Abstract
Extracellular matrix proteins form the basic structure of blood vessels. Along with providing basic structural support to blood vessels, matrix proteins interact with different sets of vascular cells via cell surface integrin or non-integrin receptors. Such interactions induce vascular cell de novo synthesis of new matrix proteins during blood vessel development or remodeling. Under pathological conditions, vascular matrix proteins undergo proteolytic processing, yielding bioactive fragments to influence vascular wall matrix remodeling. Vascular cells also produce alternatively spliced variants that induce vascular cell production of different matrix proteins to interrupt matrix homeostasis, leading to increased blood vessel stiffness; vascular cell migration, proliferation, or death; or vascular wall leakage and rupture. Destruction of vascular matrix proteins leads to vascular cell or blood-borne leukocyte accumulation, proliferation, and neointima formation within the vascular wall; blood vessels prone to uncontrolled enlargement during blood flow diastole; tortuous vein development; and neovascularization from existing pathological tissue microvessels. Here we summarize discoveries related to blood vessel matrix proteins within the past decade from basic and clinical studies in humans and animals — from expression to cross-linking, assembly, and degradation under physiological and vascular pathological conditions, including atherosclerosis, aortic aneurysms, varicose veins, and hypertension.
Keywords: extracellular matrix proteins, elastin, collagen, atherosclerosis, aortic aneurysm, varicose vein, hypertension
1. Introduction
Blood vessels deliver oxygen and nutrients to body tissues. The major constituent of the vessel wall is extracellular matrix (ECM), collectively known as stroma or matrix. In arteries or veins, the ECM constitutes more than half of the wall mass and contains mainly collagens and elastin. Other vascular wall constituents include fibronectin, microbifrils (mainly fibrillins), abundant amorphous or soluble proteoglycans, and leucine-rich glycoproteins. The normal blood vessel wall contains several functionally distinct types of vascular matrices, including subendothelial basement membrane, intima, media, adventitia, and interstitial matrix. Each of these vessel sections contains different types of cells and matrix proteins.
1.1. Vascular wall ECM components
All vessel lumens are lined with endothelial cells (ECs) that are anchored on an underlying basement membrane, a thin sheet-like structure containing mainly laminin, type IV collagen, nidogen, perlecan, type XV and type XVIII collagens, fibronectin, the heparin sulfate proteoglycan perlecan, and other macromolecules [1–3]. At least 20 ECM proteins have been identified from basement membrane preparations. Most of these proteins, if not all, have tissue-specific functions. Underneath the basement membrane is the intima, which separates ECs from the internal elastic laminae (IEL) formed by several layers of contractile vascular smooth muscle cells (SMCs) and separated by elastic fibers and collagen-rich ECM. Under the basement membrane, normal vessels contain minimal intima, and instead contain media beginning at the IEL, followed by concentric lamellar units composed of elastic fibers and SMCs separated by interlaminar matrix collagens, microfibrils, proteoglycans, glycoproteins, and ground substance [4] — although the media components can be different between different types of blood vessels. Arteries, for example, have more collagens and elastin than veins have. Outside the SMC layer of large vessels is an adventitial layer extending beyond the external elastic laminae and interstitial matrix that contains fibrillar type I and III collagen, chondroitin sulfate and dermatan sulfate proteoglycans, fibronectin, and many other ECM proteins.
The same ECM proteins at different regions of the blood vessel wall may come from different cells and be regulated by different modulators under certain circumstances. Collagen and elastin in the media are produced primarily by SMCs. TGF-β1 stimulates SMC proliferation, migration, and ECM expression, leading to luminal narrowing [5]. Attenuating TGF-β1 activity with tranilast, TGF-β3, or directly with TGF-β1 inhibitors diminishes intima SMC proliferation-associated thickening, often called blood vessel stenosis or restenosis [6, 7]. In the adventitia, however, ECMs — such as collagen, osteopontin, and fibronectin — primarily come from fibroblasts, as in other connective tissues. In cultured rat adventitial fibroblasts, vascular endothelial growth factor (VEGF) regulates the expression of osteopontin, an integrin recognition sequence arginine-glycine-asparagine (RGD)-containing ECM phosphoprotein that mediates leukocyte cell adhesion and migration, and prevents cell apoptosis.
Different ECM proteins form different types of blood vessels. Mature vessel wall ECM is a complex arrangement of fibrous proteins, associated with glycoproteins embedded in a hydrated ground substance of glycosaminoglycans and proteoglycans. Normal large arteries also contain collagen, elastin, fibronectin, and small amounts of osteopontin, thrombospondin, and tenascin. Vessel wall remodeling occurs as an adaptation to pressure and flow (e.g., vein graft) or to mechanical (e.g., angioplasty) or biochemical (e.g., atherosclerosis) injuries, all of which promote ECM-regulated SMC migration and proliferation [8]. Arterial SMCs also synthesize vitronectin. Normal quiescent vessels contain only low levels of interstitial vitronectin and fibronectin. Vitronectin receptor αvβ3 and αvβ5 integrins, and fibronectin receptor αvβ3 and α5β1 integrins, are suppressed in quiescent SMCs [9]. In normal arteries, apolipoprotein E (ApoE) and ApoE-containing high-density lipoprotein (HDL) maintain arterial elasticity by controlling the expression of ECM, reducing the expression of collagen-1, fibronectin, and the elastin/collagen cross-linking enzyme lysyl oxidase in response to substratum stiffening. In angiogenic vessels, however, fibronectin also becomes a predominant constituent of the endothelial basement membrane. Angiogenic vessels, but not quiescent vessels, also contain fibronectin alternative spliced variants: extra domain-A (ED-A) and extra domain-B (ED-B).
1.2. ECM functions
ECM is not an inert supporting network, but rather an active and dynamic structure with a fundamental role in regulating vascular function in normal and pathological conditions. One ECM protein may regulate the production of others. Homeostasis of the vascular ECM may affect intrinsic properties of the arterial wall and arterial stiffness. When elastin scaffold and collagen scaffold were prepared from porcine ascending aortas [10], and implanted subdermally into live rats for 28 days, porcine elastin scaffolds contained enhanced rat collagen fibers and bundles, and porcine collagen scaffolds contained elevated rat elastin fibers — indicating that elastin and collagen support de novo ECM synthesis [11].
Cellular interaction with ECM regulates cell adhesion, migration, proliferation, phenotype, and tissue architecture under different circumstances. From in vitro prepared SMCs, total insoluble ECM proteins stimulate RAW264.7 or thioglycolate- elicited macrophage extracellular signal regulated kinase-1/2 (EKR1/2) activation, cyclooxygenase (COX)-2 and prostaglandin (PG) E2 synthesis, and protease (e.g., urokinase plasminogen activator [uPA] and matrix metalloproteinase [MMP]-9) expression. The selective COX-2 inhibitor NS398 blocked ECM-induced protease expression. Macrophages from COX2-deficient mice showed reduced responses to SMC-ECM, demonstrating that COX-2 is an ECM target [12]. Vascular cells use matrix receptors such as integrins to detect changes in matrix rigidity and composition that occur during tissue remodeling. The resulting intracellular signaling then regulates cellular processes such as proliferation, survival, differentiation, and gene expression [13]. The basal laminae proteins collagen-IV, laminin, and perlecan limit SMC growth, enhance contractile gene expression, reduce inflammatory gene expression, reduce low-density lipoprotein (LDL) uptake in culture, and inhibit matrix calcification. In contrast, interstitial matrix proteins — such as collagen-I, collagen-III, fibronectin, and osteopontin — enhance SMC growth concomitant with elevated ERK phosphorylation and expression of cell cycle regulators [14, 15]. Inhibiting the integrins that bind to these interstitial matrix proteins sufficiently blocks SMC proliferation in response to platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and basic fibroblast growth factor (bFGF) [16], and reduces migration and neointima formation in vivo [17]. Collagen-IV, polymerized collagen-I, proteoglycans, and media elastin limit SMC growth and promote a contractile phenotype, whereas monomeric collagen-I reduces contractile gene expression.
As we will discuss further later, damage to ECM components contributes to the development of vascular diseases. ECM components such as elastin and proteoglycans undergo fragmentation or physiochemical alteration during atherogenesis. Chemically modified ECM or enzymatically degraded ECM may change the activities of parental ECM, thereby promoting ECM remodeling and vascular disease pathogenesis. Many ECM subdomains have roles independent of the parental ECM molecules; they therefore are often called matrikines, and have pathophysiological functions. Fragments from the noncollagenous (NC1) domain of the type IV collagen α1, α2, and α3 chains (also known as arresten, canstatin, tumstatin), type XV collagen (also known as restin), and type VXIII collagen (also called endostatin) all have anti-angiogenic activities to block neovascularization [18, 19]. Production of these matrikines directly affects the angiogenesis that plays physiological roles in embryogenesis and pathological roles in tumor growth, atherogenesis, abdominal aortic aneurysms (AAAs), varicose veins, hypertension, and many other large and small artery and vein disorders.
2. Elastin
Mature elastin is an insoluble and hydrophobic protein formed by cross-linking of its precursor, tropoelastin — a 68~74 kDa monomeric protein from elastin mRNA alternative splicing normally produced by SMCs in the media and by fibroblasts in the adventitia, released to the extracellular space for cross-linking and elastin fiber formation with the assistance of lysyl oxidase and the helper proteins fibulin-4 or -5. Elastin deposition is limited to the media layer extending from the internal to external elastin laminae. Elastin is the dominant ECM in the arterial wall, comprising 50% of its dry weight [20], and is the largest component of elastic fiber, comprising ~90% of elastic fiber total weight. Elastin fiber consists of fibrillin microfibrils and is embedded within an amorphous core of elastin that allows the elastic recoil. Arteries are subject to extensive mechanical stress induced by arterial blood pressure. In addition to mechanical integrity, elastic laminae contribute to the elasticity of the arteries. Recoil of the arterial wall therefore is a critical mechanism for the continuation of blood flow during diastole when cardiac ejection is ceased. Fibrillin-rich microfibrils provide a structural scaffold to guide elastin deposition and assembly. Elastic fibers are found throughout the vessel wall in the medial layer, where they arrange in concentric fenestrated elastic laminae. Each elastic laminae alternates, and is physiologically connected with a concentric ring of SMCs, forming the lamellar unit — the functional resilient unit of the arterial wall [21, 22].
Under normal conditions, elastogenesis is restricted mainly to fetal life and infancy, and mature elastic fibers last for the entire lifespan. The half-life of elastin fibers is about 40 years; elastic fibers are considered the most durable element of ECM [23]. Elastic fibers are degraded and fragmented with age and disease, leading to increased stiffness of the arterial wall [24]. Under pathological conditions, vascular cells (SMCs, ECs, and fibroblasts) make elastin as part of the reaction to increased mechanical stress [25]. In addition to vascular cells, inflammatory cells also produce tropoelastin, but these tropoelastins fail to cross-link into elastic fibers [26].
2.1. Elastin expression, cross-linking, and assembly
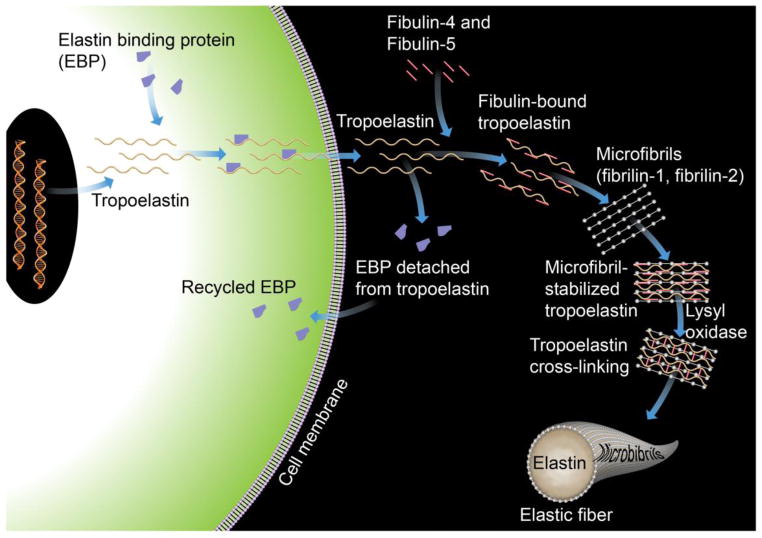
The human tropoelastin gene is located on chromosome 7. Its expression can be regulated differently in response to different cytokines, growth factors, or other bioactive molecules. Insulin-like growth factor (ILGF), transforming growth factor (TGF)-β1, cGMP, and nitric oxide (NO) all enhance elastin gene expression, whereas bFGF, EGF-like growth factor, interleukin (IL)-1β, angiotensin (Ang)-II, hypoxia, and age suppress elastin expression. After synthesis, tropoelastin traffics from the cytoplasm to the extracellular space for cross-linking and assembly, a process mediated by a 67 kDa elastin-binding protein (EBP), which protects tropoelastin from intracellular aggregation and proteolysis. After finishing its delivery, EBP is recycled to the cytoplasm to chaperon the next tropoelastin molecule [27] (Figure 1).
Figure 1.
Tropoelastin synthesis, binding with elastin-binding protein (EBP), transport, release of EBP, assembly with fibulins, binding to microfibrils, lysyl oxidase-mediated cross-linking, and final formation of an elastic fiber with microfibrils.
