD31), macrophages (CD68), and CD4+ T cells in human atherosclerotic lesions. Inserts with higher magnifications are shown on the right. C. Immunoblot analysis of CatG in human normal carotid arteries (n=6) and atherosclerotic lesions (n=7). β-actin immunoblot was used to ensure equal protein loading. CatG immunoblot gel density was quantified with ImageJ software and data was presented as relative ratio to β-actin.
3.2. CatG deficiency reduces blood pressure and plasma Ang-II and ACE levels in mice with atherosclerosis
The best-known cardiovascular activity of CatG is processing Ang-I [2]. We have recently shown that inflammatory cytokines and high glucose stimulate SMC generation of Ang-II and ACE, processes that can be fully blocked by a CatG-selective inhibitor or by CatG siRNA [17]. Therefore, increased CatG in human atherosclerotic lesions suggest a role of this protease in local production of Ang-II, thereby promoting atherogenesis and vascular dysfunction. To test whether expression of CatG affects Ang-II (and ACE) expression and blood pressures, we used atherosclerosis-prone Ldlr−/− mice and generated CatG and LDLr double-deficient (Ldlr−/−Ctsg−/−) mice and their littermates (Ldlr−/−Ctsg+/+). Before consumption of a Western diet, systolic or diastolic blood pressures did not differ significantly between these strains (data not shown). After having consumed a Western diet for 3 months, however, Ldlr−/−Ctsg−/− mice had significantly lower systolic and diastolic blood pressures compared with Ldlr−/−Ctsg+/+ mice (Fig. 2A), but plasma Ang-II and ACE levels did not differ between the groups (Fig. 2B). After 6 months on this diet, both diastolic blood pressure and plasma Ang-II and ACE levels remained significantly lower in Ldlr−/−Ctsg−/− mice than in Ldlr−/−Ctsg+/+ mice (Fig. 2A, B). The temporally disperse changes of Ang-II and ACE concentrations and systolic and diastolic blood pressures between the two groups of mice from both different time points suggest a modest role of CatG activity in Ang-II regulation and blood pressure during atherogenesis. Absence of CatG did not affect heart rate (data not shown).
Fig. 2.
CatG deficiency lowers systolic and diastolic blood pressures (A) and plasma Ang-II and ACE levels (B) in Ldlr−/−Ctsg−/− compared to Ldlr−/−Ctsg+/+ mice that consumed a Western diet for 3 and 6 months as indicated. Data are mean ± SE. The number of mice per experimental group is indicated in each bar.
3.3. Distinct roles of CatG at different stages of experimental atherosclerosis
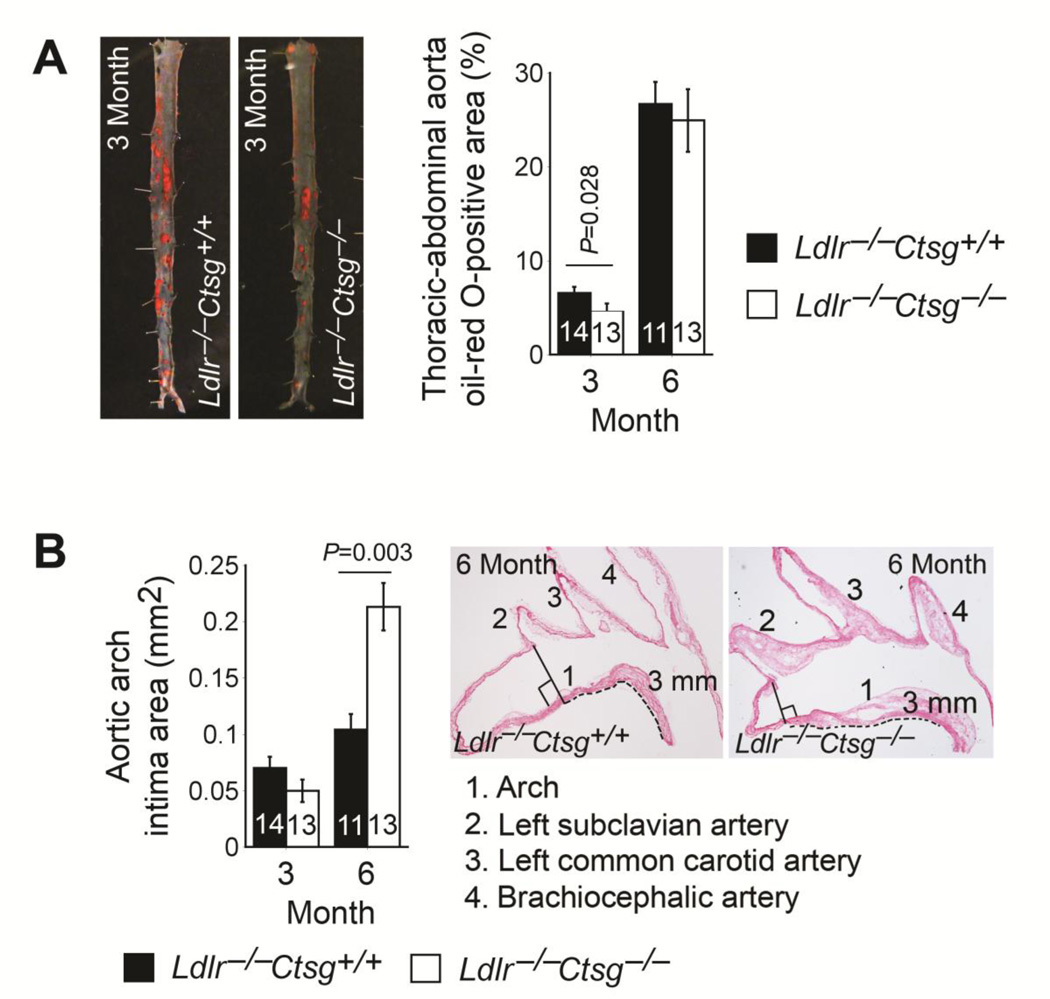
Reduced blood pressures and plasma Ang-II and ACE levels in Ldlr−/−Ctsg−/− mice suggest that these mice should develop less and smaller atherosclerotic lesions than do the Ldlr−/−Ctsg+/+ mice. En face preparation and oil-red O staining quantified thoracic-abdominal aorta atherosclerotic lesion area or lipid deposition. After consuming a Western diet for 3 months, Ldlr−/−Ctsg−/− mice showed significantly smaller oil-red O-positive areas compared with Ldlr−/−Ctsg+/+ mice. Oil-red O-positive areas between the two groups of mice did not differ, however, at a later stage of atherosclerosis, when mice had consumed a Western diet for 6 months (Fig. 3A). Reduced blood pressures in Ldlr−/−Ctsg−/− mice at the 3-month time point (Fig. 2A) might have contributed to the reduced atherosclerosis in the thoracic and abdominal aortas. At the 6-month time point, however, reduced Ang-II and ACE production and blood pressure (Fig. 2A, B) did not explain the unchanged thoracic-abdominal aorta atherosclerosis.
