Abstract
Ketamine is a non-competitive N-methyl-D-aspartate receptor (NMDAR) antagonist of interest in neuropsychiatry. In the present studies, we examined the effects of subanesthetic, low micromolar ketamine on excitatory postsynaptic potentials (EPSPs), population spikes (PSs) and synaptic plasticity in the CA1 region of rat hippocampal slices. Ketamine acutely inhibited NMDAR-mediated synaptic responses with half-maximal effects near 10 µM. When administered for 15–30 min at 1–10 µM, ketamine had no effect on baseline dendritic AMPA receptor-mediated EPSPs, but persistently enhanced somatic EPSPs in the pyramidal cell body layer and augmented PS firing. Acute low micromolar ketamine also had no effect on the induction of long-term potentiation (LTP) but blocked long-term depression (LTD). Following 30 min administration of 1–10 µM ketamine, however, a slowly developing and persistent form of LTP inhibition was observed that took two hours following ketamine washout to become manifest. This LTP inhibition did not result from prolonged or enhanced NMDAR inhibition during drug washout. Effects of low ketamine on somatic EPSPs and LTP were not mimicked by a high ketamine concentration that completely inhibited NMDARs, and both of these effects were blocked by co-administration of low ketamine with a low concentration of the competitive NMDAR antagonist, 2-amino-5-phosphonovalerate or inhibitors of nitric oxide synthase. These results indicate that concentrations of ketamine relevant to psychotropic and psychotomimetic effects have complex metaplastic effects on hippocampal function that involve activation of unblocked NMDARs during ketamine exposure.
Keywords: Synaptic plasticity, NMDA receptor, Nitric oxide, Modulation, Memory, Psychosis
1. INTRODUCTION
N-methyl-D-aspartate receptors (NMDARs) are glutamate-gated ion channels that play key roles in excitatory synaptic transmission and in forms of plasticity thought to underlie learning and memory, including long-term potentiation (LTP) and long-term depression (LTD) (Malenka and Bear, 2004). When excessively activated, however, NMDARs cause excitotoxic neuronal death and contribute to neurodegenerative illnesses. NMDARs are also involved in psychiatric disorders and are emerging as targets for novel antidepressant medications (Trullas and Skolnick, 1990; Machado-Vieira et al., 2009). Studies over the past decade provide support for ketamine, a non-competitive NMDAR antagonist and dissociative anesthetic, as a rapidly-acting antidepressant at subanesthetic doses (aan het Rot et al., 2012). Following 40 min infusion of 0.5 mg/kg ketamine, antidepressant effects are observed in about two hours. These effects can persist for days but typically fade by one week after infusion (Berman et al., 2000; Zarate et al., 2006). Ketamine is also psychotomimetic at the same doses used to treat depression (Krystal et al., 1994; Newcomer et al, 1999), and ketamine infusions result in acute delusional thinking, sensory misinterpretations and difficulties with word fluency and memory. The latter symptoms usually abate over several hours as antidepressant effects emerge. Subanesthetic blood levels of ketamine associated with psychotomimetic and antidepressant effects are in the range of 0.3–0.5 µM (80–150 ng/ml) (Zhao et al., 2012), resulting in brain concentrations of 1–10 µM (Cohen et al., 1973; Hartvig et al., 1995; Doyle et al., 2013).
Ketamine’s psychotropic effects make it important to understand how the drug produces its actions at cellular and network levels. Prior studies found that ketamine’s antidepressant-like effects in rodents are associated with enhanced excitatory synaptic responses in cortex (Li et al., 2010) and hippocampus (Autry et al., 2011). Synaptic changes are linked to several signaling systems including mTOR (mammalian target of rapamycin) (Li et al., 2010), BDNF (brain derived neurotrophic factor) and ef2 (elongation factor 2) kinase (Autry et al., 2011; Nosyreva et al., 2013). Interestingly, antidepressant effects in rodents are observed with subanesthetic ketamine but not with anesthetic doses (Li et al., 2010), and antagonists with selectivity for GluN2B (NR2B) subunits also show antidepressant actions (Li et al., 2010).
Ketamine has complex effects on NMDARs (MacDonald et al., 1987). It is an activation- and voltage-dependent open channel blocker that produces a form of trapping block akin to MK-801 in which NMDAR channels close around the blocking molecule (Huettner and Bean, 1988). Relief from block requires channel opening and is facilitated at depolarized potentials. Because of these complex actions and the importance of NMDARs in synaptic plasticity, we examined effects of ketamine on synaptic function in the CA1 hippocampal region. We found that ketamine, at concentrations relevant to psychotropic actions, acutely inhibits homosynaptic NMDAR-dependent LTD but not LTP, and modifies dendrosomatic signal propagation. Surprisingly, low ketamine also induces slower developing LTP inhibition that involves activation of unblocked NMDARs.