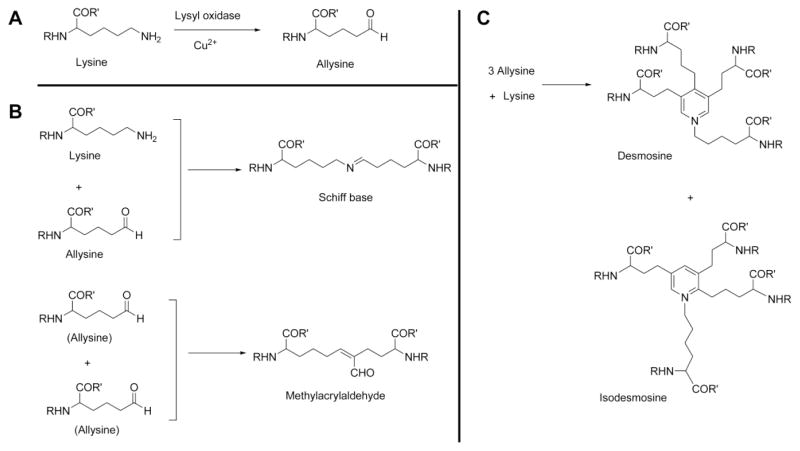
The primary structure of tropoelastin contains hydrophobic domains that include the non-polar amino acids glycine, valine, proline, and alanine, and cross-linking domains that are rich in alanine and lysine. All lysines on tropoelastin are subjected to oxidative deamination or cross-linking by lysyl oxidase [28]. The copper-dependent enzyme lysyl oxidase subjects the epsilon-amino group of the targeted lysine to deamination, producing the alpha-amino adipic delta-semialdehyde allysine [29] (Figure 2A). Under non-enzymatic condensation, one lysine molecule and one allysine molecule form a Shiff base lysine–allysine dimer, whereas two molecules of allysine form an allysine–allysine methylacrylaldehyde derivative (Figure 2B). Three allysine and one lysine form desmosine or isodesmosine (Figure 2C); both are markers of elastin cross-linking. After cross-linking, tropoelastin forms polymers that constitute the concentric rings of elastic lamellae around the arterial lumen. Such cross-links help organize the tropoelastin peptide chains into a filamentous network structure for the storage of recoiling energy under mechanical stress. Elastin assembly occurs at microfibrils that contain non-elastin components of the elastin fibers [30], such as fibrillins and microfibril-associated glycoproteins (MAGPs). These microfibrils provide a scaffold for tropoelastin deposition, alignment, and cross-linking [20]. After the organized deposition of tropoelastin at the microfibril, the final enzymatic cross-linking process ensues (Figure 1).
Figure 2.
Elastin cross-linking. A. Lysine oxidation and allysine formation. B. One lysine and one allysine form a Schiff base; two allysines together form a methylacrylaldehyde derivative. C. One lysine and three allysines cross-link into desmosine or isodesmosine.
2.2. Elastin activities
Elastin acts as more than an arterial wall structural protein for the storage of recoiling energy. In vitro, many cells exhibit migration and proliferation in response to tropoelastin, elastin degradation products, and elastin peptides. Unlike other ECM components, such as collagen, fibronectin, and laminin, elastin does not interact with integrin [31]. Vascular cells such as SMCs, and likely other cell types as well, express a non-integrin elastin/laminin receptor, which directly interacts with elastin and mediates elastin-induced cellular activities [32]. This elastin receptor has three subunits: 67 kDa EBP, 61 kDa neuraminidase, and 55 kDa protective protein [31]. Elastin may use this receptor to regulate target cell migration, chemotaxis, proliferation, myofibrillar organization, and anti-inflammatory activities.
Elastin elicits inhibitory effects on SMC migration and proliferation. In vitro, SMC proliferation and migration on collagen gels were significantly reduced by elastin degradation peptides in a dose-dependent manner [33]. SMC migration was suppressed on elastin peptide-coated surfaces, compared with those cultured on surfaces coated with collagen or fibronectin [34]. SMCs grown on elastin-coated substrates also proliferated much less than those grown on uncoated substrates [35]. In vivo, elastic fibers and laminae prohibited SMC proliferation and prevented intimal hyperplasia [36]. In a rat model of adventitial implantation of collagen, basal lamina, and elastic laminae patches, elastic laminae patches — but not laminin or collagen patches — associated with reduced neointima formation and SMC proliferation [37]. In a porcine coronary model of in-stent restenosis, stents coated with elastin sheaths prepared from carotid artery digestion elicited inflammatory and thrombotic responses lower than those from uncoated stents [38]. Growing vascular SMCs on elastin preserved the quiescent, contractile phenotypes, as shown by the presence of contractile myofilaments [39]. Elastin therefore regulates vascular SMC migration, induces contractile phenotypes, and inhibits proliferation. An elastin cleavage product called elastin-derived peptide (EDP) is also biologically active [40]. Although mature elastin fibers are key elements in the maintenance of a quiescent vascular SMC phenotype by providing a physical barrier for cellular migration, EDP binds to elastin/laminin receptors [41] and stimulates vasculature proliferation and migration. EDP also promotes the release and activation of MMPs from infiltrated leukocytes [42].
Tropoelastin and elastic fibers induce vascular SMC myofibrillar organization by activating a novel heterotrimeric G protein-coupled signaling pathway that induces actin fiber formation via intracellular Rho GTPase. On tropoelastin, a discrete hexapeptide domain, VGVAPG, is sufficient to induce vascular SMC myofibrillar organization. The hexapeptide domain stimulates a signaling pathway that is indistinguishable from that activated by the full-length gene product. Therefore, tropoelastin stimulates myofibrillar organization via a direct interaction between vascular SMCs and the VGVAPG domain [43].
Elastic matrix is also chemotactic to vascular SMCs and macrophages, anti-inflammatory on leukocytes, and anti-thrombotic. EBP in aortic tissue extracts (soluble proteins) prepared from a mouse model of Marfan syndrome and from humans with Marfan syndrome and idiopathic thoracic aortic aneurysm (TAA) has chemotaxic activity for macrophage migration in vitro. When in vitro prepared rat elastic lamina, basal lamina, and adventitial collagen were implanted into a host artery and exposed to leukocytes, elastic lamina exhibited anti-inflammatory activities. Compared with the adventitial collagen matrix and basal lamina, elastic laminae associated with lower leukocyte adhesion, reduced SMC proliferation, and inhibited neointima formation [37]. Pure porcine vascular tissue elastin scaffold subdermal implantation into rats demonstrated that these scaffolds were thrombosis-resistant [11]. Elastic laminae therefore are relatively inflammation resistant and/or thrombosis resistant, compared with collagen or laminin matrices.
2.3. Elastin gene mutations and calcification
Mutation in the elastin gene ELN in humans leads to supravalvular aortic stenosis (SVAS) and Williams syndrome. SVAS is an autosomal dominant disorder caused by intragenic deletion or a large spectrum of mutations within the elastin gene [44]. These result in functional haploinsufficiency through nonsense-mediated decay of mRNA from the mutant allele or the production of nonfunctional protein. In SVAS patients, therefore, arterial elastic fibers and laminae are composed of lower elastin levels [45]. These patients have stenosis of the ascending aorta or other arteries, or arterial narrowing, developed from uncontrolled vascular SMC proliferation and intima hyperplasia [36, 46]. If not corrected, SVAS may lead to cardiac hypertrophy and heart failure [47]. Williams syndrome is a neurodevelopmental disorder with pathological phenotypes similar to SVAS, resulting from submicroscopic deletion within the chromosome 7q11.23, involving the whole ELN gene [48]. Mice lacking the elastin gene die within days of birth from vascular occlusion due to subendothelial cell accumulation [36]. Vessel obstruction in these mice occurs due to excessive subendothelial proliferation and vascular SMC accumulation in the absence of inflammatory response. As in elastin-haploinsufficient humans with SVAS, elastin-haploinsufficient Eln+/− mice exhibited thinner arterial elastic laminae, and thereafter increased medial SMCs [45]. Arterial inner diameters were generally smaller than normal at any given intravascular pressure.
When damaged during aging or tissue injury, elastic fibers are generally not replaced, because elastin expression is turned off in adults. Instead, more collagens are made, shifting the arterial wall toward a stiffer range of collagen fibers. The arterial wall may also stiffen due to calcification of the elastic lamellae. Calcium deposits in the media in large arteries increase with age. Aortic calcium correlates with arterial stiffness in humans. In a rat calcification model, calcium accumulation in the arteries is accompanied by a concomitant increase in pulse wave velocity (PWV) [49], a measure of large artery stiffness. Additional cross-linking by advanced glycation end-products (AGEs) can increase the stiffness of elastin and collagen. AGEs form protein–protein cross-linking on collagens, which prevents collagen enzymatic digestion and increases the overall collagen in the arterial wall. AGE-mediated cross-linking also occurs in elastin, and increases with age in the human aorta [50]. There are two types of elastin calcification. In type-1 calcification, elastin undergoes self-calcification without structural changes before calcification. In type-II calcification, elastin becomes vacuolated with the accumulation of neutral lipids and unesterified cholesterol within altered elastin fibers. Type-I calcification results in elastin fiber destruction or fragmentation. Calcification of blood vessels was most prominent in small arteries in the cortex of the kidney in young mice (10 months of age), but in older mice (17 months of age) it also occurred in other areas, such as the aorta and vena cava [51].
3. Collagens
Collagen is a very stiff protein that limits vessel distension. Collagen includes at least 24 different subtypes and ~38 distinct polypeptide chains [52], depending on the structures and functions of vessels. Different cell types also express different types of collagen. In the normal and injured arterial wall, type I and type III collagen (collagen-I and collagen-III) are the main types in the media and adventitia. Arterial injury may alter the balance between the two types of collagens. For example, collagen-I in healthy arteries is a heterotrimer α1(I)2α2(I). Developing skin or healing wounds contain low levels of collagen-I homotrimers α1(I)3. In patients with ischemic heart disease, coronary artery biopsy immunohistology revealed significantly reduced collagen-III expression, reduced collagen-III/collagen-I ratio, and increased elastin/collagen-III ratio [53].
3.1. Collagen interaction with vascular cells
Collagens also interact with vascular cells (e.g., SMCs, ECs, and fibroblasts) and play important roles in vascular cell biology and pathobiology. Collagens participate in SMC differentiation, adhesion, migration, proliferation, and apoptosis. Both β1 integrin and the discoidin-domain receptor (DDR) family members mediate these collagen activities [54]. α1β1 integrin stimulates SMC proliferation by activating ERKs in the MAPK pathway [55]. The α2β1 integrin signaling pathway is important to vascular SMC adhesion, proliferation, and differentiation on polymerized fibrillar collagens [56]. α10β1 and α11β1 integrins mediate collagen-dependent mesenchymal nonmuscle cell adhesion and chemotaxis [57, 58]. In vivo, depletion of β1 integrin at the onset of SMC differentiation caused failure to assemble ECM, resulting in lethality prior to birth [59]. DDR1–collagen interaction also regulated SMC migration and proliferation, and MMP production [60]. Ddr1−/− mice had decreased SMC proliferation after vascular injury [61].
SMC contractile gene promoters are active under physiological conditions, but are temporarily silenced in response to injury [62, 63]. When rat aortic SMCs were cultured on collagen-IV and monomeric collagen-I, collagen-IV stimulated serum response factor (SRF) binding on to the promoters of SMC actin and myosin heavy chain and stimulated myocardin expression, while monomeric collagen-I stimulated SMC expression of the inflammatory adhesion molecule vascular cell adhesion molecule-1 (VCAM-1), and this collagen-I activity could be inhibited by the NF-κB inhibitor SN50 [64]. Polymerized but not monomeric collagen-I regulates SMC apoptosis. When human SMCs were cultured on polymerized collagens or monomeric collagens, polymerized collagens increased SMC apoptosis by increasing the production of active MMP-1. These MMP collagenases may degrade polymerized collagens that induce xIAP (X-chromosome-linked inhibitor of apoptosis, a caspase inhibitor) proteolysis. Therefore, collagen fragments released from ECM by MMP may propagate SMC apoptosis by calpain-mediated inactivation of anti-apoptotic xIAP [65]. While inflammatory conditions activate SMC expression of collagens, collagen matrices stimulate SMC expression of collagen, elastin, and integrins. High-glucose (22 mM) or vasoactive Ang-II (100 nM) increased mouse vascular SMC ERK1/2 phosphorylation and induced expression of latent TGF-β1, αvβ3 integrin, and collagen-I release in the medium [66]. When rat aortic SMCs were cultured on 2-D or 3-D mixtures of different combinations of collagen-I and fibrin for 7 days, SMCs expressed more collagen-III, tropoelastin, and α1, β1, and β3 integrins on 3-D matrices than on 2-D matrices. SMC expressions of tropoelastin and β1 integrin were highest on collagen matrices. Expressions of collagen-III and β3 integrin were highest on pure fibrin. Expression of collagen-I was highest on collagen-I-fibrin composite matrices [67].
Human umbilical vascular endothelial cells (HUVECs) cultured on collagen-I showed decreased nitrite synthesis, nitric oxide synthase (NOS) activity, and endothelial NOS (eNOS) protein content and mRNA, compared with those cultured on collagen-IV. This activity of collagen-I was mediated by its interaction with integrin on ECs. Interferences of collagen-I–dependent signals through the integrin-interfering peptide D6Y or an anti-integrin (α1 or β1) antibody blocked these reductions [68]. When porcine aortic ECs were grown on native or glycated collagen or exposed to shear stress using an in vitro parallel plate system, cells on native collagens, but not those on glycated collagens, elongated and aligned in the flow direction in 24 hours of 20 dynes/cm2. Shear stress-mediated NO released from ECs was also reduced by 50% when ECs were cultured on glycated collagen, which was correlated with reduced eNOS phosphorylation, likely by inhibiting sheer stress-induced focal adhesion kinase activation [69]. In a similar experiment, when porcine aortic ECs were cultured on native and glycated collagen-coated substrates in low, normal, and high glucose conditions, ECs bound to glycated collagens much more tightly than to native collagens. ECs interact with glycated collagens using αvβ3 integrin, as opposed to α2β1 with native collagen binding [70]. In addition to NO production and cell adhesion, collagens also affect EC activity in vasculogenesis. When cultured human umbilical cord blood endothelial progenitor cells (EPCs) were cultured in 3-D collagen matrices for 18 hours, in either direct analysis or at an additional 14 days after subcutaneous implantation to immunodeficient mice showed that increased collagen concentration in the 3-D collagen matrices decreased vascularization per area, but increased vessel sizes [71].
Fibroblasts also respond differently to various types of collagen. In cultured rat fibroblasts, collagen-IV induced fibroblast differentiation into myofibroblasts, while collagen-I and collagen-III induced fibroblast proliferation. Collagen-I, but not collagen-III, activated ERK1/2 in fibroblasts [72].