Fig. 3.
CatG deficiency attenuates early atherogenesis but aggravates later lesions. Atherosclerotic lesion areas in Ldlr−/−Ctsg−/− mice and Ldlr−/−Ctsg+/+ mice fed a Western diet for 3 months and 6 months. A. Thoracic-abdominal atherosclerotic lesion oil-red O-positive areas. Representative thoracic-abdominal aortas from the 3-month time point are shown on the left. B. Aortic arch intima areas from the perpendicular line between the left edge of the brachiocephalic artery and the ascending aorta to 3 mm towards the descending aorta as indicated by dotted lines (right two panels). Brachiocephalic artery, left common carotid artery, and left subclavian artery are also indicated in the representative aortic arches at the right panels. Data are mean ± SE. Number of mice per experimental group is indicated in each bar.
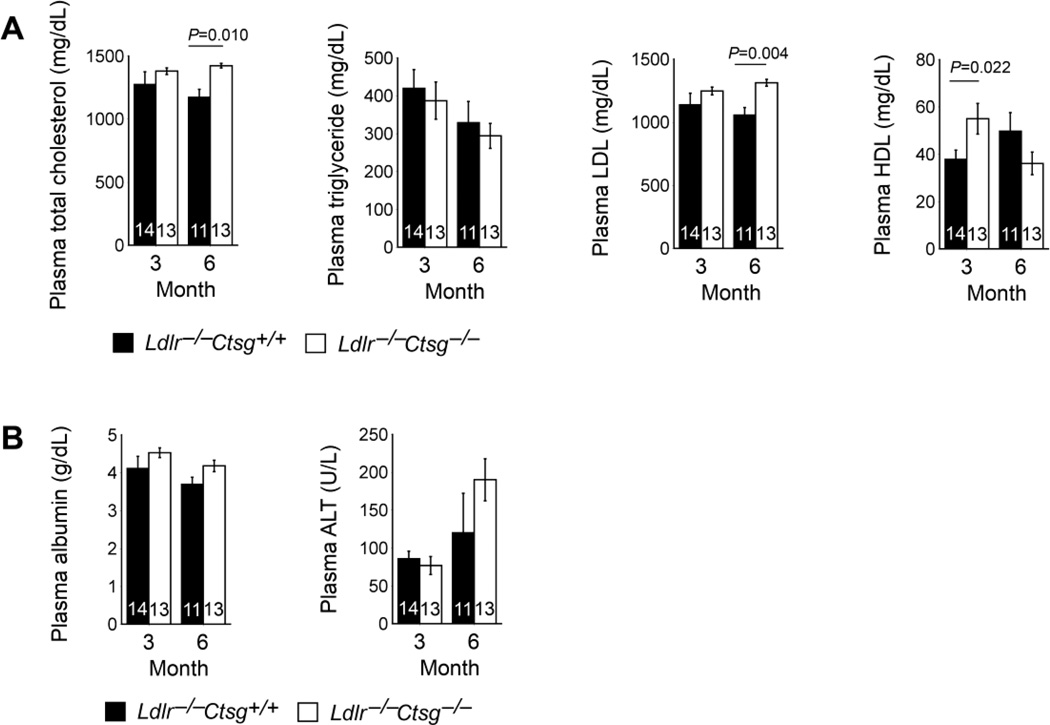
The aortic arch develops lesions earlier than the thoracic-abdominal aorta in both Ldlr−/− mice and apolipoprotein E-deficient (Apoe−/−) mice [31, 32]. After 3 months on a Western diet, aortic lesion intima areas from the 3-mm aortic arch (Fig. 3B) tended to be lower in Ldlr−/−Ctsg−/− mice, but did not reach statistical significance compared with those from Ldlr−/−Ctsg+/+ mice (P=0.084). In contrast, Ldlr−/−Ctsg−/− mice had significantly larger aortic arch intima areas than did Ldlr−/−Ctsg+/+ mice at the later stage of atherosclerosis, when mice had consumed a Western diet for 6 months (P=0.003, Fig. 3B). Although not calculated due to technical difficulties to maintain all three breaches (brachiocephalic artery, left common carotid artery, and left subclavian artery) on the exact same plane during froze tissue embedding, atherosclerotic lesion sizes in these branches were also larger in Ldlr−/−Ctsg−/− mice than in Ldlr−/−Ctsg+/+ mice at this late stage (Fig. 3B, two right panels). Since the plasma Ang-II and ACE levels and systolic and diastolic blood pressures decreased, rather than increased, in the Ldlr−/−Ctsg−/− mice at this later time point (Fig. 2A, B), these hemodynamic changes could not themselves explain the increased atherosclerosis from the aortic arch (Fig. 3B). Lesion characterization showed no significant differences in lesion Mac-3-positive macrophage content, CD4+ T-cell content, MHC class II-positive area, α-actin-positive SMC area, collagen content, or arch lipid deposition area (Fig. 4A–C), suggesting that CatG did not affect atherosclerotic lesion inflammation or collagen deposition at either the early (3 months) or the later (6 months) stages. We evaluated the degree of elastin fragmentation in longitudinal frozen sections from mouse aortae stained for elastica by Verhoeff-van Gieson and graded it as follows: grade 1, intact, well-organized elastic laminae; grade 2, elastic laminae with some interruptions and breaks; and grade 3,severe elastin fragmentation or loss as described previously [33]. Representative images are presented in Fig. 4D. Ldlr−/−Ctsg−/− mice had significantly lower aortic arch elastin fragmentation grade than did Ldlr−/−Ctsg+/+ mice at the early stage, but not at the late stage studied. CatG elastase activity [16, 17] may explain reduced early-stage atherosclerosis in both the thoracic-abdominal aorta and the aortic arch in Ldlr−/−Ctsg−/− mice (Fig. 3A, B). Increased atherosclerosis in the aortic arch in Ldlr−/−Ctsg−/− mice (Fig. 3B) suggests a different mechanism of CatG in atherogenesis, independent of Ang-II production and blood pressure changes. CatG may either promote apoptosis [34, 35], or inhibit this process via Ang-II production [36]. Prior study from CatG-insufficient Apoe−/−Ctsg+/− mice demonstrated reduced apoptosis in atherosclerotic lesions [21]. However, TUNEL staining showed increased apoptosis in atherosclerotic lesions from Ldlr−/−Ctsg−/− mice at both the 3- and 6-month time points (Fig. 4E), suggesting an indirect role of CatG deficiency in apoptosis of cells in atherosclerotic lesions, a hypothesis that merits further investigation. Consistent with increased atherosclerosis in the aortic arch of Ldlr−/−Ctsg−/− mice (Fig. 3B), we detected significantly larger necrotic core areas in atherosclerotic lesions from Ldlr−/−Ctsg−/− mice than those from Ldlr−/−Ctsg+/+ mice at the later (6 months) time point but not at the early (3 months) time point, although necrotic core compartments did not differ significantly between