2. MATERIALS and METHODS
2.1. Animals
Protocols for animal use were approved by the Washington University Animal Studies Committee in accordance with NIH guidelines for humane care and use of laboratory animals. All efforts were made to minimize animal suffering and the number of animals used for experiments.
2.2. Hippocampal slices
Hippocampal slices were prepared from postnatal day 30– 32 male Sprague-Dawley rats (Charles River Laboratories, New York, NY). Rats were anesthetized with isoflurane and decapitated. Slices were cut transversely into 500 µm slices using a rotary slicer in artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 5 KCl, 2 MgSO4, 2CaCl2, 1.25 NaH2PO4, 22 NaHCO3, 10 glucose, bubbled with 95% O2/5% CO2 at 4–6 °C (Izumi and Zorumski, 2012). Acutely prep ared slices were placed on mesh in 10 ml beakers containing gassed ACSF and maintained for at least 1 h at 30 °C before experiments. In some experiments , ketamine and other drugs were administered by preincubation with fixed concentrations of the drugs in these beakers followed by drug washout for specified durations before studies.
2.3. Electrophysiology
At the time of study, slices were transferred individually to a submerged recording chamber. Experiments were done at 30°C with continuous perfusion of ACSF at 2 ml/min. Extracellular recordings were obtained from the apical dendritic layer of CA1 (stratum radiatium) for analysis of dendritic EPSPs and from the cell body layer (stratum pyramidale) for analysis of somatic EPSPs and population spikes (PSs). EPSPs were measured by their maximal slopes and PSs were measured as a maximal height from the apex of the first positive peak to the most negative point of the spike. Somatic EPSPs were measured in the cell body layer as peak amplitudes. Using paired stimulations at an interval of 21 msec, slices showing paired pulse facilitation of EPSPs and paired pulse depression of PS, indicators of good slice health, were selected for study (Tokuda et al, 2010). EPSPs were monitored by applying single stimuli to the Schaffer collateral pathway every 60 s at half maximal intensity for evoking dendritic EPSPs. After establishing a stable baseline for at least 10 min and a control input-output curve, 1 Hz × 900 s low frequency stimulation (LFS) was applied to induce homosynaptic NMDAR-dependent LTD using the same intensity stimulus. LTP was induced using a standard 100 Hz × 1 s high frequency stimulus (HFS). The magnitude of LTD and LTP was determined 60 min following LFS or HFS. Signals were digitized and analyzed using pClamp 5.01 (Molecular Devices, Sunnyvale CA).
For investigation of paired-pulse plasticity of PSs and AMPA receptor-mediated EPSPs, dual stimuli of identical intensity were delivered at an interval of 21 msec. The stimulus intensity was initially set below threshold for evoking responses and increased in a step-wise fashion every 10 sec until 6 pairs of stimuli were administered.
Isolated NMDAR EPSPs were recorded in ACSF containing lowered Mg2+ (0.1 mM), 2.5 mM Ca2+ and 30 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) as described previously (Izumi et al., 2005; 2006). Magnesium was lowered to relieve NMDAR channel block and CNQX was included to inhibit AMPAR EPSPs.
2.4. Chemicals
2-Amino-5-phosphonovaleric acid (APV), ZD 7288, rapamycin, 3-bromo-7-nitroindazole and L-N-monomethylarginine were purchased from Tocris Bioscience (Ellisville, MO). Finasteride was purchased from Steraloids Inc. (Newport, RI). Other chemicals were purchased from Sigma Chemical Company (St. Louis, MO). (RS)-ketamine was used for all experiments.
2.5. Data Analysis
Data are expressed as mean ± s.e.m and reported as percent of baseline control responses (set at 100%). In these studies, “n” represents the number of slices studied in a given condition, and, unless stated otherwise, data were normalized with respect to initial control responses. Points in the graphs without error bars have s.e.m. smaller than the symbol size. Statistical comparisons are based on analysis of input / output curves at baseline and 60 min following LFS or drug exposure, and represent the degree of change at the half-maximal point on the input / output curves compared with baseline responses. In some experiments, we also analyzed paired-pulse plasticity using the baseline stimulation intensity to changes in EPSP to PS relationships. Where appropriate, Student’s t-test was used for comparisons between two groups. If a test of equal variance failed, the non-parametric Mann–Whitney rank sum test was applied. Statistical analyses and plots were performed using commercial software (SigmaPlot 5.01 and 9.0, and SigmaStat 3.1; Systat Software Inc., Richmond, CA). P-values of less than 0.05 were considered statistically significant.