3.2. Bioactive collagen fragments
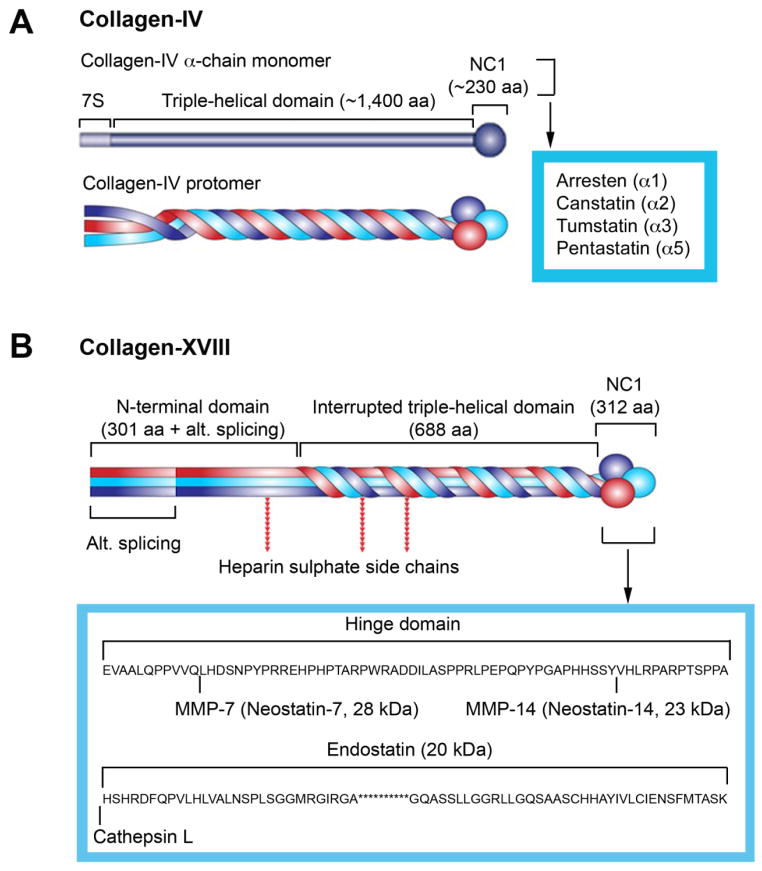
When intact collagens affect vascular cell activities, the pathophysiological activities of proteolytic fragments from both type IV and type XVIII collagens are well characterized. Collagen-IV contains six chains (α1 to α6), and each has three functional domains: the cysteine-rich N-terminal 7S domain, the central triple-helical domain, and the globular C-terminal non-collagenous domain (NCl) (Figure 3A). The NCl domain is critical for cellular interaction, matrix association, and network assembly [73]. Three α chains form a collagen-IV protomer (Figure 3A), the basic unit of collagen-IV superstructure. Endogenously produced NCl fragments of human α1 (IV), α2 (IV), and α3 (IV) have been identified as anti-angiogenic [74–76], corresponding to 26 kDa arresten, 24 kDa canstatin, and 28 kDa long tumstatin, respectively (Figure 3A). When α2 (IV) NCl was added to bovine retinal microvascular ECs, it inhibited EC early attachment, proliferation, and in vitro angiogenesis, and induced EC apoptosis and inhibited angiogenesis in an oxygen-induced retinopathy (OIR) model [77]. Pentastatin is a 20-amino acid peptide from the α5 fibril of collagen-IV. It suppressed vessel growth in an angiogenesis in vitro assay and in an in vivo tumor model (small lung cancer xenograft) [78].
Figure 3.
Schematic structures of collagen types IV (A) and XVIII (B) and locations of their identified bioactive peptides.
Collagen-XVIII is a 133 kDa non-fibrillar collagen that forms an α-chain trimer and is located mainly in the basement membrane, in the perivascular region of blood vessels, and in adult and embryonic basal laminae. Mice and humans with defective collagen-XVIII had ocular defects and poor ocular vessel growth [79, 80]. Unlike collagen-IV, alternative splicing at the N-terminal of collagen-XVIII yields two additional variants with an extra 20 kDa or 44 kDa N-terminal peptide. Endostatin is a 20 kDa fragment of the C-terminal of collagen-XVIII and has tumor-suppressing properties [81]. Elastase or cysteine protease cathepsin L (CatL) generates endostatin by cleaving the collagen-XVIII at the hinge domain between the association domain and endostatin domain [82]. Neostatins are MMP-derived cleavage products of collagen-XVIII. Neostatin-7 is a C-terminal 28 kDa endostatin-spanning fragment generated from collagen-XVIII by MMP-7 [83]. MMP-14 (also known as MT1-MMP) generates 23 kDa neostatin-14 from collagen-XVIII [84] (Figure 3B). Murine neostatin-7 inhibits calf pulmonary artery EC proliferation. Microinjection of neostatin-7 and neostatin-14 naked DNA into the corneal stroma reduced FGF-induced neovascularization [84].
3.3. Collagen mutation in humans and animals
Mutations, deficiencies, or different composites of collagens directly or indirectly affect the vasculatures. Collagen-I (COL1A1 or COL1A2) mutations in humans cause osteogenesis imperfecta with aortic dilation, dissection, and rupture [85]. Ehlers-Danlos syndrome (EDS) is an inherited connective tissue disorder caused by a defect in collagen synthesis. Most EDS subtypes in humans are caused by mutations of collagen-I, collagen-III, and collagen-V, or in genes responsible for their post-translational modification [86]. Collagen-III pro-collagen mutation (COL3A1) causes EDS [87]. Patients are particularly susceptible to dilation and rupture of arteries throughout the vasculature. Defects in collagen-III are responsible for type IV EDS [88]. In humans, collagen-I homotrimers associate with a pro-α2(I)-chain–defective variant of EDS [89], and homozygous α1(I) collagen deficiency associates with osteogenesis imperfecta [90].
The presence of collagen-I homotrimers [α1(I)]3 in mice significantly weakens the aorta. Mice with a mutant Col1a1 allele that lacks the first intron showed reduced age-dependent and tissue-dependent expression of collagen-I [91]. Mice die at 18 weeks of age, without aortic dilation as determined by high-resolution magnetic resonance imaging (MRI); mice with both collagen-I and collagen-III mutations die prematurely from ruptured blood vessels [92]. Collagen-VI–dependent basal lamina assembly is a critical aspect of vessel development. In a B16F10 melanoma tumor model, absence of collagen-VI in mice reduced vascular basal lamina by twofold, reduced EC sprouting and survival, increased vessel leakage by threefold, and increased tumor hypoxia 10-fold [93].
3.4. Collagen excessive production, synthesis and degradation markers, and imaging
Excessive collagen in the vascular wall leads to vessel fibrosis and increased stiffness. Increased expression of lysyl oxidase is a mechanism of tissue fibrosis and stiffness [94, 95]. High lysyl oxidase expression increases collagen cross-linking and collagen stiffness. TGF-β is another possible element involved in collagen deposition via stimulation of ECM synthesis and reduction of its degradation [96]. In a rat common carotid artery balloon cathether injury model, which involves SMC migration, proliferation, and neointima formation, elastin synthesis was twice as much as collagen 7 days after injury, but both significantly increased by 21 days and 60 days post-injury. At 60 days, collagen was nearly twice as much as control artery, and elastin was more than twofold as the controls [97]. Expression of collagenase-resistant collagen in experimental models provided a powerful tool to test the role of excessive collagen in the vasculatures. For example, Apoe−/− mice overexpressing MMP collagenase-resistant collagen (Apoe−/−Col1a1r/r) showed increased adventitia collagen content (picrosirius red, masson trichrome, and α1 pro-collagen mRNA) [98].
Although markers for synthesis or metabolism have not been identified for every type of collagen, markers for collagen-I and collagen-III have been studied thoroughly. C-terminal procollagen type I (PICP) and free amino-terminal collagen-I pro-peptide (PINP) have been used as markers of collagen-I synthesis. The telopeptide of collagen-I (ICTP) is an index of collagen-I degradation, and free amino-terminal collagen-III pro-peptide (PIIINP) reflects collagen-III metabolism.
Oregon green 488-conjugated CNA35 is a collagen-specific probe. Administration ex vivo to freshly prepared mouse tissues, or intravenous injection into mice, allowed detection of tissue collagen contents. In ex vivo tissue culture, CNA35 labeled collagens I, III, and IV as confirmed by antibody immunostaining [99]. Two-photon laser scanning microscopy allowed the use of specific dyes for collagen (Oregon Green 488-conjugated CNA35) on isolated intact viable carotid arteries from mice [100].
4. Fibronectin
Fibronectin is a dimeric multidomain glycoprotein in plasma and in tissue ECM. This 440 kDa glycoprotein of the ECM is linked by two disulfide bonds located at the C-terminus. It is produced and secreted by numerous cell types including SMCs, fibroblasts, and myofibroblasts, and is widely distributed in ECM. Fibronectin function in the vasculatures is mediated by α5β1 integrin, which is expressed by ECs, SMCs, and fibroblasts. Fibronectin binding to cell surface α5β1 integrin uses the RGD sequence within the 10th type III globular β-sheet repeat of fibronectin. This binding is required for fibronectin matrix assembly and signaling.
4.1. Fibronectin pathophysiological activities
Fibronectin plays roles in cell adhesion, migration, growth, and differentiation. Bone marrow-derived EPCs involve in vascular repair. When EPCs were cultured on AGE-modified fibronectin, cells showed reduced attachment, spread, and chemotaxis. On wounded retinal microvascular ECs, EPCs showed clustering at the wound sites, but AGE-fibronectin reduced this targeting response [101]. Fibronectin in blood vessels modifies the mean stress and elastic modulus of the vessel wall, and associates closely with arterial SMCs to detect and react to mechanical forces via integrin receptors [102]. Deposition of fibronectin on ECM controls the deposition, organization, and stability of other matrix proteins, including collagen-I, collagen-III, and thrombospondin-1 [103]; and modulates leukocyte infiltration, expression of adhesion molecules, cell proliferation, and SMC phenotype, all which are involved in vascular remolding [104]. In freshly isolated rat aortic SMCs, when cells were seeded on fibronectin, cells in serum-free medium remained in G0/G1 phase for the first 6 days with increased expression of cyclin D1 and p27KIP1. Addition of serum enhanced the expression of cyclins D1, A, and D3, reduced p27KIP1 expression and retinoblastoma protein hyperphosphorylation, and promoted cell cycle progression into S phase. Fibronectin therefore promotes cell cycle entry of SMCs in primary culture [105].
Plasma fibronectin supports platelet thrombus formation. In a model of arterial thrombosis, plasma fibronectin deficiency (plasma fibronectin conditional knockout mice) delayed thrombus formation and growth and subsequent occlusion of injured arteries [106]. In a mouse arteriole endothelium denudation-induced local vessel injury thrombotic response model, normal thrombosis was observed in the venules from WT mice, but in FN+/− mice, a decrease by half of plasma fibronectin delayed the appearance of thrombi in arterioles and their occlusion. Giving mice rat plasma fibronectin could prevent this defect. Fluorescent-labeled plasma fibronectin demonstrated incorporation of exogenous fibronectin into the developing thrombi [107].
4.2. Fibronectin alternative splicing variants
Human fibronectin variants result from RNA alternative splicing of the IIICS, ED-A, and ED-B segments, which are also known as V, EIIIA, and EIIIB from rat fibronectin. Each of these fibronectin variants has distinct effects on the vasculatures or vascular cells. The IIICS segment alone contains at least four different variants, with or without cell binding sites CS-1 and/or CS-5. In vitro, fibronectin CS-1 acts like VCAM-1 by binding to α4 integrin on leukocytes [108], which may indirectly affect vascular wall remodeling. CS-1 therefore mediates leukocyte adhesion and chemotaxis. ED-A and ED-B are highly enhanced around newly developing vasculature during embryogenesis and in pathological conditions. ED-A and ED-B may be important in facilitating SMC phenotype differentiation [109]. Embryos lacking both ED-A and ED-B are e9.5-e10.5 lethal [110]. During wound healing in the liver, ED-A induced stellate cell differentiation into myofibroblasts and promoted fibrosis [111]. Skin fibroblasts responded to ED-A in vitro to differentiate to a fibrotic phenotype of myofibroblasts [110]. In vitro, macrophage foam cell formation increased ED-A expression. Macrophages from ED-A–deficient Apoe−/− mice accumulated less intracellular lipids, suggesting that fibronectin ED-A contributed to macrophage lipid metabolism and foam cell formation [112]. In vitro, Ang-II and tumor necrosis factor-α (TNF-α) induce SMC expression of ED-B [113]. Unlike ED-A, ED-B plays a regulatory role in the differentiation of immature acinar epithelial cells into type II pneumocytes in lung alveolar [114]. Genetic depletion of ED-B reduced fibroblast growth and fibronectin production [115], but whether ED-B also affects vascular cell differentiation is unknown.
5. Fibulins
All seven fibulins, except fibulin-3, have been found during cardiovascular development, and mostly are induced after injury. Like most other vascular wall matrix proteins, fibulins exhibit their functions by interacting with integrin, but the activity most characteristic of fibulins is interaction with other ECM proteins, such as elastin, fibronectin, and proteoglycan. Fibulins participate with fibronectin in blood clotting. Interaction between fibulins (e.g., fibulin-4 and fibulin-5) and tropoelastin assist elastin assembly (Figure 1). Fibulin-1 and fibulin-2 also bind to the C-type lectin-like domain of aggregating proteoglycans including versican, aggrecan, and probably other lecticans.
Lack of fibulin-4 abolishes elastogenesis. Fibulin-4–deficient (Fbln4−/−) mice exhibit lung and vascular defects, including emphysema, artery tortuosity, irregularity, aneurysm, rupture, and resulting hemorrhage. Mice die prenatally. E12.5 mice show descending aorta narrowing. Mice do not develop intact elastin, but only contain irregular elastin aggregates in skin and lung. Desmosine analysis showed that elastin cross-links in these mice were largely diminished, although expression of tropoelastin and lysyl oxidase mRNA was not affected in the lungs [116]. SMCs isolated from SMC-specific fibulin-4–deficient mice exhibited an immature SMC phenotype with reduced smooth muscle-myosin heavy chain and increased proliferation. In mice with fibulin-4 deficiency only in SMCs, aortic wall showed reduced expression of smooth muscle–specific contractile genes, and focal SMC proliferation with degenerative medial wall. These mice also developed ascending TAAs and tortuousity, with markedly increased ERK1/2 [117]. Transcription interference through placement of a TKneo targeting construct in a downstream of Mus81 gene generated Fbln4R/R mice with reduced expression of fibulin-4. These mice showed dilation of the ascending aorta and tortuous and stiffened aorta, resulting from a disorganized elastin fiber network. They displayed thickened aortic valvular leaflets with aortic valve stenosis and insufficiency [118].