the two groups at both time points (Fig. 4F). To seek the mechanism(s) by which CatG protects against late-stage atherosclerosis in Ldlr−/−Ctsg−/− mice (Fig. 3B, Fig. 4F), we measured mouse plasma lipid profiles and found that the absence of CatG increased plasma levels of total and LDL cholesterol significantly at 6 months, but did not affect these levels at 3 months — nor did it alter plasma triglyceride levels at either time point or HDL cholesterol levels at the 6-month time point (Fig. 5A). Plasma total cholesterol and LDL concentration increases in Ldlr−/−Ctsg−/− mice at a later time point (6 months) suggest that CatG can modulate LDL metabolism. Ldlr−/−Ctsg−/− mice had higher concentrations HDL, however, than Ldlr−/−Ctsg+/+ mice at the 3-month time point (Fig. 5A), in association with reduced atherosclerosis in Ldlr−/−Ctsg−/− mice at this early time point (Fig. 3A, 3B). Increased plasma total or LDL cholesterol levels from Ldlr−/−Ctsg−/− mice did not result from altered liver function, as indicated by the lack of significant differences in plasma albumin and alanine aminotransferase levels of Ldlr−/−Ctsg−/− mice and Ldlr−/−Ctsg+/+ mice either at the 3-month or 6-month time points (Fig. 5B). These observations suggest that increased atherosclerotic lesion sizes at the 6-month time point were resulted at least in part from increased plasma total and LDL cholesterol levels.
Fig. 4.
Mouse aortic arch atherosclerotic lesion characterization. Aortic arch lesion Mac-3+ macrophage areas, CD4+ T-cell numbers, and MHC class-II-positive areas (A), SMC and collagen contents (B), oil-red O-positive areas (C), elastin fragmentation grades (D), TUNEL-positive cell numbers (E), and lesion necrotic core compartments and areas (F) from Ldlr−/−Ctsg−/− mice and Ldlr−/−Ctsg+/+ mice fed a Western diet for 3 months and 6 months. Elastin fragmentation grading keys (arrows indicate elastica breaks) are shown to the right of panel D, and representative data for TUNEL staining and necrotic cores are shown to right of panels E and F. Data are mean ± SE. Number of mice per experimental group is indicated in each bar.
Fig. 5.
CatG deficiency raises plasma LDL but not HDL levels in LDL receptor deficient mice. Plasma total cholesterol, triglyceride, LDL, and HDL levels (A), and plasma albumin and ALT (alanine aminotransferase) levels (B) from Ldlr−/−Ctsg−/− mice and Ldlr−/−Ctsg+/+ mice fed a Western diet for 3 months and 6 months. Data are mean ± SE. Number of mice per experimental group is indicated in each bar.
3.4. Correlation between plasma CatG and LDL in patients with atherosclerosis
The increased LDL and total cholesterol in conjunction with accentuated atherosclerosis in Ldlr−/−Ctsg−/− mice well agrees with the well-known pathogenic role of LDL in Ldlr−/−mice. These findings suggested the possibility that CatG also participates in LDL metabolism by decreasing its concentration in humans, and that CatG could thus protect humans from atherogenesis. To test this hypothesis, we measured plasma CatG levels and lipid profiles in patients with acute myocardial infarction (AMI), unstable angina pectoris (UAP), and stable angina pectoris (SAP). Patients with AMI, SAP, and UAP all had significantly lower plasma CatG levels than those without coronary heart diseases (non-CHD group) (Table 1). Pearson’s correlation test demonstrated that plasma CatG levels correlated significantly and negatively with plasma total cholesterol (r = − 0.535, P=1.88E-15) and LDL (r = −0.559, P=4.99E-17), but not with triglyceride (r = − 0.096, P=0.186) or HDL (r = −0.009, P=0.901) (Table 2). Among this population, some received statins to lower plasma lipid levels, a treatment that might alter their associations with plasma CatG levels. Therefore, we performed partial correlation test by adjusting for statin treatment as a controlling variable. The negative correlations between plasma CatG and plasma total cholesterol (r = −0.504, P=1.37E-13) and LDL (r = −0.502, P=1.91E-13) remained significant after adjusting for statin use (Table 2). These human data agree with the observations from experimental atherosclerosis. Low plasma CatG or genetic deficiency of CatG consistently associated with plasma total cholesterol and LDL, but not plasma HDL and triglyceride, in CHD patients (Table 1) or in Ldlr−/−Ctsg−/− mice after consuming a Western diet for 6 months (Fig. 5A)
Table 1.