3. RESULTS
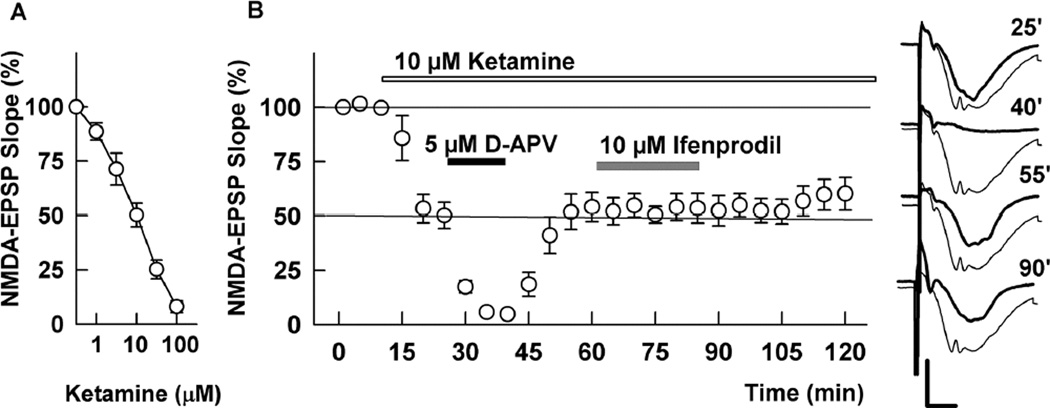
To determine how ketamine affects hippocampal function, we initially examined the concentration-dependence of ketamine against isolated NMDAR-mediated synaptic responses in the CA1 region. Ketamine inhibited NMDAR EPSPs with clearly detectable effects at 1 µM, an EC50 near 10 µM, and complete block at 100 µM (Figure 1A). Because antidepressant effects of ketamine are mimicked by NMDAR antagonists with GluN2B selectivity, we examined interactions of ketamine with 2-amino-5-phosphonovalerate (APV), a broad spectrum competitive NMDAR antagonist, and ifenprodil, an antagonist with selectivity for receptors expressing GluN2B subunits at low micromolar concentrations (Priestley et al., 1995; Paoleti et al., 2013). At 10 µM, ketamine inhibited NMDAR EPSPs by about 50% (Figure 1B). We previously found that 10 µM ifenprodil and 5 µM D-APV, administered individually, also produce about 50% NMDAR inhibition (Izumi et al., 2005; 2006). In the presence of10 µM ketamine, addition of 5 µM D-APV resulted in complete, but reversible NMDAR inhibition. In contrast, administration of 10 µM ifenprodil in the presence of ketamine resulted in no further increase in NMDAR block compared to ketamine alone (Figure 1B). The complete block of NMDARs with ketamine and D-APV is similar to what we have observed previously with a combination of 10 µM ifenprodil and 5 µM D-APV (Izumi et al., 2005; 2006). Taken together, these results suggest that low micromolar ketamine may have some preference for GluN2B containing receptors in the CA1 region.
Figure 1.
Effects of ketamine on NMDAR EPSPs. A. In the presence of CNQX and low Mg2+ ketamine inhibited isolated NMDAR EPSPs in a concentration dependent manner with an EC50 near 10 µM (N = 5 each). B. At 10 µM, ketamine (white bar) depressed NMDAR EPSPs by about 50%. Application of 5 µM D-APV (black bar), a concentration that also inhibits CA1 NMDAR EPSPs by about 50%, caused nearly complete, reversible depression in the presence of ketamine. Application of 10 µM ifenprodil (gray bar), however, did not further depress NMDAR EPSPs in the presence of ketamine (N = 5). Traces show representative waveforms at designated times with the initial control traces as thin lines. Calibration: 1 mV, 5 msec.