Fibulin-5 (also known as developing arteries and neural crest EGF-like, DANCE) is an ECM from the blood vessel basement membrane, ECs, and SMCs that, like fibulin-4, regulates elastic fiber assembly. Developing vessels and adult vessels express it prominently; it thereby plays an important role in vasculogenesis. Fibulin-5 binds to integrin and localizes tropoelastin to microfibrils (Figure 1), and therefore participates in elastin assembly. Without fibulin-5, elastin cannot form functional fiber [119]. In humans, altered expression of fibulin-5 correlated with thoracic aortic dissection [120]. Studies from organ donors from TAA patients demonstrated that aortic elastin and fibulin-5 mRNA levels decreased. Decreased fibulin-5 expression correlated strongly with decreased elastin. Primary cultured SMCs from TAA patients showed a decrease in fibulin-5 mRNA, compared with normal SMCs [120]. Fibulin-5–deficient (Fbln5−/−) mice displayed enhanced SMC proliferation and migration [121], loose skin, and tortuous arteries with disrupted elastic lamellae [119]. Fibulin-5–deficient mice, which lacked functional cross-linked elastin-containing fibers, abrogated microstructural properties and the biaxial mechanical response of the common carotid arteries, although multiphoton microscopy revealed only negligible changes in collagen organization [122].
Fibulin-2 in the vasculature is regulated by injury. Treatment with peptides (FN III 3-5 and aggrecan C-type lectin-like domain) interferes fibulin-2 and versican interaction, thereby blocking SMC migration [123]. In Fbln2−/−Fbln5−/− double-knockout mice, all elastin laminae, including internal elastic laminae, were severely disorganized, but elastic lamina disruption did not appear in Fbln2−/− mice and was much milder in Fbln5−/− single-knockout mice. Fbln2−/−Fbln5−/− mice also had enhanced vascular adhesion molecules (ICAM-1) and tissue factors, thrombus formation, marked dilation, and vessel wall thinning after carotid artery ligation injury [124]. Therefore, fibulin-2, -4, and -5 are important ECM in the vascular wall, contributing directly to elastic fiber cross-linking and assembly and associated cell biology.
6. Other vascular ECM proteins
Elastin, collagen, fibronectin, and fibulin have been among the most studied ECM proteins from the blood vessel wall during the past decade. But other ECM proteins, such as laminin, fibrillin, fibrinogen, and vitronectin, also play important roles during vessel wall development and remodeling, although relatively fewer studies of these proteins have been performed.
Laminin is a family of large cross-like heterotrimeric glycoproteins composed of 1 α chain, 1 β chain, and 1 γ chain derived from 11 genes, 5 α chains, 3 β chains, and 3 γ chains. Laminin α4, α5, and α2 are the major isoforms in large blood vessels [125]. We have found that cysteinyl cathepsin S (CatS) generates 100 kDa, 80 kDa, and 50 kDa proteolytic fragments from the 150 kDa full-length laminin γ2 chain. Both the 100 kDa and 80 kDa fragments were pro-angiogenic in an in vitro mouse aortic ring assay [126]. Monoclonal antibodies against these γ2 fragments are now widely used to detect microvessels.
Fibrillins are extracellular microfibrils that associate with elastic fibers. Both fibrillin-1 and fibrillin-2 interact with elastin, fibronectin, vitronectin, and collagens. Fibrillin-1 also interacts with integrin receptors [127]. Fibrillin-1 stabilizes elastic fiber structure in mature vessels (Figure 1). Fibrillin-1 mutation causes postnatal death in mice from vessel dissection and rupture [128]. In humans, fibrillin-1 mutation is linked to Marfan syndrome [129]. Therefore, fibrillin-1 maintains elastic fiber structure and vessel integrity, stabilizing the interaction between vascular cells and matrix scaffold. The role of fibrillin-2 in vascular morphogenesis or disease, however, remains unknown.
Vitronectin is a glycoprotein found in the circulation and ECM. The best-known function of this ECM protein is promoting cell adhesion and spreading. Vitronectin plays a role in arterial wall remodeling [130], by promoting SMC migration, but has no effect on SMC proliferation [131]. Vitronectin also contributes to thrombosis. In the mesenteric arteriole FeCl3-induced injury real-time intravital microscopy thrombosis model, Vn−/− mice had unstable thrombi and greater numbers of emboli. Vessel occlusion was delayed and frequent vessel re-opening occurred in Vn−/− mice. In a nitrogen dye laser-induced cremaster muscle arteriole injury model, Vn−/− mice had fewer platelets, lower fibrin content, and more unstable fibrin in the thrombi than did WT control mice. In vitro, thrombin-induced aggregation was abolished at a low concentration of thrombin in Vn−/− platelets [132].
Fibrinogen is a 340 kDa plasma protein that is converted into fibrin during blood clotting. But when porcine ECs were cultured on fibrinogen, cells expressed high levels of the chemokines monocyte chemoattractant protein-1 (MCP-1), PDGF-AB, and IL-8, leading to a two-fold increase in monocyte chemotactic activity [133]. In human patients with primary systemic vasculitis, such as Churg-Strauss syndrome (CSS) and Wegener’s granulomatosis (WG), blood antibodies against fibrinogen and vitronectin were increased [134].
7. ECM protein synthesis and assembly in aortic and venous diseases
ECM proteins are the main structural proteins of large blood vessels, such as aortic and venous arteries. Here, we provide a brief overview of ECM production and assembly in few selected large blood vessel diseases — atherosclerosis, AAA, varicose veins, and hypertension.
7.1. Atherosclerosis
Arterial wall elastin and collagen are the best-studied ECM proteins in atherogenesis. Uncontrolled degradation of these ECM proteins promotes atherogenesis by increasing blood-borne leukocyte transendothelium migration, SMC migration and proliferation, neovascularization, vascular cell apoptosis, and ultimately neointima formation and aortic wall rupture. Elastic fibers are made of elastin molecules organized in long cross-linked filaments; their cross-links are desmosine and isodesmosine. Pyridinoline and deoxypyridinoline — two forms of non-reducible cross-links — stabilize mature collagen. Change in collagen structure may predispose to arterial rupture in atherosclerosis.
Human carotid plaques contain reduced elastin. In atherosclerotic lesions, the inability of the cells within the lesion to produce mechanically stable matrix may lead to plaque rupture. As mentioned earlier, elastin cross-linking is prerequisite for elastic fiber assembly and is mediated by lysyl oxidase, which is diminished by inflammatory cytokines in human ECs. Atorvastatin and simvastatin, two common statin medications used as lipid-lowering regimens among hyperlipidemia patients, increased inflammatory cytokine-induced lysyl oxidase expression reduction in porcine, bovine, and human aortic ECs and abrogated reduction of lysyl oxidase in hypercholesterolemic animals [135]. In a rabbit model of atherosclerotic femoral artery balloon injury, animals fed with a lysyl oxidase inhibitor, β-APN, demonstrated increased femoral artery lysine and decreased desmosine and isodesmosine, indicating inhibition of elastin cross-linking (Figure 2).
Human aortic intima contains mainly collagen-I and collagen-III, but also other collagens (e.g., collagen-IV, V, VI, VII, and VIII) and proteoglycan. Laser-induced fluorescent spectroscopy (LIFS) combined with microscopy, birefringence, and gene expression profiling demonstrated that human carotid plaques contain reduced elastin, but increased collagen-I and collagen-III. Picrosirius red staining showed the association of collagen content with mechanical rigidity of the fibrous cap [136]. Although SMCs, ECs, and fibroblasts all express collagens, SMCs are likely dominant in producing collagen in the media. One important role of SMCs is to stabilize the artery wall by elaborating collagen-I fibrils; but in atherosclerotic lesions, SMCs are loaded with lipid, which may affect SMC biology. In vitro, when SMCs were incubated with LDL or very low-density lipoprotein (VLDL), the ability of SMCs in collagen fibril assembly decreased, and fibronectin assembly decreased. Although actin cytoskeleton expression and vinculin-containing focal adhesion complex formation were unaffected in these SMCs, they failed to assemble fibrillar adhesion complexes and developed disorganized clustering of αvβ1 integrin and tensin [137], a primary component of fibrillar adhesion complex that is critical in fibronectin assembly. SMCs from atherosclerotic lesions therefore may express a different set of collagens than do healthy aortas. Differential display and RT-PCR of SMCs isolated from atherosclerotic plaque portions and non-atheroma portions from atherosclerosis-prone Apoe−/− mice demonstrated that collagen-VIII was expressed in atherosclerotic lesion SMCs, but not in normal SMCs; in situ hybridization showed their expression on the plaque luminal surface [138]. In a rabbit model of atherosclerotic femoral artery balloon injury, inhibition of collagen cross-linking with a lysyl oxidase inhibitor β-APN reduced femoral artery tissue extract levels of pyridinoline and pentosidine. Scanning and transmission electron microscopy showed profound disorganization of artery collagen fibers from β-APN–treated mice, with high remodeling index, low neointimal collagen density, and decreased restenosis [139].
7.2. Aortic aneurysms
Elastin degradation is one of the most important signatures of human AAAs and other small and large blood vessel aneurysms. ELISA demonstrated that serum elastin peptides were significantly higher in AAA patients than in normal controls. Elastin cross-linking and elastic fiber formation directly affect AAA expansion and rupture. Aortic tissue extract HPLC analysis showed a reduction of elastin cross-links in human AAAs [140]. Increase of elastin synthesis or prevention of elastinolysis helps to stabilize AAA progression. In a rat model of aortic elastase-perfusion–induced AAA, adenoviral expression of tropoelastin in vascular SMCs reduced AAA growth [141]. Polyphenolic tannins, such as pentagalloyl glucose, are novel elastin stabilizing agents. Tannins bind to elastin and render elastin resistant to enzymatic degradation [142]. In CaCl2 periaortic injury–induced experimental AAA in rats after 28 or 56 days, one-time periadventitial delivery of noncytotoxic pentagalloyl glucose inhibited elastin degeneration and attenuated AAAs without interfering with inflammation (macrophage and T cell contents), calcification, or high metalloproteinase activities [143].
Although media elastin loss is considered a hallmark of AAA, AAA growth and ultimate rupture also associate with impaired collagen homeostasis. 3-D confocal imaging showed that collagen fibers are organized in a loose braiding of collagen ribbons in normal aorta adventitia. These ribbons encage the vessel, allowing it to dilate easily without overstretching. AAA and aneurysms of Marfan syndrome, however, show altered collagen architectures with loss of collagen knitting. Atomic force microscopy showed that the AAA wall lost its ability to stretch easily, but collagen content changes in aneurysmal lesions have been controversial. This may be due to the type of collagens and the methods applied. Plasma ELISA showed that both collagen-IV and collagen-XVIII levels are significantly higher in AAA patients than in healthy donors or even those with peripheral arterial disease. Colorimetric analysis and gas chromatography demonstrated that, in human AAA lesion tissue extracts, levels of the collagen synthesis markers collagen hydroxyproline (4-hypro) and 5-hydrpxylysine (5-hylys) were reduced by 50%, but pyridinoline collagen increased by 350% and deoxypyridinolines increased by 100%. These observations suggest reduced new collagen synthesis but accumulation of old collagen in human AAA lesions [140]. Picrosirius red staining of aortic tissue sections from AAAs, aortic dissection (cleavage of wall into two sheets: the inner half and the outer half), and control patients showed that collagen levels in the inner and outer halves in AAA and aortic dissection lesions were much lower than those from control patients. Decrease of collagen content in dissected aortas was mainly located at the external portion of the media (site of cleavage), and in aneurysm it was more diffused, which is consistent with global expansion. In a rat left common carotid artery bilateral renal arteries posterior branch ligation-induced cerebral aneurysms, both RT-PCR and immunohistological analysis revealed diminished expression of collagen-I, collagen-III, and lysyl oxidase over time. IL-1β inhibited the expression of collagen-I, collagen-III, and lysyl oxidase in rat aortic SMCs by activating the NF-κB pathway [144].
7.3. Varicose veins
Depending on the definition, 14%–50% adults have varicose veins, and 3%–11% have clinical signs of chronic insufficiency, such as eczema, hyperpigmentation, or ulceration. These conditions result from venous reflux and hypertension due to abnormal dilation of the superficial veins or deep vein thrombosis, valvular incompetence, and reflux through the perforating veins. The major structural proteins of the vein wall are collagen, elastin, and SMC actin. Dilatation and enhanced distensibility are biophysical properties of varicose veins, which can be detected using ultrasonography.
Histology, morphometric, and tissue microarray of vein wall tissue in patients with varicose veins showed that varicose veins have significant elastin loss in the adventitia and reduced collagen-III in the intima and media. Elastin loss correlated negatively with vein diameter at rest. Collagen-III loss in the intima correlated negatively with the increase in vein diameter during the Valsalva maneuver [145]. In contrast, collagen-I mRNA levels increase in media from varicose veins [146], leading to decrease of the ratio of collagen-III to collagen-I that may affect the vein resistance to stretch.