Clinical characteristics and plasma cathepsin G levels in patients with or without coronary heart disease (CHD).*
| Variables | Non-CHDz (n = 61) |
SAP (n = 45) |
UAP (n = 67) |
AMI (n = 59) |
|---|---|---|---|---|
| Age (years) | 45 (22, 74) | 57 (36, 78)*** | 60 (25, 69)*** | 62 (29, 79)*** |
| Sex (female/male) | 22/39 | 21/24 | 32/35 | 9/50*** |
| Type 2 diabetes mellitus (%) | – | 8.89 | 13.43 | 13.59 |
| Hypertension (%) | – | 62.22 | 71.64 | 50.85 |
| Total cholesterol (mmol/L) | 4.22 (5.59, 2.74) |
4.73** (6.40, 2.56) |
4.89*** (8.46, 2.91) |
4.64** (7.55, 3.03) |
| Triglyceride (mmol/L) | 1.44 (2.18, 0.50) |
1.74*** (4.66, 0.56) |
1.57 (4.29, 0.37) |
2.03*** (7.90, 0.28) |
| LDL (mmol/L) | 2.47 (3.64, 1.08) |
2.81** (4.81, 1.82) |
3.02*** (5.43, 1.72) |
2.77** (5.45, 1.59) |
| HDL (mmol/L) | 1.25 2.07, 0.20) |
1.30 (2.34, 0.68) |
1.18 (2.52, 0.61) |
1.03*** (2.31, 0.35) |
| Cathepsin G (U/mL) | 440.70 (40.00, 1874.70) |
241.90*** (10.45, 1617.70) |
330.00*** (74.05, 2468.00) |
284.90*** (28.60, 1350.45) |
AMI, acute myocardial infarction; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SAP, stable angina pectoris; UAP, unstable angina pectoris.
Values are expressed as median (minimum, maximum) or a percentage.
P < 0.05 compared with the non-CHD group.
P < 0.01 compared with the non-CHD group.
Table 2.
Correlation analysis of plasma cathepsin G with plasma lipid levels among all 232 patients.
| Before adjustment* |
After adjustment** |
|||
|---|---|---|---|---|
| Lipid | Correlation coefficient |
P value | Correlation coefficient |
P value |
| Total cholesterol (mmol/L) | −0.535 | 1.886E-15 | −0.504 | 1.369E-13 |
| Triglyceride (mmol/L) | −0.096 | 0.186 | −0.052 | 0.475 |
| Low-density lipoprotein (mmol/L) | −0.559 | 4.997E-17 | −0.502 | 1.911E-13 |
| High-density lipoprotein (mmol/L) | −0.009 | 0.901 | −0.044 | 0.552 |
Pearson’s correlation analysis was performed before adjusting for lipid lowering (statin) treatment.
Partial correlation test was performed with statin treatment as the controlling variable.
3.5. CatG degrades LDL particles, but not HDL particles
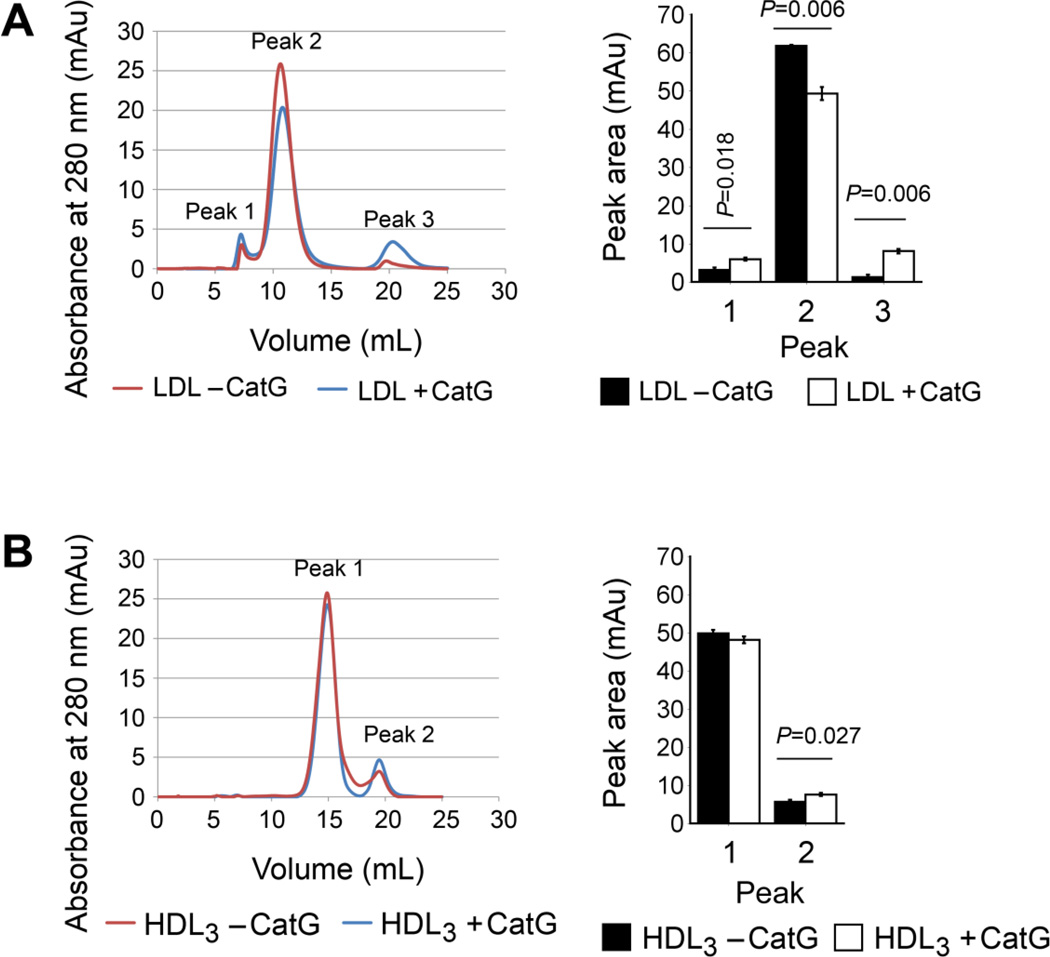
To investigate whether CatG can catabolize plasma lipoproteins, we purified human plasma LDL and HDL3 and incubated these lipoproteins for 18 hours with and without 2 mU/mL human neutrophil CatG, after which the samples underwent FPLC analysis using a Superose 6 column. Brief incubation of LDL with CatG significantly reduced the quantity of native LDL (Peak 2, Fig. 6A). Since a fraction of LDL eluted in the void volume of the column (Peak 1), CatG must have induced aggregation of the protease-treated LDL [37]. Of note, a third peak (Peak 3) appeared after CatG incubation — the material in this peak representing apoB-100 degradation products and eluted CatG. In contrast, incubation of HDL3 with CatG did not significantly reduce the quantity of native HDL3 (Peak 1), while the quantity of HDL3-derived peptides increased slightly (Peak 2, Fig. 6B). Ultracentrifugally isolated native HDL3 fraction contained lower molecular weight material (Peak 2), which we have shown to contain lipid-free and lipid-poor apoA-I detached from the HDL3 particles [38]. Moreover, a portion of the increase in peak 2 can be explained by the presence of eluted CatG. Taken together, while CatG degrades apoB-100 and generates fragments, this enzyme does not alter HDL3 under the conditions used. This finding established a previously unrecognized role of CatG in proteolysis of LDL, but not HDL, and explained increased LDL cholesterol but not HDL cholesterol in Ldlr−/−Ctsg−/− mice and negative association between plasma CatG and LDL cholesterol but not HDL in patients with atherosclerotic coronary artery disease.