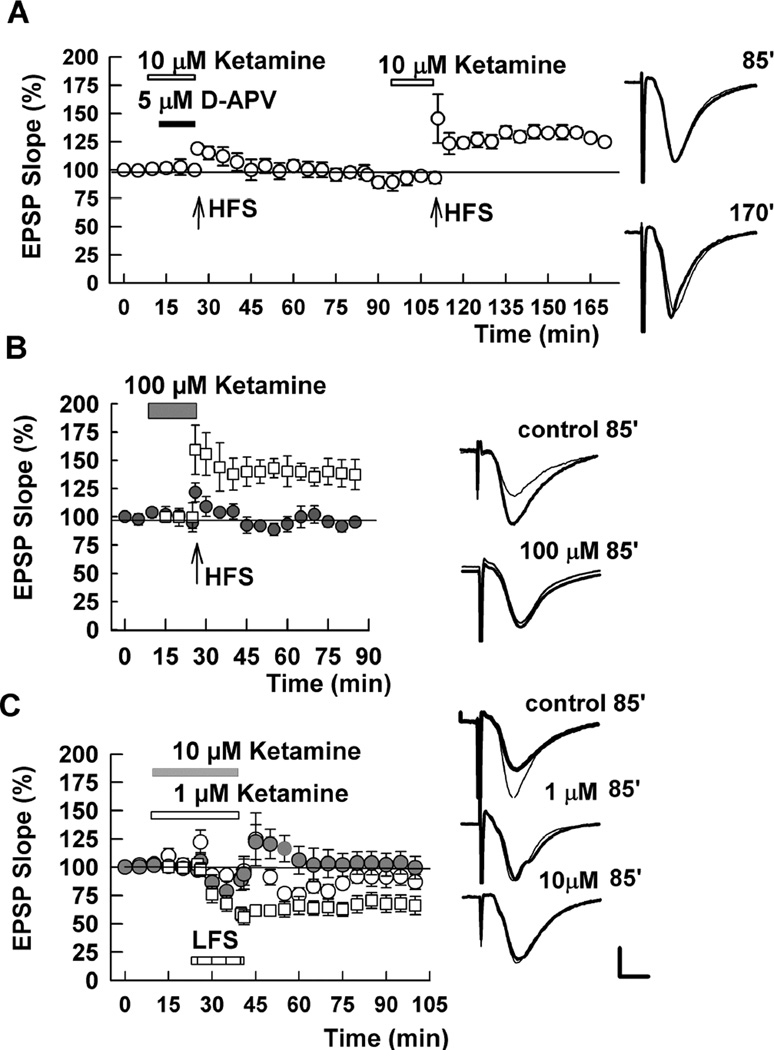
We next examined acute effects of ketamine against NMDAR-dependent LTP and LTD in the CA1 region (Figure 2). LTP was induced with a single 100 Hz × 1 s HFS that typically results in a 40–50% increase in the slope of AMPA receptor (AMPAR)-mediated EPSPs measured 60 min following HFS (e.g. Figure 2B). Ketamine acutely blocked LTP only at high concentrations. When administered for 15 min prior to HFS, a combination of 10 µM ketamine plus 5 µM D-APV completely blocked LTP induction, consistent with the complete block of synaptic NMDARs shown in Figure 1. At 10 µM ketamine, a single HFS readily induced LTP (Figure 2A). Similarly, 30 µM ketamine, administered for 30 min prior to HFS, failed to block LTP induction (EPSP slope: 136.3 ± 7.7% of baseline 60 min following HFS, N = 5; not shown). A high concentration of ketamine (100 µM) had no acute effect on baseline AMPAR-mediated EPSPs but completely inhibited LTP (Figure 2B).
Figure 2.
Acute effects of ketamine on LTP and LTD. A. HFS (100 Hz, 1s; arrows) failed to induce LTP in the presence of 10 µM ketamine (white bar) plus 5 µM D-APV (black bar), but readily induced LTP in the presence of 10 µM ketamine alone. B. HFS (arrow) induced LTP in control slices (white squares) but not in the presence of 100 µM ketamine (black circles). C. In contrast to LTP, LFS (1 Hz, 900s; hatched bar) failed to induce LTD in the presence of 1 µM (open circles) or 10 µM ketamine (gray circles). Control LTD is shown in white squares. Traces show representative waveforms at designated times with the initial control traces as thin lines. Calibration: 1 mV, 5 msec.
Homosynaptic NMDAR-dependent LTD was induced using a standard protocol of 1 Hz stimulation × 15 min. This LFS resulted in a persistent decrease in AMPAR EPSP slopes measured 60 min following stimulation (53.6 ± 6.5% of baseline responses, N = 5) (Figure 2B). In contrast to LTP, LTD was inhibited by 1 µM and 10 µM ketamine administered for 15 min before and during LFS (Figure 2C). Prior studies have indicated that NMDARs expressing GluN2B subunits are preferentially involved in LTD (Liu et al., 2004; Massey et al., 2004; Berberich et al., 2005; Izumi et al., 2005; 2006; Bartlett et al., 2007) and the effects of ketamine against LTD are consistent with its apparent preference for GluN2B containing receptors at low concentrations (Figure 1).