As in the aortic wall, SMCs are important cells that produce elastin, fibrillin-1, and collagen. Immunostaining and in situ hybridization demonstrated expression of tropoelastin and fibrillin-1 in SMCs boarding the elastic laminae, and SMCs expressing these ECM occurred in patchy disorganized patterns. Varicose veins show high latent TGF-β binding protein (LTBP)-2 and TGFβ expression, particularly in the subendothelium and media, and in areas with marked injury. Development of varicose veins therefore involves elastin component reconstruction [147]. Increased TGF-β and LTBP-2 in varicose veins may regulate SMC expression of elastin and collagens, and these SMCs may express different sets of ECM than those expressed by healthy veins. In vitro cultured SMCs from varicose veins showed increased levels of collagen-I mRNA, but no difference in collagen-III mRNA levels compared with normal vessels [145]. At protein levels, both collagen-III and fibronectin were decreased in cultured SMCs from varicose veins due to proteolytic degradation by collagenases, such as MMP-3 [148]. Decreased collagen-III expression in varicose vein SMCs is also responsible for collagen-I overexpression [145]. SMCs from varicose veins also showed increased proliferation and enhanced matrix mineralization, a process that may be mediated by matrix Gla protein (MGP). MGP is a calcium-binding protein that participates in bone tissue organization, but this protein is highly expressed in human atherosclerotic lesions and is increased in plasma from atherosclerosis patients. Plasma MGP levels increase with age and associate with increasing Framingham coronary heart disease risk score [149]. In vitro, MGP inhibition or using siRNA reduces varicose vein SMC proliferation [150].
Compared with studies in aortic arteries or microvessels, very little information is available regarding varicose vein elastin and collagen degradations. Among proteolysis events, MMPs and their TIMPs have garnered the most attention and have been linked with the pathological events of varicose veins, but different groups have reported contradictory observations. Some studies have shown that MMP-1, MMP-2, MMP-3, MMP-7, TIMP-1, and TIMP-3 increase in varicose veins, but another study showed reduced expression of MMP-1 and MMP-2 in varicose veins. We have recently shown that human varicose veins contain large quantities of cysteinyl cathepsins S, K, L, and B, but reduced levels of their endogenous inhibitor, cystatin C [151]. Elevated expression of these proteases in human varicose veins may contribute to reduced elastin and collagen-III — a hypothesis that, to our knowledge, has not been tested.
7.4. Hypertension
The main function of the large elastic arteries is to serve as capacitance vessels that distend and retract during systole and diastole. During aging, arterial wall elastin laminae become fragmented, thereby transferring mechanical load to collagen fibers, which are much more stiffer than elastic fibers. Such alterations of the arterial wall ECM structure lead to high systolic and pulse pressure or hypertension, which increases circumferential wall stress, elastic fiber further breakdown, and high risk of local arterial wall fatigue and endothelial damage [152]. Therefore, arterial remodeling has to occur in response to arterial wall hypertensive hemodynamic changes to adapt increased mechanical load, a process involving ECM degradation and reorganization, vascular wall hypertrophy, and skewed aortic collagen to elastin content [153, 154]. An important feature of hypertensive vascular remodeling is an increase in the media/lumen ratio, which can occur with or without vessel growth (e.g. hypertrophic and eutrophic) due to changes in SMC and ECM components [155, 156]. The stiffer the artery, the more pressure is required to distend the wall, a pathological change relaying on mainly the expression, deposition, and functional construction of elastin and collagen fibers.
Elastin provides reversible extensibility during cardiac cyclic loading, while collagen provides strength and prevents failure at high pressure. Elastin gene expression and degradation, elastic fiber assembly, and unbalanced elastin synthesis relative to collagens all determine arterial wall stiffness and thickening. For example, arterial wall thickening is associated with increased elastin degradation products (elastin-derived peptides), which also stimulate chemotaxis of leukocytes into the vascular wall, producing proteinases and further contributing to elastic fiber degradation [157].
Reduced elastin level in SVAS patients and elastin haploinsufficiency from Eln+/− mice have increased arterial stiffness and hypertension, although they have mild cardiac hypertrophy and a normal lifespan [45, 158, 159]. As discussed, SVAS is caused by mutations including point mutations, translocations, and partial deletions within the eln gene [160–163]. Hypertension is present in large proportion of SVAS patients usually from early age. Eln+/− mouse arterial stiffness increases by 7 days after birth, while systolic blood pressure is not significantly increased until one week after arterial stiffness, suggesting that increased large artery stiffness is required for the development of hypertension in these mice [164]. Expression of human elastin gene in Eln+/− mice increases elastin level to 60~80% of the normal level and reverses the changes in both arterial stiffness and hypertension [165]. Therefore, defective elastinogenesis in humans and animals could be an initiating event of hypertension [166, 167].
Not only expression, but also altered spatial organization of elastic fibers, improper assembly, or even skewed synthesis relative to collagen all compromise vascular elasticity and hypertension. In resistance [168, 169] and conduit vessels from spontaneous hypertensive rats (SHRs) [170, 171], more compact elastic laminae with reduced relative volume of fenestrae are associated with increased vessel stiffness and correlate with vessel narrowing. Abnormal lamellae organization was also observed after chronic Ang II infusion [172] and present in neonatal SHRs before the establishment of hypertension and alteration in collagen content [171, 173]. Fibulin-5 participates in elastin fiber assembly (Figure 1). Similar to those in aged human arteries [174, 175], aortas of fibulin-5-deficient mice demonstrate abnormal morphology and altered elastic lamella [119, 176], accompanied by a significant increase in systolic blood pressure and pulse pressure [119]. In some animals of induced hypertension, elastin expression is increased but less than collagen, pushing the elastin-to-collagen ratio skewed toward increased collagen amounts [177]. In monocrotaline-induced pulmonary hypertension, the ratio of elastin to collagens remains constant, but the newly synthesized elastin does not assemble properly [178].
Collagen is a stiff protein and has the physiological role to limit vessel distension. Excessive collagen in the vascular wall leads to vessel fibrosis and overly stiffness. Increased collagen deposition in the vascular wall has been demonstrated in the conduit and resistance arteries from patients [179–181], or animals [182, 183] with hypertension. In addition to altered content, collagen undulation, orientation, cross-linking, and interactions with other ECM elements are fundamental to defining arterial wall stiffness and hypertension. Ang II induces hypertension by increasing collagen and decreasing elastin content, creating an imbalance between elastic and rigid ECM components in the vascular wall [184]. The stiffness of elastin and collagen fibers can be increased through additional crosslinking by AGEs. Unlike normal crosslinking at discrete sites and the end of collagen molecules, AGEs form protein-protein crosslinks throughout the collagen molecules in hypertensive arterial wall [185].
In addition to elastin and collagens, several other ECM proteins have also been implicated in arterial wall structural changes in hypertensive subjects. Arteries from SHRs [186] or from Ang II-infused animals [187, 188] contain increased levels of total fibronectin. Ang II stimulates proliferative signaling pathway in part through TGF-β [189], which increases the synthesis of fibronectin and collagens [190, 191]. The deposition of fibronectin controls the deposition, organization, and stability of other ECM, such as type I and type III collagens [189]. Treatment of hypertensive mice with neutralizing TGF-β antibody or with a TGF-β inhibitor reduces resistance artery stiffness [66] and collagen deposition [66, 192]. However, since collagen confers rigidity to the vascular wall, most hypertension therapeutic studies have been focused on the reduction of vascular collagen content.
8. Conclusions
Vascular wall matrix protein synthesis and metabolism are strictly regulated to maintain the blood vessel structure and functions in assisting blood flow diastole and systole. Such blood vessel homeostasis is organized and protected by vascular wall interlaminal matrix protein fibers or filaments, surrounded by layers of vascular cells (SMCs, ECs, and fibroblasts). Chemical, mechanical, and enzymatic injuries interrupt blood vessel homeostasis, leading to altered structure of existing matrix proteins or changes in the production of newly synthesized matrix proteins, their proteolytic bioactive fragments, and alternatively spliced variants from vascular cells. As a consequence, blood vessels may narrow (e.g. atherosclerosis) or become stiff (hypertension) and easy to rupture, or they may expand (e.g. aneurysms) or become tortuous (e.g. varicose veins). Interactions among different matrix proteins or between matrix proteins and neighboring vascular cells may prevent or progress the destruction of vascular wall matrix, depending on the timing of vascular wall development and on the types of cells and matrix proteins or fragments. Injury or inflammatory mediators often yield uncontrolled expression of proteolytic enzymes that directly affect the accumulation of matrix proteins and the production of bioactive matrix peptides, a potential future focus of research in controling effectively vascular wall matrix remodeling in human vascular diseases.
Highlights.
We summarize the biology of several common vascular wall matrix proteins.
These matrix proteins form the basic structure of blood vessels.
They also interact each other and regulate vascular cell activities.
These matrix proteins exert different roles in vascular diseases.
Acknowledgments
Authors thank Ms. Sara Karwacki for editorial assistance. Our studies cited in this article are supported by grants from the National Institutes of Health (HL60942, HL81090, and HL88547, to G.P.S.); and by an Established Investigator Award (0840118N) from the American Heart Association (to G.P.S.).
Footnotes
Conflict of Interest: The authors have declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moulton KS, Olsen BR, Sonn S, Fukai N, Zurakowski D, Zeng X. Loss of collagen XVIII enhances neovascularization and vascular permeability in atherosclerosis. Circulation. 2004;110:1330–1336. doi: 10.1161/01.CIR.0000140720.79015.3C. [DOI] [PubMed] [Google Scholar]
- 3.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 4.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991;11:1223–1230. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- 6.Ward MR, Agrotis A, Kanellakis P, Hall J, Jennings G, Bobik A. Tranilast prevents activation of transforming growth factor-beta system, leukocyte accumulation, and neointimal growth in porcine coronary arteries after stenting. Arterioscler Thromb Vasc Biol. 2002;22:940–948. doi: 10.1161/01.atv.0000019405.84384.9c. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh J, Baguneid M, Khwaja N, Murphy MO, Turner N, Halka A, Ferguson MW, Kielty CM, Walker MG. Reduction of myointimal hyperplasia after arterial anastomosis by local injection of transforming growth factor beta3. J Vasc Surg. 2006;43:142–149. doi: 10.1016/j.jvs.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Newby AC, Zaltsman AB. Fibrous cap formation or destruction--the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc Res. 1999;41:345–360. [PubMed] [Google Scholar]
- 9.Dufourcq P, Couffinhal T, Alzieu P, Daret D, Moreau C, Duplaa C, Bonnet J. Vitronectin is up-regulated after vascular injury and vitronectin blockade prevents neointima formation. Cardiovasc Res. 2002;53:952–962. doi: 10.1016/s0008-6363(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 10.Lu Q, Ganesan K, Simionescu DT, Vyavahare NR. Novel porous aortic elastin and collagen scaffolds for tissue engineering. Biomaterials. 2004;25:5227–5237. doi: 10.1016/j.biomaterials.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Simionescu DT, Lu Q, Song Y, Lee JS, Rosenbalm TN, Kelley C, Vyavahare NR. Biocompatibility and remodeling potential of pure arterial elastin and collagen scaffolds. Biomaterials. 2006;27:702–713. doi: 10.1016/j.biomaterials.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Khan KM, Howe LR, Falcone DJ. Extracellular matrix-induced cyclooxygenase-2 regulates macrophage proteinase expression. J Biol Chem. 2004;279:22039–22046. doi: 10.1074/jbc.M312735200. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz MA. Integrin signaling revisited. Trends Cell Biol. 2001;11:466–470. doi: 10.1016/s0962-8924(01)02152-3. [DOI] [PubMed] [Google Scholar]
- 14.Barnes MJ, Farndale RW. Collagens and atherosclerosis. Exp Gerontol. 1999;34:513–525. doi: 10.1016/s0531-5565(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 15.Moiseeva EP. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res. 2001;52:372–386. doi: 10.1016/s0008-6363(01)00399-6. [DOI] [PubMed] [Google Scholar]