Fig. 6.
CatG processes plasma LDL but not HDL. Gel filtration chromatography of purified human plasma LDL (A) and HDL3 (B) before and after incubation for 18 hours with and without 2 mU/ml of CatG. Independent incubations were performed and each sample was analyzed once or twice, resulting in 4–6 chromatograms per sample. The areas of peaks 1–3 (LDL) and peaks 1 and 2 (HDL3) were calculated and presented as mean ± SD. Representative chromatograms of LDL and HDL3 are shown on the left. P<0.05 is considered statistically significant. Mann-Whitney U test.
4. Discussion
As an elastase [16, 17], CatG may promote arterial wall elastinolysis and exacerbate atherosclerosis. An enzyme capable of producing Ang-II [2, 4, 6], CatG may also enhance blood pressures and increase the risk of CHD. This study used CatG-deficient Ldlr−/−Ctsg−/− mice and established a role of CatG in elastin fragmentation at early stage of atherogenesis. Yet, when these mice consumed a Western diet for 6 months, absence of CatG actually enlarged atherosclerotic lesion areas in the aortic arch by nearly 2-fold (Fig. 3B) and increased plasma levels of total cholesterol and LDL cholesterol, but not HDL cholesterol or triglyceride levels (Fig. 5A), although CatG- deficiency still reduced diastolic blood pressure and plasma Ang-II and ACE levels in these mice. The mechanistic studies presented here disclose a heretofore unrecognized role for CatG in the regulation of plasma total and LDL cholesterol levels directly by degrading apoB on LDL (while not affecting HDL), although our study did not prove a role of CatG activity in Ang-II production in systolic or diastolic blood pressure changes during atherogenesis. A recent study using Apoe−/−Ctsg+/+ and CatG-haploinsufficient Apoe−/−Ctsg+/− mice suggested an insignificant contribution of CatG in atherosclerosis in these animals that accumulate primarily beta-VLDL rather than LDL [21]. CatG may behave differently depending on the type of hyperlipidemia, as does the cysteine protease cathepsin S (CatS) which we demonstrated significantly reduces atherosclerosis in CatS-deficient Ldlr−/−Ctss−/− mice [23], but not in Apoe−/−Ctss−/− mice (unpublished data) compared with corresponding CatS-sufficient control mice.
Coronary angiographic studies often dichotomize those with <50% coronary stenosis as “insignificant” CHD. Yet, some 40~50% of acute coronary syndromes occur at sites with <50% luminal narrowing [39–41] and increased stenosis correlates with reduced risk of coronary rupture [42]. Elastin fragmentation correlates with human and experimental atherosclerotic lesion ruptures [43, 44]. CatG appears to contribute to elastin fragmentation at the early stage (3 months) of atherogenesis [16, 17]. At this time point CatG deficiency in Ldlr−/−Ctsg−/− mice showed significantly reduced aortic arch elastin fragmentation, compared to that in CatG-sufficient Ldlr−/−Ctsg+/+ mice (Fig. 4D). In contrast, at a later stage in atherogenesis (6 months), arterial wall elastin fragmentation increased over time in Ldlr−/−Ctsg−/− mice, but showed no significant difference between Ldlr−/−Ctsg−/− and Ldlr−/−Ctsg+/+ mice, suggesting that elastin fragmentation by other elastinolytic proteases such as cysteinyl cathepsins and MMPs becomes dominant in more advanced atherosclerotic lesions [45, 46]. Therefore, CatG elastinolytic activity in non-flow-limiting lesions may contribute to their propensity to rupture in humans, a hypothesis that merits detailed investigation.