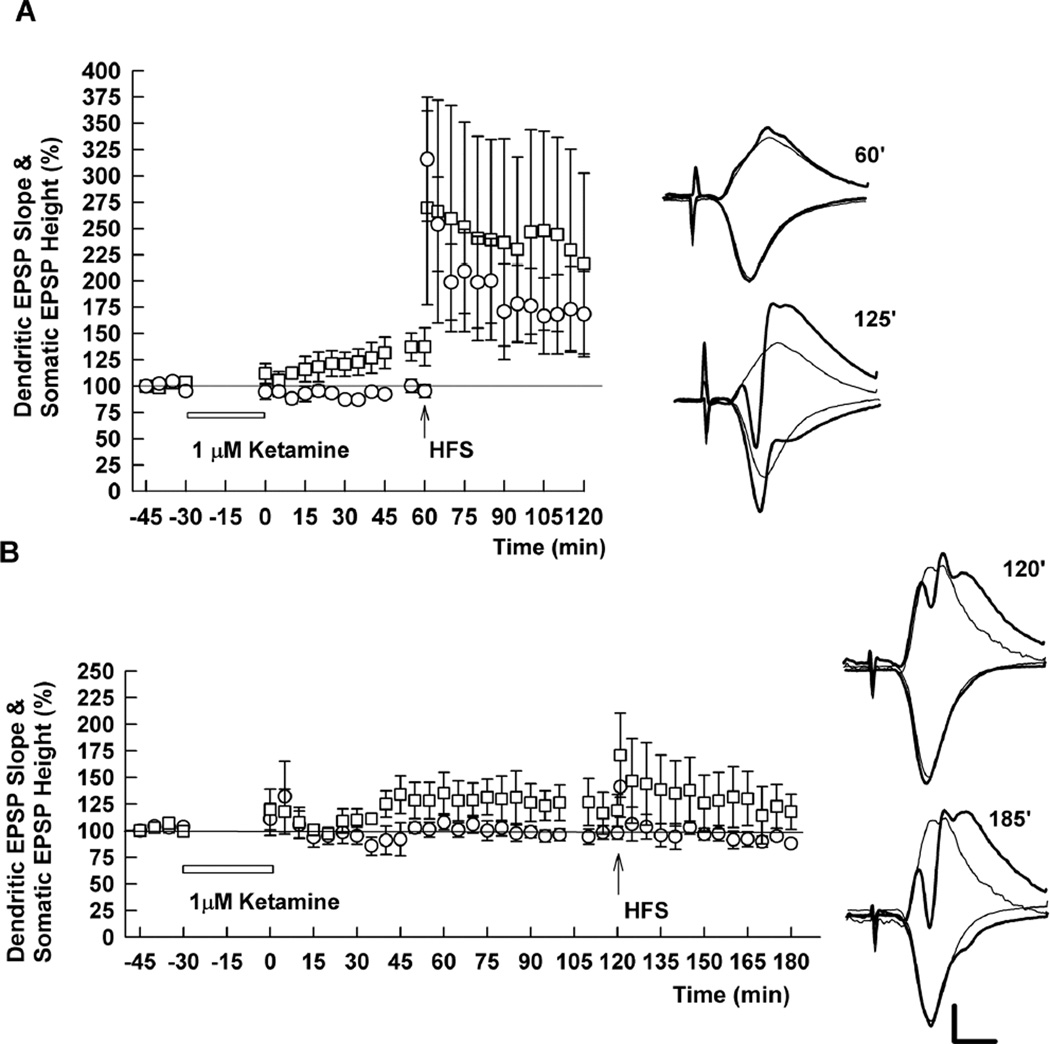
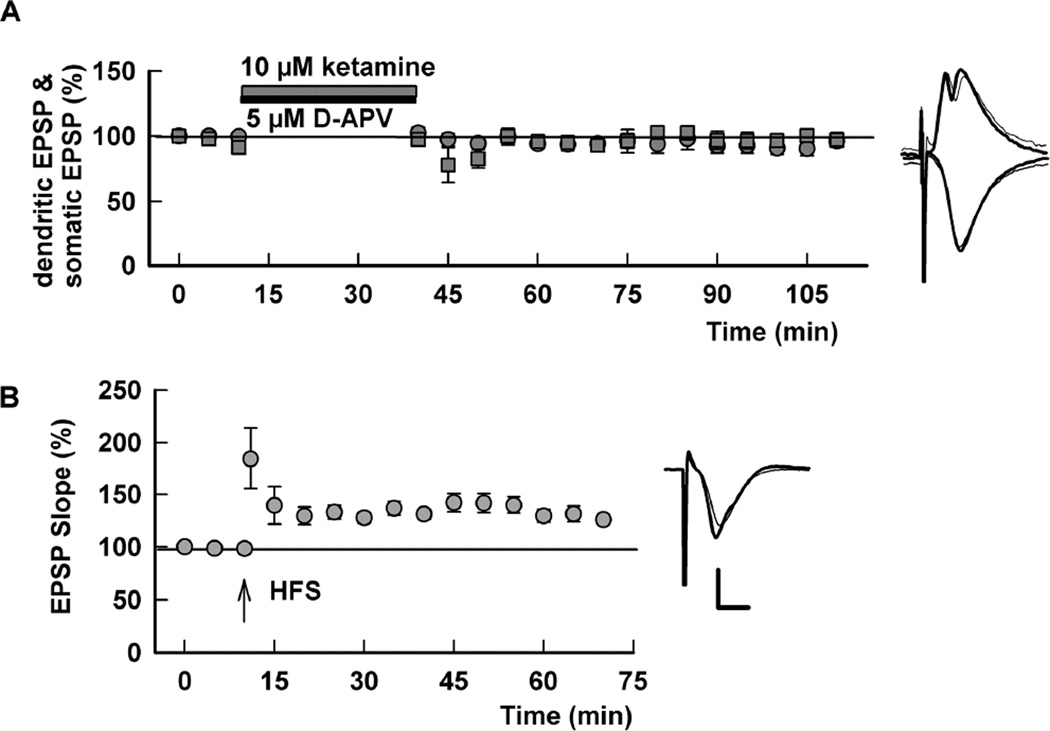
Prior studies indicate that ketamine may act by modulating spontaneous synaptic transmission (Autry et al., 2011; Kavalali and Monteggia, 2012; Nosyreva et al., 2013). In these earlier studies, treatment of hippocampal slices with 20 µM ketamine for 30 min in the absence of stimulation of the Schaffer collateral pathway resulted in persistently enhanced evoked synaptic responses following drug washout (Autry et al., 2011; Nosyreva et al., 2013). We pursued these observations by examining effects of low micromolar ketamine on synaptic transmission and LTP in the absence of stimulation during ketamine perfusion. With this paradigm, we found that 1–10 µM ketamine administered for 30 min has two additional effects on CA1 responses. First, low ketamine produces an early and persistent enhancement of somatic EPSPs recorded in the pyramidal cell body layer (145.2 ± 17.8% of control with 1 µM ketamine 60 min following washout, N=5), without effect on dendritic EPSPs recorded in stratum radiatum (94.0 ± 4.2% of control) (Figure 3A). The enhanced somatic EPSPs persisted for at least two hours, the longest duration studied systematically (Figure 3B). Second, when