- 16.Mawatari K, Liu B, Kent KC. Activation of integrin receptors is required for growth factor-induced smooth muscle cell dysfunction. J Vasc Surg. 2000;31:375–381. doi: 10.1016/s0741-5214(00)90167-8. [DOI] [PubMed] [Google Scholar]
- 17.Kappert K, Blaschke F, Meehan WP, Kawano H, Grill M, Fleck E, Hsueh WA, Law RE, Graf K. Integrins alphavbeta3 and alphavbeta5 mediate VSMC migration and are elevated during neointima formation in the rat aorta. Basic Res Cardiol. 2001;96:42–49. doi: 10.1007/s003950170076. [DOI] [PubMed] [Google Scholar]
- 18.Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci U S A. 2001;98:1024–1029. doi: 10.1073/pnas.031564998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J. 1993;7:1208–1218. [PubMed] [Google Scholar]
- 21.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20:99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- 22.Clark JM, Glagov S. Transmural organization of the arterial media. The lamellar unit revisited. Arteriosclerosis. 1985;5:19–34. doi: 10.1161/01.atv.5.1.19. [DOI] [PubMed] [Google Scholar]
- 23.Arribas SM, Hinek A, Gonzalez MC. Elastic fibres and vascular structure in hypertension. Pharmacol Ther. 2006;111:771–791. doi: 10.1016/j.pharmthera.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2101. [DOI] [PubMed] [Google Scholar]
- 25.Keeley FW, Johnson DJ. The effect of developing hypertension on the synthesis and accumulation of elastin in the aorta of the rat. Biochem Cell Biol. 1986;64:38–43. doi: 10.1139/o86-006. [DOI] [PubMed] [Google Scholar]
- 26.Krettek A, Sukhova GK, Libby P. Elastogenesis in human arterial disease: a role for macrophages in disordered elastin synthesis. Arterioscler Thromb Vasc Biol. 2003;23:582–587. doi: 10.1161/01.ATV.0000064372.78561.A5. [DOI] [PubMed] [Google Scholar]
- 27.Hinek A, Keeley FW, Callahan J. Recycling of the 67-kDa elastin binding protein in arterial myocytes is imperative for secretion of tropoelastin. Exp Cell Res. 1995;220:312–324. doi: 10.1006/excr.1995.1321. [DOI] [PubMed] [Google Scholar]
- 28.Rosenbloom J, Bashir M, Yeh H, Rosenbloom J, Ornstein-Goldstein N, Fazio M, Kahari VM, Uitto J. Regulation of elastin gene expression. Ann N Y Acad Sci. 1991;624:116–136. doi: 10.1111/j.1749-6632.1991.tb17012.x. [DOI] [PubMed] [Google Scholar]
- 29.Vrhovski B, Weiss AS. Biochemistry of tropoelastin. Eur J Biochem. 1998;258:1–18. doi: 10.1046/j.1432-1327.1998.2580001.x. [DOI] [PubMed] [Google Scholar]
- 30.Robb BW, Wachi H, Schaub T, Mecham RP, Davis EC. Characterization of an in vitro model of elastic fiber assembly. Mol Biol Cell. 1999;10:3595–3605. doi: 10.1091/mbc.10.11.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mochizuki S, Brassart B, Hinek A. Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. The J Biol Chem. 2002;277:44854–44863. doi: 10.1074/jbc.M205630200. [DOI] [PubMed] [Google Scholar]
- 32.Hinek A. Biological roles of the non-integrin elastin/laminin receptor. Biol Chem. 1996;377:471–480. [PubMed] [Google Scholar]
- 33.Ito S, Ishimaru S, Wilson SE. Inhibitory effect of type 1 collagen gel containing alpha-elastin on proliferation and migration of vascular smooth muscle and endothelial cells. Cardiovasc Surg. 1997;5:176–183. doi: 10.1016/s0967-2109(97)00004-5. [DOI] [PubMed] [Google Scholar]
- 34.Ooyama T, Fukuda K, Oda H, Nakamura H, Hikita Y. Substratum-bound elastin peptide inhibits aortic smooth muscle cell migration in vitro. Arteriosclerosis. 1987;7:593–598. doi: 10.1161/01.atv.7.6.593. [DOI] [PubMed] [Google Scholar]
- 35.Ito S, Ishimaru S, Wilson SE. Effect of coacervated alpha-elastin on proliferation of vascular smooth muscle and endothelial cells. Angiology. 1998;49:289–297. doi: 10.1177/000331979804900407. [DOI] [PubMed] [Google Scholar]
- 36.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 37.Liu SQ, Tieche C, Alkema PK. Neointima formation on vascular elastic laminae and collagen matrices scaffolds implanted in the rat aortae. Biomaterials. 2004;25:1869–1882. doi: 10.1016/j.biomaterials.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 38.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M, Fujita K, Shinkai T, Yamamoto K, Noumura T. Identification of the phenotypic modulation of rabbit arterial smooth muscle cells in primary culture by flow cytometry. Exp Cell Res. 1992;198:43–51. doi: 10.1016/0014-4827(92)90147-z. [DOI] [PubMed] [Google Scholar]
- 40.Robert L. Cell-elastin interaction and signaling. Pathol Biol (Paris) 2005;53:399–404. doi: 10.1016/j.patbio.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 41.Faury G, Ristori MT, Verdetti J, Jacob MP, Robert L. Effect of elastin peptides on vascular tone. J Vasc Res. 1995;32:112–119. doi: 10.1159/000159084. [DOI] [PubMed] [Google Scholar]
- 42.Brassart B, Fuchs P, Huet E, Alix AJ, Wallach J, Tamburro AM, Delacoux F, Haye B, Emonard H, Hornebeck W, Debelle L. Conformational dependence of collagenase (matrix metalloproteinase-1) up-regulation by elastin peptides in cultured fibroblasts. J Biol Chem. 2001;276:5222–5227. doi: 10.1074/jbc.M003642200. [DOI] [PubMed] [Google Scholar]
- 43.Karnik SK, Wythe JD, Sorensen L, Brooke BS, Urness LD, Li DY. Elastin induces myofibrillogenesis via a specific domain. VGVAPG. Matrix Biol. 2003;22:409–425. doi: 10.1016/s0945-053x(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 44.Curran ME, Atkinson DL, Ewart AK, Morris CA, Leppert MF, Keating MT. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell. 1993;73:159–168. doi: 10.1016/0092-8674(93)90168-p. [DOI] [PubMed] [Google Scholar]
- 45.Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest. 1998;102:1783–1787. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urban Z, Riazi S, Seidl TL, Katahira J, Smoot LB, Chitayat D, Boyd CD, Hinek A. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am J Hum Genet. 2002;71:30–44. doi: 10.1086/341035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chowdhury T, Reardon W. Elastin mutation and cardiac disease. Pediatr Cardiol. 1999;20:103–107. doi: 10.1007/s002469900415. [DOI] [PubMed] [Google Scholar]
- 48.Lowery MC, Morris CA, Ewart A, Brothman LJ, Zhu XL, Leonard CO, Carey JC, Keating M, Brothman AR. Strong correlation of elastin deletions, detected by FISH, with Williams syndrome: evaluation of 235 patients. Am J Hum Genet. 1995;57:49–53. [PMC free article] [PubMed] [Google Scholar]
- 49.Dao HH, Essalihi R, Bouvet C, Moreau P. Evolution and modulation of age-related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res. 2005;66:307–317. doi: 10.1016/j.cardiores.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Konova E, Baydanoff S, Atanasova M, Velkova A. Age-related changes in the glycation of human aortic elastin. Exp Gerontol. 2004;39:249–254. doi: 10.1016/j.exger.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Gorgels TG, Hu X, Scheffer GL, van der Wal AC, Toonstra J, de Jong PT, van Kuppevelt TH, Levelt CN, de Wolf A, Loves WJ, Scheper RJ, Peek R, Bergen AA. Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet. 2005;14:1763–1773. doi: 10.1093/hmg/ddi183. [DOI] [PubMed] [Google Scholar]
- 52.Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann Med. 2001;33:7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- 53.Kong CH, Lin XY, Woo CC, Wong HC, Lee CN, Richards AM, Sorokin VA. Characteristics of aortic wall extracellular matrix in patients with acute myocardial infarction: tissue microarray detection of collagen I, collagen III and elastin levels. Interact Cardiovasc Thorac Surg. 2013;16:11–15. doi: 10.1093/icvts/ivs421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 55.Pozzi A, Wary KK, Giancotti FG, Gardner HA. Integrin alpha1beta1 mediates a unique collagen-dependent proliferation pathway in vivo. J Cell Biol. 1998;142:587–594. doi: 10.1083/jcb.142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 57.Tiger CF, Fougerousse F, Grundstrom G, Velling T, Gullberg D. alpha11beta1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev Biol. 2001;237:116–129. doi: 10.1006/dbio.2001.0363. [DOI] [PubMed] [Google Scholar]
- 58.Gullberg DE, Lundgren-Akerlund E. Collagen-binding I domain integrins--what do they do? Prog Histochem Cytochem. 2002;37:3–54. doi: 10.1016/s0079-6336(02)80008-0. [DOI] [PubMed] [Google Scholar]
- 59.Turlo KA, Noel OD, Vora R, LaRussa M, Fassler R, Hall-Glenn F, Iruela-Arispe ML. An essential requirement for beta1 integrin in the assembly of extracellular matrix proteins within the vascular wall. Dev Biol. 2012;365:23–35. doi: 10.1016/j.ydbio.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou G, Vogel WF, Bendeck MP. Tyrosine kinase activity of discoidin domain receptor 1 is necessary for smooth muscle cell migration and matrix metalloproteinase expression. Circ Res. 2002;90:1147–1149. doi: 10.1161/01.res.0000022166.74073.f8. [DOI] [PubMed] [Google Scholar]
- 61.Hou G, Vogel W, Bendeck MP. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 2001;107:727–735. doi: 10.1172/JCI10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C element mediates repression of the SM22alpha promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res. 2004;95:981–988. doi: 10.1161/01.RES.0000147961.09840.fb. [DOI] [PubMed] [Google Scholar]
- 64.Orr AW, Lee MY, Lemmon JA, Yurdagul A, Jr, Gomez MF, Bortz PD, Wamhoff BR. Molecular mechanisms of collagen isotype-specific modulation of smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2009;29:225–231. doi: 10.1161/ATVBAHA.108.178749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.von Wnuck Lipinski K, Keul P, Lucke S, Heusch G, Wohlschlaeger J, Baba HA, Levkau B. Degraded collagen induces calpain-mediated apoptosis and destruction of the X-chromosome-linked inhibitor of apoptosis (xIAP) in human vascular smooth muscle cells. Cardiovasc Res. 2006;69:697–705. doi: 10.1016/j.cardiores.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Belmadani S, Zerfaoui M, Boulares HA, Palen DI, Matrougui K. Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, alphavbeta3-integrin, and TGF-beta1 in response to ANG II and high glucose. Am J Physiol Heart Circ Physiol. 2008;295:H69–76. doi: 10.1152/ajpheart.00341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hong H, Stegemann JP. 2D and 3D collagen and fibrin biopolymers promote specific ECM and integrin gene expression by vascular smooth muscle cells. J Biomater Sci Polym Ed. 2008;19:1279–1293. doi: 10.1163/156856208786052380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez-Santiago L, Lopez-Ongil S, Rodriguez-Puyol M, Rodriguez-Puyol D. Decreased nitric oxide synthesis in human endothelial cells cultured on type I collagen. Circ Res. 2002;90:539–545. doi: 10.1161/01.res.0000012445.68979.9d. [DOI] [PubMed] [Google Scholar]
- 69.Kemeny SF, Figueroa DS, Andrews AM, Barbee KA, Clyne AM. Glycated collagen alters endothelial cell actin alignment and nitric oxide release in response to fluid shear stress. J Biomech. 2011;44:1927–1935. doi: 10.1016/j.jbiomech.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 70.Kemeny SF, Cicalese S, Figueroa DS, Clyne AM. Glycated collagen and altered glucose increase endothelial cell adhesion strength. J Cell Physiol. 2013;228:1727–1736. doi: 10.1002/jcp.24313. [DOI] [PubMed] [Google Scholar]
- 71.Critser PJ, Kreger ST, Voytik-Harbin SL, Yoder MC. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res. 2010;80:23–30. doi: 10.1016/j.mvr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naugle JE, Olson ER, Zhang X, Mase SE, Pilati CF, Maron MB, Folkesson HG, Horne WI, Doane KJ, Meszaros JG. Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol. 2006;290:H323–330. doi: 10.1152/ajpheart.00321.2005. [DOI] [PubMed] [Google Scholar]
- 73.Sundaramoorthy M, Meiyappan M, Todd P, Hudson BG. Crystal structure of NC1 domains. Structural basis for type IV collagen assembly in basement membranes. J Biol Chem. 2002;277:31142–31153. doi: 10.1074/jbc.M201740200. [DOI] [PubMed] [Google Scholar]
- 74.Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, Dhanabal M, Simons M, Post M, Kufe DW, Weichselbaum RR, Sukhatme VP, Kalluri R. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60:2520–2526. [PubMed] [Google Scholar]
- 75.Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J Biol Chem. 2000;275:21340–21348. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- 76.Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Jr, Hudson BG, Brooks PC. New functions for non-collagenous domains of human collagen type IV. Novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J Biol Chem. 2000;275:8051–8061. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- 77.Coleman G, Gardiner TA, Boutaud A, Stitt AW. Recombinant alpha2(IV)NC1 domain of type IV collagen is an effective regulator of retinal capillary endothelial cell proliferation and inhibits pre-retinal neovascularisation. Graefes Arch Clin Exp Ophthalmol. 2007;245:581–587. doi: 10.1007/s00417-006-0396-1. [DOI] [PubMed] [Google Scholar]
- 78.Koskimaki JE, Karagiannis ED, Tang BC, Hammers H, Watkins DN, Pili R, Popel AS. Pentastatin-1, a collagen IV derived 20-mer peptide suppresses tumor growth in a small cell lung cancer xenograft model. BMC cancer. 2010;10:29. doi: 10.1186/1471-2407-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fukai N, Eklund L, Marneros AG, Oh SP, Keene DR, Tamarkin L, Niemela M, Ilves M, Li E, Pihlajaniemi T, Olsen BR. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 2002;21:1535–1544. doi: 10.1093/emboj/21.7.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Menzel O, Bekkeheien RC, Reymond A, Fukai N, Boye E, Kosztolanyi G, Aftimos S, Deutsch S, Scott HS, Olsen BR, Antonarakis SE, Guipponi M. Knobloch syndrome: novel mutations in COL18A1, evidence for genetic heterogeneity, and a functionally impaired polymorphism in endostatin. Hum Mutat. 2004;23:77–84. doi: 10.1002/humu.10284. [DOI] [PubMed] [Google Scholar]