An LDL-lowering effect of CatG could protect animals and humans from atherosclerosis, a hypothesis supported by several observations from this study and prior studies. In mice, CatG deficiency accentuated late-stage atherosclerosis, in association with increased plasma LDL and total cholesterol levels (Fig. 5A). Human patients with CHD had significantly lower plasma CatG levels than did those without CHD (Table 1), and plasma CatG levels correlated significantly and negatively with plasma LDL and total cholesterol levels before and after adjusting for lipid-lowering medications (Table 2). In vitro, sub-physiological concentration of human neutrophil CatG degraded purified human LDL (Fig. 6A). This pathway of CatG in contributing to plasma LDL level changes may not operate in the presence of plasma protease inhibitors — such as α1-antichymotrypsin, an endogenous inhibitor of CatG (and of neutrophil elastase and mast cell chymase) derived from mast cells, neutrophils [47], hepatocytes, bronchial epithelial cells, and neuronal cells [48, 49]. But several lines of evidence support the hypothesis that plasma α1-anti-chymotrypsin [50, 51] has limited inhibitory activity on CatG-mediated LDL catabolism in vivo. First, a fraction of CatG from human polymorphonuclear neutrophils [52], and probably other inflammatory cells and vascular cells [53], may remain bound to cell-surface negatively charged heparin proteoglycans. Such cell-surface-bound CatG is catalytically active and remarkably resistant to naturally occurring inhibitors from the plasma [29]. Second, other cell surface heparin proteoglycan-bound proteases, such as chymase, have proven most effective in LDL and HDL degradation when heparin-bound [54, 55]. After separation from heparin proteoglycan, chymase becomes susceptible to physiological chymase inhibitors present in blood plasma [56]. Third, ApoB-100 of LDL interacts nonspecifically with the negatively charged glycosaminoglycan chains of proteoglycan in the aortic intima at sites of cholesterol accumulation [57, 58], and probably on the surface of CatG-positive cells in the circulation. This protection from endogenous inhibitors may facilitate ApoB proteolysis by proteoglycan-bound CatG. In addition to inflammatory cells, vascular cell CatG may also mediate LDL catabolism in a similar manner. Subendothelial retention of LDL by binding to intimal proteoglycan occurs during atherogenesis [53], and ECs from human atherosclerotic lesions contain CatG (Fig. 1B). Significantly lower systemic CatG in CHD patients compared with non-CHD patients (Table 1) may contribute directly to enhanced plasma LDL levels (Fig, 5A, Table 1), thereby exacerbating atherosclerotic lesion progression, as observed in the Ctsg−/− mice (Fig. 3B).
Although CatG-mediated extracellular elastin degradation may contribute to atherogenesis, increased levels of the CatG inhibitor α1-anti-chymotrypsin in human and experimental atherosclerotic lesions [59] may limit secreted CatG’s contribution to elastin degradation in patients and animals with advanced atherosclerosis. In contrast, proteolysis of LDL by cell surface-associated CatG in various compartments protects the enzyme from α1-anti-chymotrypsin.
5. Conclusion
The results of this study reveal several novel cellular sources of CatG expression in addition to mast cells and neutrophils in atherosclerotic lesions, notably SMCs, ECs, macrophages, and T cells. The results also disclose novel roles of CatG in elastin degradation and cholesterol metabolism, providing mechanistic insight into the diverse and previously unknown functions of this serine proteinase in atherosclerosis.
Highlights.
This study demonstrates increased expression of CatG in human atherosclerotic lesions.
CatG contributes to experimental atherogenesis differently at different stages.
CatG can degrade arterial wall elastin to promote early stage atherogenesis.
CatG can also degrade plasma LDL to reduce late stage atherogenesis.
Acknowledgements
The authors thank Drs. Timothy J. Ley, M.D. and Christine T. Pham, M.D. from Washington University Medical School, St Louis, MO 63110, USA, for providing the cathepsin G-deficient mice, Wendy Yu and Eugenia Shvartz for technical assistance, and Sara Karwacki for editorial assistance. This study is supported by grants from the National Institutes of Health (HL60942, HL81090, HL88547, to G.P.S.; HL56985, to P.L.), the Academy of Finland (265940 to K.Ö.), and by an EIA award (0840118N) from the American Heart Association (to GPS). Wihuri Research Institute is maintained by the Jenny and Antti Wihuri Foundation. Dr. Sara Sjöberg was supported by the Swedish research council #K2010-78PK-21625-01-2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Helske S, Syvaranta S, Kupari M, Lappalainen J, Laine M, Lommi J, Turto H, Mayranpaa M, Werkkala K, Kovanen PT, Lindstedt KA. Possible role for mast cell-derived cathepsin G in the adverse remodelling of stenotic aortic valves. Eur Heart J. 2006;27:1495–1504. doi: 10.1093/eurheartj/ehi706. [DOI] [PubMed] [Google Scholar]
- 2.Owen CA, Campbell EJ. Angiotensin II generation at the cell surface of activated neutrophils: novel cathepsin G-mediated catalytic activity that is resistant to inhibition. J Immunol. 1998;160:1436–1443. [PubMed] [Google Scholar]
- 3.Lindstedt KA, Mayranpaa MI, Kovanen PT. Mast cells in vulnerable atherosclerotic plaques--a view to a kill. J Cell Mol Med. 2007;11:739–758. doi: 10.1111/j.1582-4934.2007.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W, Drexler H. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101:1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 6.Mazzolai L, Duchosal MA, Korber M, Bouzourene K, Aubert JF, Hao H, Vallet V, Brunner HR, Nussberger J, Gabbiani G, Hayoz D. Endogenous angiotensin II induces atherosclerotic plaque vulnerability and elicits a Th1 response in ApoE-/- mice. Hypertension. 2004;44:277–282. doi: 10.1161/01.HYP.0000140269.55873.7b. [DOI] [PubMed] [Google Scholar]