HFS is administered one hour after ketamine washout, LTP of both dendritic EPSPs (175.8 ± 20.8% of pre-HFS baseline 60 min following HFS, N=5) and somatic EPSPs (168.0 ± 10.2% of baseline) is observed (Figure 3A), consistent with data shown in Figure 2. When HFS is administered two to four hours after ketamine washout, however, LTP of dendritic (92.6 ± 2.7% of pre-HFS baseline, N=5) and somatic EPSPs (103.6 ± 11.7%) is completely inhibited (Figure 3B).
Figure 3.
Effects of 1 µM ketamine on dendritic EPSPs, somatic EPSPs and LTP after drug washout. A. LTP of dendritic EPSPs (circles) was induced when HFS (100 Hz, 1s; arrow) was delivered 1 hour after washout of 1 µM ketamine, which was administered for 30 min (white bar) in the absence of stimulation. Somatic EPSPs (squares) were augmented without effect on dendritic EPSPs after washout of ketamine, and also exhibited LTP following HFS. B. The enhancement of somatic EPSPs (squares) persisted for at least 2 hours after washout of 1 µM ketamine. However, HFS delivered 2 hours after ketamine washout failed to induce LTP of either dendritic or somatic EPSPs. Traces show representative waveforms at designated times with the initial control traces as thin lines. Calibration: 1 mV, 5 msec.