- 81.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 82.Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaisse J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- 83.Lin HC, Chang JH, Jain S, Gabison EE, Kure T, Kato T, Fukai N, Azar DT. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest Ophthalmol Vis Sci. 2001;42:2517–2524. [PubMed] [Google Scholar]
- 84.Chang JH, Javier JA, Chang GY, Oliveira HB, Azar DT. Functional characterization of neostatins, the MMP-derived, enzymatic cleavage products of type XVIII collagen. FEBS Lett. 2005;579:3601–3606. doi: 10.1016/j.febslet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 85.Kuivaniemi H, Tromp G, Prockop DJ. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat. 1997;9:300–315. doi: 10.1002/(SICI)1098-1004(1997)9:4<300::AID-HUMU2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 86.De Paepe A, Malfait F. Bleeding and bruising in patients with Ehlers-Danlos syndrome and other collagen vascular disorders. Br J Haematol. 2004;127:491–500. doi: 10.1111/j.1365-2141.2004.05220.x. [DOI] [PubMed] [Google Scholar]
- 87.Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342:673–680. doi: 10.1056/NEJM200003093421001. [DOI] [PubMed] [Google Scholar]
- 88.Pope FM, Burrows NP. Ehlers-Danlos syndrome has varied molecular mechanisms. J Med Genet. 1997;34:400–410. doi: 10.1136/jmg.34.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hata R, Kurata S, Shinkai H. Existence of malfunctioning pro alpha2(I) collagen genes in a patient with a pro alpha 2(I)-chain-defective variant of Ehlers-Danlos syndrome. Eur J Biochem. 1988;174:231–237. doi: 10.1111/j.1432-1033.1988.tb14087.x. [DOI] [PubMed] [Google Scholar]
- 90.Nicholls AC, Osse G, Schloon HG, Lenard HG, Deak S, Myers JC, Prockop DJ, Weigel WR, Fryer P, Pope FM. The clinical features of homozygous alpha 2(I) collagen deficient osteogenesis imperfecta. J Med Genet. 1984;21:257–262. doi: 10.1136/jmg.21.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rahkonen O, Su M, Hakovirta H, Koskivirta I, Hormuzdi SG, Vuorio E, Bornstein P, Penttinen R. Mice with a deletion in the first intron of the Col1a1 gene develop age-dependent aortic dissection and rupture. Circ Res. 2004;94:83–90. doi: 10.1161/01.RES.0000108263.74520.15. [DOI] [PubMed] [Google Scholar]
- 92.Lohler J, Timpl R, Jaenisch R. Embryonic lethal mutation in mouse collagen I gene causes rupture of blood vessels and is associated with erythropoietic and mesenchymal cell death. Cell. 1984;38:597–607. doi: 10.1016/0092-8674(84)90514-2. [DOI] [PubMed] [Google Scholar]
- 93.You WK, Bonaldo P, Stallcup WB. Collagen VI ablation retards brain tumor progression due to deficits in assembly of the vascular basal lamina. The Am J Pathol. 2012;180:1145–1158. doi: 10.1016/j.ajpath.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ooshima A, Midorikawa O. Increased lysyl oxidase activity in blood vessels of hypertensive rats and effect of beta-aminopropionitrile on arteriosclerosis. Jpn Circ J. 1977;41:1337–1340. doi: 10.1253/jcj.41.1337. [DOI] [PubMed] [Google Scholar]
- 95.Lopez B, Querejeta R, Gonzalez A, Beaumont J, Larman M, Diez J. Impact of treatment on myocardial lysyl oxidase expression and collagen cross-linking in patients with heart failure. Hypertension. 2009;53:236–242. doi: 10.1161/HYPERTENSIONAHA.108.125278. [DOI] [PubMed] [Google Scholar]
- 96.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32:1805–1819. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 97.Nili N, Zhang M, Strauss BH, Bendeck MP. Biochemical analysis of collagen and elastin synthesis in the balloon injured rat carotid artery. Cardiovasc Pathol. 2002;11:272–276. doi: 10.1016/s1054-8807(02)00119-9. [DOI] [PubMed] [Google Scholar]
- 98.Deguchi JO, Huang H, Libby P, Aikawa E, Whittaker P, Sylvan J, Lee RT, Aikawa M. Genetically engineered resistance for MMP collagenases promotes abdominal aortic aneurysm formation in mice infused with angiotensin II. Lab Invest. 2009;89:315–326. doi: 10.1038/labinvest.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Megens RT, Oude Egbrink MG, Cleutjens JP, Kuijpers MJ, Schiffers PH, Merkx M, Slaaf DW, van Zandvoort MA. Imaging collagen in intact viable healthy and atherosclerotic arteries using fluorescently labeled CNA35 and two-photon laser scanning microscopy. Mol Imaging. 2007;6:247–260. [PubMed] [Google Scholar]
- 100.Megens RT, oude Egbrink MG, Merkx M, Slaaf DW, van Zandvoort MA. Two-photon microscopy on vital carotid arteries: imaging the relationship between collagen and inflammatory cells in atherosclerotic plaques. J Biomed Opt. 2008;13:044022. doi: 10.1117/1.2965542. [DOI] [PubMed] [Google Scholar]
- 101.Bhatwadekar AD, Glenn JV, Li G, Curtis TM, Gardiner TA, Stitt AW. Advanced glycation of fibronectin impairs vascular repair by endothelial progenitor cells: implications for vasodegeneration in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:1232–1241. doi: 10.1167/iovs.07-1015. [DOI] [PubMed] [Google Scholar]
- 102.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 2009;24:45–57. doi: 10.1152/physiol.00029.2008. [DOI] [PubMed] [Google Scholar]
- 103.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13:3546–3559. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chiang HY, Korshunov VA, Serour A, Shi F, Sottile J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1074–1079. doi: 10.1161/ATVBAHA.108.181081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roy J, Tran PK, Religa P, Kazi M, Henderson B, Lundmark K, Hedin U. Fibronectin promotes cell cycle entry in smooth muscle cells in primary culture. Exp Cell Res. 2002;273:169–177. doi: 10.1006/excr.2001.5427. [DOI] [PubMed] [Google Scholar]
- 106.Ni H, Yuen PS, Papalia JM, Trevithick JE, Sakai T, Fassler R, Hynes RO, Wagner DD. Plasma fibronectin promotes thrombus growth and stability in injured arterioles. Proc Natl Acad Sci U S A. 2003;100:2415–2419. doi: 10.1073/pnas.2628067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matuskova J, Chauhan AK, Cambien B, Astrof S, Dole VS, Piffath CL, Hynes RO, Wagner DD. Decreased plasma fibronectin leads to delayed thrombus growth in injured arterioles. Arterioscler Thromb Vasc Biol. 2006;26:1391–1396. doi: 10.1161/01.ATV.0000216282.58291.c6. [DOI] [PubMed] [Google Scholar]
- 108.Shih PT, Elices MJ, Fang ZT, Ugarova TP, Strahl D, Territo MC, Frank JS, Kovach NL, Cabanas C, Berliner JA, Vora DK. Minimally modified low-density lipoprotein induces monocyte adhesion to endothelial connecting segment-1 by activating beta1 integrin. J Clin Invest. 1999;103:613–625. doi: 10.1172/JCI5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Astrof S, Crowley D, Hynes RO. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev Biol. 2007;311:11–24. doi: 10.1016/j.ydbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood. 2004;104:11–18. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- 113.Dietrich T, Perlitz C, Licha K, Stawowy P, Atrott K, Tachezy M, Meyborg H, Stocker C, Grafe M, Fleck E, Schirner M, Graf K. ED-B fibronectin (ED-B) can be targeted using a novel single chain antibody conjugate and is associated with macrophage accumulation in atherosclerotic lesions. Basic Res Cardiol. 2007;102:298–307. doi: 10.1007/s00395-007-0652-5. [DOI] [PubMed] [Google Scholar]
- 114.Arai H, Hirano H, Mushiake S, Nakayama M, Takada G, Sekiguchi K. Loss of EDB+ fibronectin isoform is associated with differentiation of alveolar epithelial cells in human fetal lung. Am J Pathol. 1997;151:403–412. [PMC free article] [PubMed] [Google Scholar]
- 115.Fukuda T, Yoshida N, Kataoka Y, Manabe R, Mizuno-Horikawa Y, Sato M, Kuriyama K, Yasui N, Sekiguchi K. Mice lacking the EDB segment of fibronectin develop normally but exhibit reduced cell growth and fibronectin matrix assembly in vitro. Cancer Res. 2002;62:5603–5610. [PubMed] [Google Scholar]
- 116.McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol Cell Biol. 2006;26:1700–1709. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang J, Davis EC, Chapman SL, Budatha M, Marmorstein LY, Word RA, Yanagisawa H. Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ Res. 2010;106:583–592. doi: 10.1161/CIRCRESAHA.109.207852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hanada K, Vermeij M, Garinis GA, de Waard MC, Kunen MG, Myers L, Maas A, Duncker DJ, Meijers C, Dietz HC, Kanaar R, Essers J. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circ Res. 2007;100:738–746. doi: 10.1161/01.RES.0000260181.19449.95. [DOI] [PubMed] [Google Scholar]
- 119.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 120.Wang X, LeMaire SA, Chen L, Carter SA, Shen YH, Gan Y, Bartsch H, Wilks JA, Utama B, Ou H, Thompson RW, Coselli JS, Wang XL. Decreased expression of fibulin-5 correlates with reduced elastin in thoracic aortic dissection. Surgery. 2005;138:352–359. doi: 10.1016/j.surg.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 121.Spencer JA, Hacker SL, Davis EC, Mecham RP, Knutsen RH, Li DY, Gerard RD, Richardson JA, Olson EN, Yanagisawa H. Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc Natl Acad Sci U S A. 2005;102:2946–2951. doi: 10.1073/pnas.0500058102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wan W, Gleason RL., Jr Dysfunction in elastic fiber formation in fibulin-5 null mice abrogates the evolution in mechanical response of carotid arteries during maturation. Am J Physiol Heart Circ Physiol. 2013;304:H674–686. doi: 10.1152/ajpheart.00459.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Strom A, Olin AI, Aspberg A, Hultgardh-Nilsson A. Fibulin-2 is present in murine vascular lesions and is important for smooth muscle cell migration. Cardiovasc Res. 2006;69:755–763. doi: 10.1016/j.cardiores.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 124.Chapman SL, Sicot FX, Davis EC, Huang J, Sasaki T, Chu ML, Yanagisawa H. Fibulin-2 and fibulin-5 cooperatively function to form the internal elastic lamina and protect from vascular injury. Arterioscler Thromb Vasc Biol. 2010;30:68–74. doi: 10.1161/ATVBAHA.109.196725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yousif LF, Di Russo J, Sorokin L. Laminin isoforms in endothelial and perivascular basement membranes. Cell Adh Migr. 2013;7:101–110. doi: 10.4161/cam.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, Kalluri R, Shi GP. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem. 2006;281:6020–6029. doi: 10.1074/jbc.M509134200. [DOI] [PubMed] [Google Scholar]
- 127.Pfaff M, Reinhardt DP, Sakai LY, Timpl R. Cell adhesion and integrin binding to recombinant human fibrillin-1. FEBS Lett. 1996;384:247–250. doi: 10.1016/0014-5793(96)00325-0. [DOI] [PubMed] [Google Scholar]
- 128.Pereira L, Andrikopoulos K, Tian J, Lee SY, Keene DR, Ono R, Reinhardt DP, Sakai LY, Biery NJ, Bunton T, Dietz HC, Ramirez F. Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat Genet. 1997;17:218–222. doi: 10.1038/ng1097-218. [DOI] [PubMed] [Google Scholar]
- 129.Robinson PN, Godfrey M. The molecular genetics of Marfan syndrome and related microfibrillopathies. J Med Genet. 2000;37:9–25. doi: 10.1136/jmg.37.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ekmekci H, Ekmekci OB, Sonmez H, Ozturk Z, Domanic N, Kokoglu E. Evaluation of fibronectin, vitronectin, and leptin levels in coronary artery disease: impacts on thrombosis and thrombolysis. Clin Appl Thromb Hemost. 2005;11:63–70. doi: 10.1177/107602960501100107. [DOI] [PubMed] [Google Scholar]
- 131.Peng L, Bhatia N, Parker AC, Zhu Y, Fay WP. Endogenous vitronectin and plasminogen activator inhibitor-1 promote neointima formation in murine carotid arteries. Arterioscler Thromb Vasc Biol. 2002;22:934–939. doi: 10.1161/01.atv.0000019360.14554.53. [DOI] [PubMed] [Google Scholar]
- 132.Reheman A, Gross P, Yang H, Chen P, Allen D, Leytin V, Freedman J, Ni H. Vitronectin stabilizes thrombi and vessel occlusion but plays a dual role in platelet aggregation. J Thromb Haemost. 2005;3:875–883. doi: 10.1111/j.1538-7836.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 133.Seeger FH, Blessing E, Gu L, Bornhold R, Denger S, Kreuzer J. Fibrinogen induces chemotactic activity in endothelial cells. Acta Physiol Scand. 2002;176:109–115. doi: 10.1046/j.1365-201X.2002.01023.x. [DOI] [PubMed] [Google Scholar]
- 134.Maehnss K, Kobarg J, Schmitt WH, Hansen HP, Lange H, Csernok E, Gross WL, Lemke H. Vitronectin- and fibronectin-containing immune complexes in primary systemic vasculitis. J Autoimmun. 2002;18:239–250. doi: 10.1006/jaut.2002.0582. [DOI] [PubMed] [Google Scholar]
- 135.Rodriguez C, Alcudia JF, Martinez-Gonzalez J, Guadall A, Raposo B, Sanchez-Gomez S, Badimon L. Statins normalize vascular lysyl oxidase down-regulation induced by proatherogenic risk factors. Cardiovasc Res. 2009;83:595–603. doi: 10.1093/cvr/cvp136. [DOI] [PubMed] [Google Scholar]
- 136.Korol RM, Canham PB, Liu L, Viswanathan K, Ferguson GG, Hammond RR, Finlay HM, Baker HV, Lopez C, Lucas AR. Detection of altered extracellular matrix in surface layers of unstable carotid plaque: an optical spectroscopy, birefringence and microarray genetic analysis. Photochem Photobiol. 2011;87:1164–1172. doi: 10.1111/j.1751-1097.2011.00960.x. [DOI] [PubMed] [Google Scholar]
- 137.Frontini MJ, O’Neil C, Sawyez C, Chan BM, Huff MW, Pickering JG. Lipid incorporation inhibits Src-dependent assembly of fibronectin and type I collagen by vascular smooth muscle cells. Circ Res. 2009;104:832–841. doi: 10.1161/CIRCRESAHA.108.187302. [DOI] [PubMed] [Google Scholar]