- 7.Daien V, Duny Y, Ribstein J, du Cailar G, Mimran A, Villain M, Daures JP, Fesler P. Treatment of hypertension with renin-angiotensin system inhibitors and renal dysfunction: a systematic review and meta-analysis. Am J Hypertens. 2012;25:126–132. doi: 10.1038/ajh.2011.180. [DOI] [PubMed] [Google Scholar]
- 8.Kranzhofer R, Schmidt J, Pfeiffer CA, Hagl S, Libby P, Kubler W. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1623–1629. doi: 10.1161/01.atv.19.7.1623. [DOI] [PubMed] [Google Scholar]
- 9.Okada Y, Gonoji Y, Naka K, Tomita K, Nakanishi I, Iwata K, Yamashita K, Hayakawa T. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells. Purification and activation of the precursor and enzymic properties. J Biol Chem. 1992;267:21712–21719. [PubMed] [Google Scholar]
- 10.Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, Mignatti P. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189:197–206. doi: 10.1002/jcp.10014. [DOI] [PubMed] [Google Scholar]
- 11.Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118:2325–2340. doi: 10.1242/jcs.02360. [DOI] [PubMed] [Google Scholar]
- 12.Okada Y, Nakanishi I. Activation of matrix metalloproteinase 3 (stromelysin) and matrix metalloproteinase 2 (‘gelatinase’) by human neutrophil elastase and cathepsin G. FEBS Lett. 1989;249:353–356. doi: 10.1016/0014-5793(89)80657-x. [DOI] [PubMed] [Google Scholar]
- 13.Son ED, Kim H, Choi H, Lee SH, Lee JY, Kim S, Closs B, Lee S, Chung JH, Hwang JS, Cathepsin Gincreases MMP expression in normal human fibroblasts through fibronectin fragmentation. and induces the conversion of proMMP-1 to active MMP-1. J Dermatol Sci. 2009;53:150–152. doi: 10.1016/j.jdermsci.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2008;28:2108–2114. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 15.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 16.Boudier C, Godeau G, Hornebeck W, Robert L, Bieth JG. The elastolytic activity of cathepsin G: an ex vivo study with dermal elastin. Am J Respir Cell Mol Biol. 1991;4:497–503. doi: 10.1165/ajrcmb/4.6.497. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Sukhova GK, Liu J, Ozaki K, Lesner A, Libby P, Kovanen PT, Shi GP. Cathepsin G deficiency reduces peri-aortic calcium chloride injury-induced abdominal aortic aneurysms in mice. J Vasc Surg. 2014 doi: 10.1016/j.jvs.2014.06.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatham WW, Blackburn WD, Jr., Heck LW. Additive enhancement of neutrophil collagenase activity by HOCl and cathepsin G. Biochem Biophys Res Commun. 1992;184:560–567. doi: 10.1016/0006-291x(92)90626-v. [DOI] [PubMed] [Google Scholar]
- 19.Kaschina E, Scholz H, Steckelings UM, Sommerfeld M, Kemnitz UR, Artuc M, Schmidt S, Unger T. Transition from atherosclerosis to aortic aneurysm in humans coincides with an increased expression of RAS components. Atherosclerosis. 2009;205:396–403. doi: 10.1016/j.atherosclerosis.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Mayranpaa MI, Heikkila HM, Lindstedt KA, Walls AF, Kovanen PT. Desquamation of human coronary artery endothelium by human mast cell proteases: implications for plaque erosion. Coron Artery Dis. 2006;17:611–621. doi: 10.1097/01.mca.0000224420.67304.4d. [DOI] [PubMed] [Google Scholar]
- 21.Rafatian N, Karunakaran D, Rayner KJ, Leenen FH, Milne RW, Whitman SC. Cathepsin G deficiency decreases complexity of atherosclerotic lesions in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol. 2013;305:H1141–H1148. doi: 10.1152/ajpheart.00618.2012. [DOI] [PubMed] [Google Scholar]
- 22.MacIvor DM, Shapiro SD, Pham CT, Belaaouaj A, Abraham SN, Ley TJ. Normal neutrophil function in cathepsin G-deficient mice. Blood. 1999;94:4282–4293. [PubMed] [Google Scholar]
- 23.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 25.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radding CM, Steinberg D. Studies on the synthesis and secretion of serum lipoproteins by rat liver slices. J Clin Invest. 1960;39:1560–1569. doi: 10.1172/JCI104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayranpaa MI, Trosien JA, Fontaine V, Folkesson M, Kazi M, Eriksson P, Swedenborg J, Hedin U. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. J Vasc Surg. 2009;50:388–395. doi: 10.1016/j.jvs.2009.03.055. discussion 395–386. [DOI] [PubMed] [Google Scholar]
- 28.Lindmark A, Gullberg U, Olsson I. Processing and intracellular transport of cathepsin G and neutrophil elastase in the leukemic myeloid cell line U-937-modulation by brefeldin A, ammonium chloride and monensin. J Leukoc Biol. 1994;55:50–57. doi: 10.1002/jlb.55.1.50. [DOI] [PubMed] [Google Scholar]
- 29.Owen CA, Campbell MA, Sannes PL, Boukedes SS, Campbell EJ. Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol. 1995;131:775–789. doi: 10.1083/jcb.131.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas MP, Whangbo J, McCrossan G, Deutsch AJ, Martinod K, Walch M, Lieberman J. Leukocyte protease binding to nucleic acids promotes nuclear localization and cleavage of nucleic acid binding proteins. J Immunol. 2014;192:5390–5397. doi: 10.4049/jimmunol.1303296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y, Wang W, Zhang J, Lu Y, Wu W, Yan H, Wang Y. Hyperlipidemia and atherosclerotic lesion development in Ldlr-deficient mice on a long-term high-fat diet. PLoS One. 2012;7:e35835. doi: 10.1371/journal.pone.0035835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choy K, Beck K, Png FY, Wu BJ, Leichtweis SB, Thomas SR, Hou JY, Croft KD, Mori TA, Stocker R. Processes involved in the site-specific effect of probucol on atherosclerosis in apolipoprotein E gene knockout mice. Arterioscler Thromb Vasc Biol. 2005;25:1684–1690. doi: 10.1161/01.ATV.0000174125.89058.b6. [DOI] [PubMed] [Google Scholar]
- 33.Sukhova GK, Wang B, Libby P, Pan JH, Zhang Y, Grubb A, Fang K, Chapman HA, Shi GP. Cystatin C deficiency increases elastic lamina degradation and aortic dilatation in apolipoprotein E-null mice. Circ Res. 2005;96:368–375. doi: 10.1161/01.RES.0000155964.34150.F7. [DOI] [PubMed] [Google Scholar]