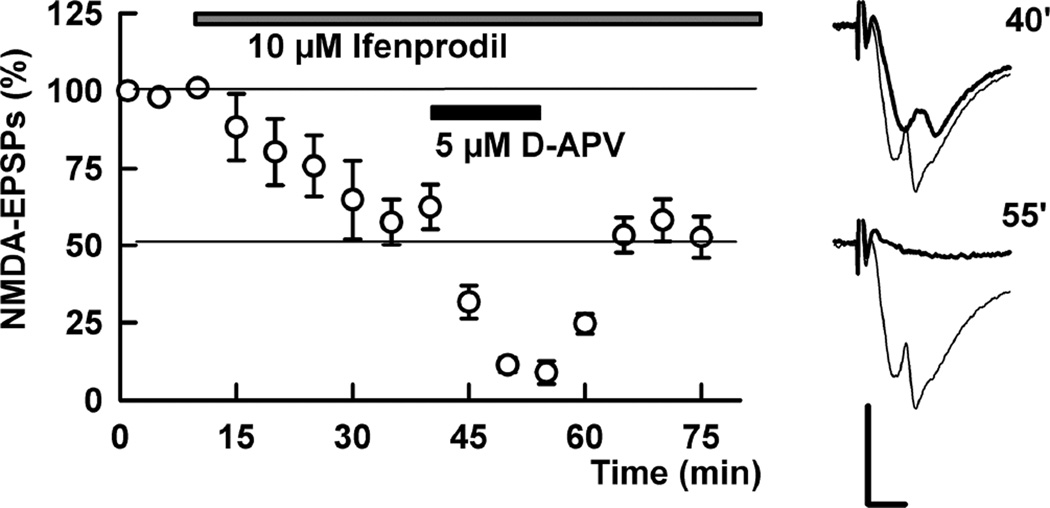
The finding that LTP is intact one hour following ketamine but inhibited two or more hours after ketamine washout strongly suggests that delayed LTP inhibition does not result from persistent or accumulating NMDAR channel block by residual ketamine. To test this further, we examined isolated NMDAR-mediated EPSPs two hours after washout of 1 µM ketamine (x 30 min), and found that NMDAR responses could be reliably recorded and that 10 µM ifenprodil inhibited these NMDAR EPSPs by 43.4 ± 7.3% (N=5), similar to control slices. A combination of ifenprodil plus 5 µM D-APV blocked these NMDAR EPSPs by more than 90% (91.1 ± 3.7% inhibition, Figure 4), also consistent with effects in naïve slices (Izumi et al., 2005; 2006).
Figure 4.
NMDAR EPSPs are not eliminated in slices pretreated with low ketamine. In the presence of CNQX and low Mg2+ NMDAR EPSPs were reliably recorded in ketamine pretreated slices (N=5). For these studies, slices were pretreated with 10 µM ketamine for 30 min and ketamine was washed out for 2 hours prior to recording. In these slices, administration of 10 µM ifenprodil (gray bar) partially depressed NMDAR EPSPs and addition of 5 µM D-APV (black bar) almost completely depressed NMDAR EPSPs in a reversible manner. Traces show representative waveforms at designated times with the initial control traces as thin lines. Calibration: 1 mV, 5 msec.
These studies indicate that ketamine (Figure 1) results in sustained changes in somatic EPSPs and a slowly developing inhibition of CA1 LTP (Figure 3). Because low micromolar ketamine only partially inhibits synaptic NMDARs, we reasoned that activation of the remaining unblocked NMDARs may contribute to our observations (Tokuda et al., 2011). To test this, we co-administered low micromolar ketamine plus a low concentration of D-APV. We found that both the effects of ketamine on somatic EPSPs and the slower developing LTP inhibition are overcome by complete NMDAR block during ketamine exposure (Figure 5). Co-administration of low micromolar APV with 10 µM ketamine prevented the acute enhancement of somatic EPSPs while having no effect on dendritic EPSPs (somatic EPSPs: 98.4 ± 6.7% of baseline; dendritic EPSPs: 98.2 ± 6.5% 60 min following 10 µM ketamine + 5 µM D-APV × 30 min, N=5) (Figure 5A). Furthermore, in slices pre-treated with 10 µM ketamine plus 5 µM D-APV for 30 min, we found that a single 100 Hz × 1s HFS resulted in LTP two to four hours following washout of both drugs (Figure 5B).
Figure 5.
Effects of pretreatment with low ketamine plus APV on synaptic responses and LTP. A. Unlike low ketamine alone, administration of 10 µM ketamine plus 5 µM D-APV for 30 min had no acute effect on either dendritic (circles) or somatic (squares) EPSPs. Traces show representative EPSPs and PSs recorded before (thin lines) and 60 min after (thick lines) drug washout. B. In slices pretreated with 10 µM ketamine plus 5 µM D-APV for 30 min followed by 2–4 hour drug washout, a single HFS (arrow) successfully induced LTP. Traces depict representative waveforms during the baseline period (thin) and 60 min following HFS (thick). Calibration: 1 mV, 5 msec.