- 138.Yasuda O, Fukuo K, Maeda N, Ogihara T. Elevated expression of type VIII collagen gene in the atherosclerotic plaque of the ApoE-deficient mouse. Ann N Y Acad Sci. 2001;947:312–315. doi: 10.1111/j.1749-6632.2001.tb03954.x. [DOI] [PubMed] [Google Scholar]
- 139.Brasselet C, Durand E, Addad F, Al Haj Zen A, Smeets MB, Laurent-Maquin D, Bouthors S, Bellon G, de Kleijn D, Godeau G, Garnotel R, Gogly B, Lafont A. Collagen and elastin cross-linking: a mechanism of constrictive remodeling after arterial injury. Am J Physiol Heart Circ Physiol. 2005;289:H2228–2233. doi: 10.1152/ajpheart.00410.2005. [DOI] [PubMed] [Google Scholar]
- 140.Carmo M, Colombo L, Bruno A, Corsi FR, Roncoroni L, Cuttin MS, Radice F, Mussini E, Settembrini PG. Alteration of elastin, collagen and their cross-links in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2002;23:543–549. doi: 10.1053/ejvs.2002.1620. [DOI] [PubMed] [Google Scholar]
- 141.Xiong J, Wang SM, Chen LH, Lin Y, Zhu YF, Ye CS. Elastic fibers reconstructed using adenovirus-mediated expression of tropoelastin and tested in the elastase model of abdominal aortic aneurysm in rats. J Vasc Surg. 2008;48:965–973. doi: 10.1016/j.jvs.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 142.Isenburg JC, Simionescu DT, Vyavahare NR. Elastin stabilization in cardiovascular implants: improved resistance to enzymatic degradation by treatment with tannic acid. Biomaterials. 2004;25:3293–3302. doi: 10.1016/j.biomaterials.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 143.Isenburg JC, Simionescu DT, Starcher BC, Vyavahare NR. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation. 2007;115:1729–1737. doi: 10.1161/CIRCULATIONAHA.106.672873. [DOI] [PubMed] [Google Scholar]
- 144.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Morishita R, Hashimoto N. Reduced collagen biosynthesis is the hallmark of cerebral aneurysm: contribution of interleukin-1beta and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol. 2009;29:1080–1086. doi: 10.1161/ATVBAHA.108.180760. [DOI] [PubMed] [Google Scholar]
- 145.Jeanneret C, Baldi T, Hailemariam S, Koella C, Gewaltig J, Biedermann BC. Selective loss of extracellular matrix proteins is linked to biophysical properties of varicose veins assessed by ultrasonography. Br J Surg. 2007;94:449–456. doi: 10.1002/bjs.5630. [DOI] [PubMed] [Google Scholar]
- 146.Sansilvestri-Morel P, Rupin A, Badier-Commander C, Kern P, Fabiani JN, Verbeuren TJ, Vanhoutte PM. Imbalance in the synthesis of collagen type I and collagen type III in smooth muscle cells derived from human varicose veins. J Vasc Res. 2001;38:560–568. doi: 10.1159/000051092. [DOI] [PubMed] [Google Scholar]
- 147.Bujan J, Gimeno MJ, Jimenez JA, Kielty CM, Mecham RP, Bellon JM. Expression of elastic components in healthy and varicose veins. World J Surg. 2003;27:901–905. doi: 10.1007/s00268-003-6897-8. [DOI] [PubMed] [Google Scholar]
- 148.Sansilvestri-Morel P, Rupin A, Jullien ND, Lembrez N, Mestries-Dubois P, Fabiani JN, Verbeuren TJ. Decreased production of collagen Type III in cultured smooth muscle cells from varicose vein patients is due to a degradation by MMPs: possible implication of MMP-3. J Vasc Res. 2005;42:388–398. doi: 10.1159/000087314. [DOI] [PubMed] [Google Scholar]
- 149.O’Donnell CJ, Shea MK, Price PA, Gagnon DR, Wilson PW, Larson MG, Kiel DP, Hoffmann U, Ferencik M, Clouse ME, Williamson MK, Cupples LA, Dawson-Hughes B, Booth SL. Matrix Gla protein is associated with risk factors for atherosclerosis but not with coronary artery calcification. Arterioscler Thromb Vasc Biol. 2006;26:2769–2774. doi: 10.1161/01.ATV.0000245793.83158.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cario-Toumaniantz C, Boularan C, Schurgers LJ, Heymann MF, Le Cunff M, Leger J, Loirand G, Pacaud P. Identification of differentially expressed genes in human varicose veins: involvement of matrix gla protein in extracellular matrix remodeling. J Vasc Res. 2007;44:444–459. doi: 10.1159/000106189. [DOI] [PubMed] [Google Scholar]
- 151.Xu N, Zhang YY, Lin Y, Bao B, Zheng L, Shi GP, Liu J. Increased levels of lysosomal cysteinyl cathepsins in human varicose veins: a histology study. Thromb Haemost. 2014;111:333–344. doi: 10.1160/TH13-04-0309. [DOI] [PubMed] [Google Scholar]
- 152.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S–10S. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 153.Castro MM, Rizzi E, Figueiredo-Lopes L, Fernandes K, Bendhack LM, Pitol DL, Gerlach RF, Tanus-Santos JE. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis. 2008;198:320–331. doi: 10.1016/j.atherosclerosis.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 154.Castro MM, Rizzi E, Rodrigues GJ, Ceron CS, Bendhack LM, Gerlach RF, Tanus-Santos JE. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic Biol Med. 2009;46:1298–1307. doi: 10.1016/j.freeradbiomed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 155.Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension. 2000;36:312–318. doi: 10.1161/01.hyp.36.3.312. [DOI] [PubMed] [Google Scholar]
- 156.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 157.Hinek A. Nature and the multiple functions of the 67-kD elastin-/laminin binding protein. Cell Adhes Commun. 1994;2:185–193. doi: 10.3109/15419069409004436. [DOI] [PubMed] [Google Scholar]
- 158.Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest. 2003;112:1419–1428. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1209–1217. doi: 10.1152/ajpheart.00046.2005. [DOI] [PubMed] [Google Scholar]
- 160.Ewart AK, Morris CA, Ensing GJ, Loker J, Moore C, Leppert M, Keating M. A human vascular disorder, supravalvular aortic stenosis, maps to chromosome 7. Proc Natl Acad Sci U S A. 1993;90:3226–3230. doi: 10.1073/pnas.90.8.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li DY, Toland AE, Boak BB, Atkinson DL, Ensing GJ, Morris CA, Keating MT. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet. 1997;6:1021–1028. doi: 10.1093/hmg/6.7.1021. [DOI] [PubMed] [Google Scholar]
- 162.Metcalfe K, Rucka AK, Smoot L, Hofstadler G, Tuzler G, McKeown P, Siu V, Rauch A, Dean J, Dennis N, Ellis I, Reardon W, Cytrynbaum C, Osborne L, Yates JR, Read AP, Donnai D, Tassabehji M. Elastin: mutational spectrum in supravalvular aortic stenosis. Eur J Hum Genet. 2000;8:955–963. doi: 10.1038/sj.ejhg.5200564. [DOI] [PubMed] [Google Scholar]
- 163.Urban Z, Zhang J, Davis EC, Maeda GK, Kumar A, Stalker H, Belmont JW, Boyd CD, Wallace MR. Supravalvular aortic stenosis: genetic and molecular dissection of a complex mutation in the elastin gene. Hum Genet. 2001;109:512–520. doi: 10.1007/s00439-001-0608-z. [DOI] [PubMed] [Google Scholar]
- 164.Le VP, Knutsen RH, Mecham RP, Wagenseil JE. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficient mice. Am J Physiol Heart Circ Physiol. 2011;301:H221–229. doi: 10.1152/ajpheart.00119.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hirano E, Knutsen RH, Sugitani H, Ciliberto CH, Mecham RP. Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circ Res. 2007;101:523–531. doi: 10.1161/CIRCRESAHA.107.153510. [DOI] [PubMed] [Google Scholar]
- 166.Martyn CN, Greenwald SE. Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet. 1997;350:953–955. doi: 10.1016/s0140-6736(96)10508-0. [DOI] [PubMed] [Google Scholar]
- 167.Martyn CN, Greenwald SE. A hypothesis about a mechanism for the programming of blood pressure and vascular disease in early life. Clin Exp Pharmacol Physiol. 2001;28:948–951. doi: 10.1046/j.1440-1681.2001.03555.x. [DOI] [PubMed] [Google Scholar]
- 168.Briones AM, Gonzalez JM, Somoza B, Giraldo J, Daly CJ, Vila E, Gonzalez MC, McGrath JC, Arribas SM. Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. J Physiol. 2003;552:185–195. doi: 10.1113/jphysiol.2003.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jimenez-Altayo F, Martin A, Rojas S, Justicia C, Briones AM, Giraldo J, Planas AM, Vila E. Transient middle cerebral artery occlusion causes different structural, mechanical, and myogenic alterations in normotensive and hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293:H628–635. doi: 10.1152/ajpheart.00165.2007. [DOI] [PubMed] [Google Scholar]
- 170.Boumaza S, Arribas SM, Osborne-Pellegrin M, McGrath JC, Laurent S, Lacolley P, Challande P. Fenestrations of the carotid internal elastic lamina and structural adaptation in stroke-prone spontaneously hypertensive rats. Hypertension. 2001;37:1101–1107. doi: 10.1161/01.hyp.37.4.1101. [DOI] [PubMed] [Google Scholar]
- 171.Arribas SM, Briones AM, Bellingham C, Gonzalez MC, Salaices M, Liu K, Wang Y, Hinek A. Heightened aberrant deposition of hard-wearing elastin in conduit arteries of prehypertensive SHR is associated with increased stiffness and inward remodeling. Am J Physiol Heart Circ Physiol. 2008;295:H2299–2307. doi: 10.1152/ajpheart.00155.2008. [DOI] [PubMed] [Google Scholar]
- 172.Briones AM, Xavier FE, Arribas SM, Gonzalez MC, Rossoni LV, Alonso MJ, Salaices M. Alterations in structure and mechanics of resistance arteries from ouabain-induced hypertensive rats. Am J Physiol Heart Circ Physiol. 2006;291:H193–201. doi: 10.1152/ajpheart.00802.2005. [DOI] [PubMed] [Google Scholar]
- 173.Gonzalez JM, Briones AM, Somoza B, Daly CJ, Vila E, Starcher B, McGrath JC, Gonzalez MC, Arribas SM. Postnatal alterations in elastic fiber organization precede resistance artery narrowing in SHR. Am J Physiol Heart Circ Physiol. 2006;291:H804–812. doi: 10.1152/ajpheart.01262.2005. [DOI] [PubMed] [Google Scholar]
- 174.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 175.Franklin SS. Pulse pressure as a risk factor. Clin Exp Hypertens. 2004;26:645–652. doi: 10.1081/ceh-200031962. [DOI] [PubMed] [Google Scholar]
- 176.Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 177.Wolinsky H. Response of the rat aortic media to hypertension. Morphological and chemical studies. Circ Res. 1970;26:507–522. doi: 10.1161/01.res.26.4.507. [DOI] [PubMed] [Google Scholar]
- 178.Todorovich-Hunter L, Johnson DJ, Ranger P, Keeley FW, Rabinovitch M. Altered elastin and collagen synthesis associated with progressive pulmonary hypertension induced by monocrotaline. A biochemical and ultrastructural study. Lab Invest. 1988;58:184–195. [PubMed] [Google Scholar]
- 179.Olsen MH, Christensen MK, Wachtell K, Tuxen C, Fossum E, Bang LE, Wiinberg N, Devereux RB, Kjeldsen SE, Hildebrandt P, Dige-Petersen H, Rokkedal J, Ibsen H. Markers of collagen synthesis is related to blood pressure and vascular hypertrophy: a LIFE substudy. J Hum Hypertens. 2005;19:301–307. doi: 10.1038/sj.jhh.1001819. [DOI] [PubMed] [Google Scholar]
- 180.Rizzoni D, Muiesan ML, Porteri E, Salvetti M, Castellano M, Bettoni G, Tiberio G, Giulini SM, Monteduro C, Garavelli G, Agabiti-Rosei E. Relations between cardiac and vascular structure in patients with primary and secondary hypertension. J Am Coll Cardiol. 1998;32:985–992. doi: 10.1016/s0735-1097(98)00322-2. [DOI] [PubMed] [Google Scholar]
- 181.Intengan HD, Deng LY, Li JS, Schiffrin EL. Mechanics and composition of human subcutaneous resistance arteries in essential hypertension. Hypertension. 1999;33:569–574. doi: 10.1161/01.hyp.33.1.569. [DOI] [PubMed] [Google Scholar]
- 182.Intengan HD, Thibault G, Li JS, Schiffrin EL. Resistance artery mechanics, structure and extracellular components in spontaneously hypertensive rats : effects of angiotensin receptor antagonism and converting enzyme inhibition. Circulation. 1999;100:2267–2275. doi: 10.1161/01.cir.100.22.2267. [DOI] [PubMed] [Google Scholar]
- 183.Rossi GP, Cavallin M, Belloni AS, Mazzocchi G, Nussdorfer GG, Pessina AC, Sartore S. Aortic smooth muscle cell phenotypic modulation and fibrillar collagen deposition in angiotensin II-dependent hypertension. Cardiovasc Res. 2002;55:178–189. doi: 10.1016/s0008-6363(02)00400-5. [DOI] [PubMed] [Google Scholar]
- 184.Volpe M. Treatment of systolic hypertension: spotlight on recent studies with angiotensin II antagonists. J Hum Hypertens. 2005;19:93–102. doi: 10.1038/sj.jhh.1001781. [DOI] [PubMed] [Google Scholar]
- 185.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 186.Bezie Y, Lamaziere JM, Laurent S, Challande P, Cunha RS, Bonnet J, Lacolley P. Fibronectin expression and aortic wall elastic modulus in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 1998;18:1027–1034. doi: 10.1161/01.atv.18.7.1027. [DOI] [PubMed] [Google Scholar]
- 187.Ruperez M, Lorenzo O, Blanco-Colio LM, Esteban V, Egido J, Ruiz-Ortega M. Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation. 2003;108:1499–1505. doi: 10.1161/01.CIR.0000089129.51288.BA. [DOI] [PubMed] [Google Scholar]
- 188.Brassard P, Amiri F, Schiffrin EL. Combined angiotensin II type 1 and type 2 receptor blockade on vascular remodeling and matrix metalloproteinases in resistance arteries. Hypertension. 2005;46:598–606. doi: 10.1161/01.HYP.0000176744.15592.7d. [DOI] [PubMed] [Google Scholar]
- 189.Touyz RM. Molecular and cellular mechanisms in vascular injury in hypertension: role of angiotensin II. Curr Opin Nephrol Hypertens. 2005;14:125–131. doi: 10.1097/00041552-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 190.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 191.ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 192.Fu K, Corbley MJ, Sun L, Friedman JE, Shan F, Papadatos JL, Costa D, Lutterodt F, Sweigard H, Bowes S, Choi M, Boriack-Sjodin PA, Arduini RM, Sun D, Newman MN, Zhang X, Mead JN, Chuaqui CE, Cheung HK, Zhang X, Cornebise M, Carter MB, Josiah S, Singh J, Lee WC, Gill A, Ling LE. SM16, an orally active TGF-beta type I receptor inhibitor prevents myofibroblast induction and vascular fibrosis in the rat carotid injury model. Arterioscler Thromb Vasc Biol. 2008;28:665–671. doi: 10.1161/ATVBAHA.107.158030. [DOI] [PubMed] [Google Scholar]