- 34.Biggs JR, Yang J, Gullberg U, Muchardt C, Yaniv M, Kraft AS. The human brm protein is cleaved during apoptosis: the role of cathepsin G. Proc Natl Acad Sci U S A. 2001;98:3814–3819. doi: 10.1073/pnas.071057398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabri A, Alcott SG, Elouardighi H, Pak E, Derian C, Andrade-Gordon P, Kinnally K, Steinberg SF. Neutrophil cathepsin G promotes detachment-induced cardiomyocyte apoptosis via a protease-activated receptor-independent mechanism. J Biol Chem. 2003;278:23944–23954. doi: 10.1074/jbc.M302718200. [DOI] [PubMed] [Google Scholar]
- 36.Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens. 1999;12:205S–213S. doi: 10.1016/s0895-7061(99)00103-x. [DOI] [PubMed] [Google Scholar]
- 37.Oorni K, Pentikainen MO, Ala-Korpela M, Kovanen PT, Aggregation fusion. and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactions. J Lipid Res. 2000;41:1703–1714. [PubMed] [Google Scholar]
- 38.Lee M, Metso J, Jauhiainen M, Kovanen PT. Degradation of phospholipid transfer protein (PLTP) and PLTP-generated pre-beta-high density lipoprotein by mast cell chymase impairs high affinity efflux of cholesterol from macrophage foam cells. J Biol Chem. 2003;278:13539–13545. doi: 10.1074/jbc.M210847200. [DOI] [PubMed] [Google Scholar]
- 39.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 40.van der Wal AC, Becker AE. Atherosclerotic plaque rupture--pathologic basis of plaque stability and instability. Cardiovasc Res. 1999;41:334–344. doi: 10.1016/s0008-6363(98)00276-4. [DOI] [PubMed] [Google Scholar]
- 41.Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, Virmani R. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 42.Cheng GC, Loree HM, Kamm RD, Fishbein MC, Lee RT. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation. 1993;87:1179–1187. doi: 10.1161/01.cir.87.4.1179. [DOI] [PubMed] [Google Scholar]
- 43.Van der Donckt C, Van Herck JL, Schrijvers DM, Vanhoutte G, Verhoye M, Blockx I, Van Der Linden A, Bauters D, Lijnen HR, Sluimer JC, Roth L, Van Hove CE, Fransen P, Knaapen MW, Hervent AS, De Keulenaer GW, Bult H, Martinet W, Herman AG, De Meyer GR. Elastin fragmentation in atherosclerotic mice leads to intraplaque neovascularization, plaque rupture, myocardial infarction, stroke, and sudden death. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson JL, Jackson CL. Atherosclerotic plaque rupture in the apolipoprotein E knockout mouse. Atherosclerosis. 2001;154:399–406. doi: 10.1016/s0021-9150(00)00515-3. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1359–1366. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 46.Luttun A, Lutgens E, Manderveld A, Maris K, Collen D, Carmeliet P, Moons L. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein E-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation. 2004;109:1408–1414. doi: 10.1161/01.CIR.0000121728.14930.DE. [DOI] [PubMed] [Google Scholar]
- 47.Horvath AJ, Irving JA, Rossjohn J, Law RH, Bottomley SP, Quinsey NS, Pike RN, Coughlin PB, Whisstock JC. The murine orthologue of human antichymotrypsin: a structural paradigm for clade A3 serpins. J Biol Chem. 2005;280:43168–43178. doi: 10.1074/jbc.M505598200. [DOI] [PubMed] [Google Scholar]
- 48.Chandra T, Stackhouse R, Kidd VJ, Robson KJ, Woo SL. Sequence homology between human alpha 1-antichymotrypsin, alpha 1-antitrypsin, and antithrombin III. Biochemistry. 1983;22:5055–5061. doi: 10.1021/bi00291a001. [DOI] [PubMed] [Google Scholar]
- 49.Schechter NM, Jordan LM, James AM, Cooperman BS, Wang ZM, Rubin H. Reaction of human chymase with reactive site variants of alpha 1-antichymotrypsin. Modulation of inhibitor versus substrate properties. The Journal of biological chemistry. 1993;268:23626–23633. J. Biol. Chem. 268: 23626–23633. [PubMed] [Google Scholar]
- 50.Patston PA. Studies on inhibition of neutrophil cathepsin G by alpha 1-antichymotrypsin. Inflammation. 1995;19:75–81. doi: 10.1007/BF01534382. [DOI] [PubMed] [Google Scholar]
- 51.Hollander C, Westin U, Wallmark A, Piitulainen E, Sveger T, Janciauskiene SM. Plasma levels of alpha1-antichymotrypsin and secretory leukocyte proteinase inhibitor in healthy and chronic obstructive pulmonary disease (COPD) subjects with and without severe alpha1-antitrypsin deficiency. BMC Pulm Med. 2007;7:1. doi: 10.1186/1471-2466-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owen CA, Campbell MA, Boukedes SS, Campbell EJ. Inducible binding of bioactive cathepsin G to the cell surface of neutrophils. A novel mechanism for mediating extracellular catalytic activity of cathepsin G. J Immunol. 1995;155:5803–5810. [PubMed] [Google Scholar]
- 53.Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Boren J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 54.Lindstedt L, Lee M, Castro GR, Fruchart JC, Kovanen PT, Chymase in exocytosed rat mast cell granules effectively proteolyzes apolipoprotein AI-containing lipoproteins. so reducing the cholesterol efflux-inducing ability of serum and aortic intimal fluid. J Clin Invest. 1996;97:2174–2182. doi: 10.1172/JCI118658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paananen K, Kovanen PT. Proteolysis and fusion of low density lipoprotein particles independently strengthen their binding to exocytosed mast cell granules. J Biol Chem. 1994;269:2023–2031. [PubMed] [Google Scholar]
- 56.Lindstedt L, Lee M, Kovanen PT. Chymase bound to heparin is resistant to its natural inhibitors and capable of proteolyzing high density lipoproteins in aortic intimal fluid. Atherosclerosis. 2001;155:87–97. doi: 10.1016/s0021-9150(00)00544-x. [DOI] [PubMed] [Google Scholar]
- 57.Vijayagopal P, Srinivasan SR, Radhakrishnamurthy B, Berenson GS. Interaction of serum lipoproteins and a proteoglycan from bovine aorta. J Biol Chem. 1981;256:8234–8241. [PubMed] [Google Scholar]
- 58.Camejo G. The interaction of lipids and lipoproteins with the intercellular matrix of arterial tissue: its possible role in atherogenesis. Adv Lipid Res. 1982;19:1–53. doi: 10.1016/b978-0-12-024919-0.50007-2. [DOI] [PubMed] [Google Scholar]
- 59.Wagsater D, Johansson D, Fontaine V, Vorkapic E, Backlund A, Razuvaev A, Mayranpaa MI, Hjerpe C, Caidahl K, Hamsten A, Franco-Cereceda A, Wilbertz J, Swedenborg J, Zhou X, Eriksson P. Serine protease inhibitor A3 in atherosclerosis and aneurysm disease. Int J Mol Med. 2012;30:288–294. doi: 10.3892/ijmm.2012.994. [DOI] [PubMed] [Google Scholar]