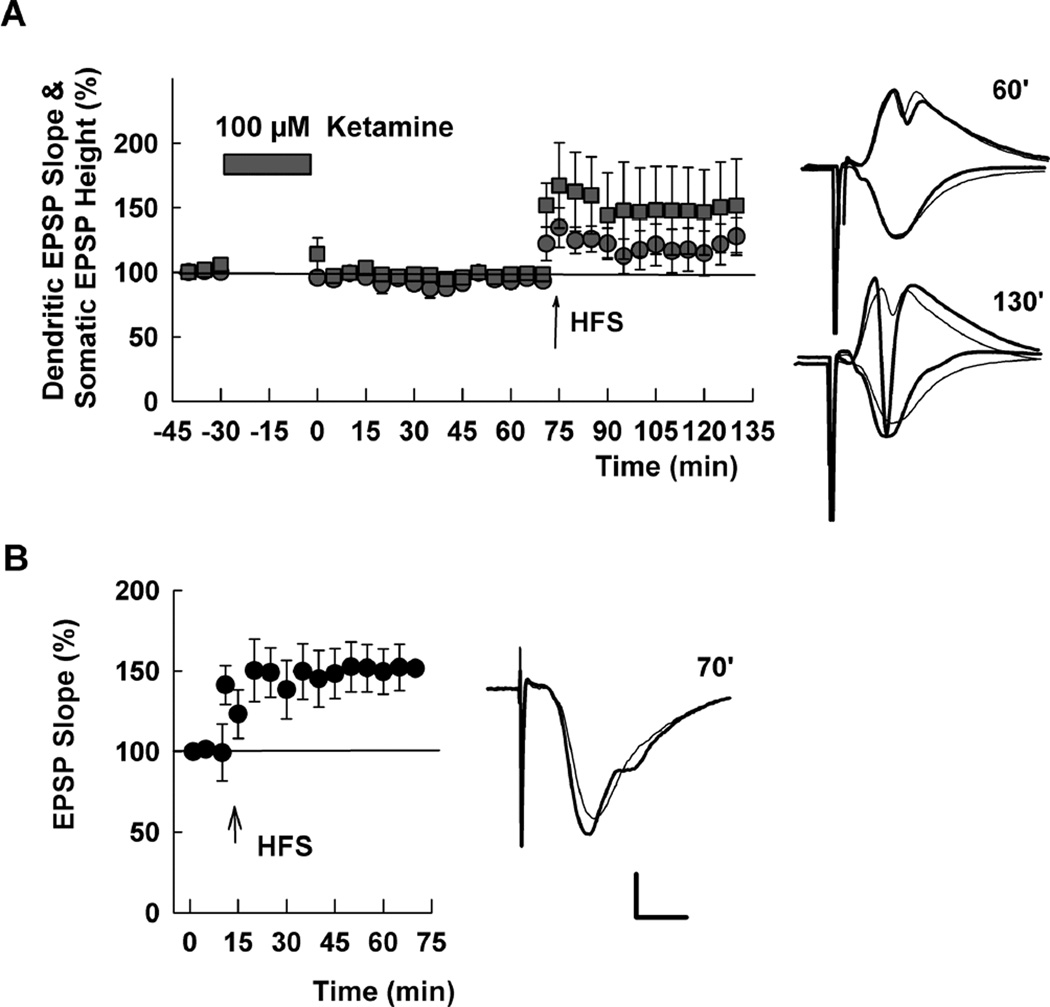
Because complete NMDAR antagonism during low ketamine administration overcomes the change in somatic EPSPs and the slowly developing block of LTP (Figure 5), it is possible that these effects may not occur with high ketamine concentrations that block NMDARs completely (Figure 1). To test this, we treated slices with 100 µM ketamine for 30 min in the absence of synaptic stimulation. In contrast to low micromolar ketamine, 100 µM ketamine eliminated changes in somatic EPSPs observed with low ketamine (somatic EPSPs = 98.4 ± 4.3% 60 min after washout, N=5) (Figure 6A). LTP of both somatic and dendritic EPSPs was also induced in these slices (Figure 6A), again supporting the idea that the late-developing effects of low micromolar ketamine on LTP do not simply result from persistent block of NMDAR channels. To determine whether high ketamine overcomes the metaplastic LTP inhibition, we also examined slices that were pretreated with 100 µM ketamine for 30 min followed by two to four hours of washout prior to synaptic studies. In these slices, 100 Hz × 1 s HFS reliably induced LTP of dendritic EPSPs (141.5 ± 9.0% of baseline, N=5, Figure 6B).
Figure 6.
A high concentration of ketamine does not reproduce the effects of low ketamine on EPSPs or LTP. A. Administration of 100 µM ketamine in the absence of synaptic stimulation for 30 min did not alter dendritic (circles) or somatic EPSPs (squares). HFS (100 Hz, 1s; arrow) delivered 75 min after washout of 100 µM ketamine induced LTP. B. HFS also induced LTP in slices pretreated with 100 µM ketamine × 30 min (circles) administered 2–4 hours before experiments. Traces show representative waveforms at designated times with the initial control traces as thin lines. Calibration: 1 mV, 5 msec.
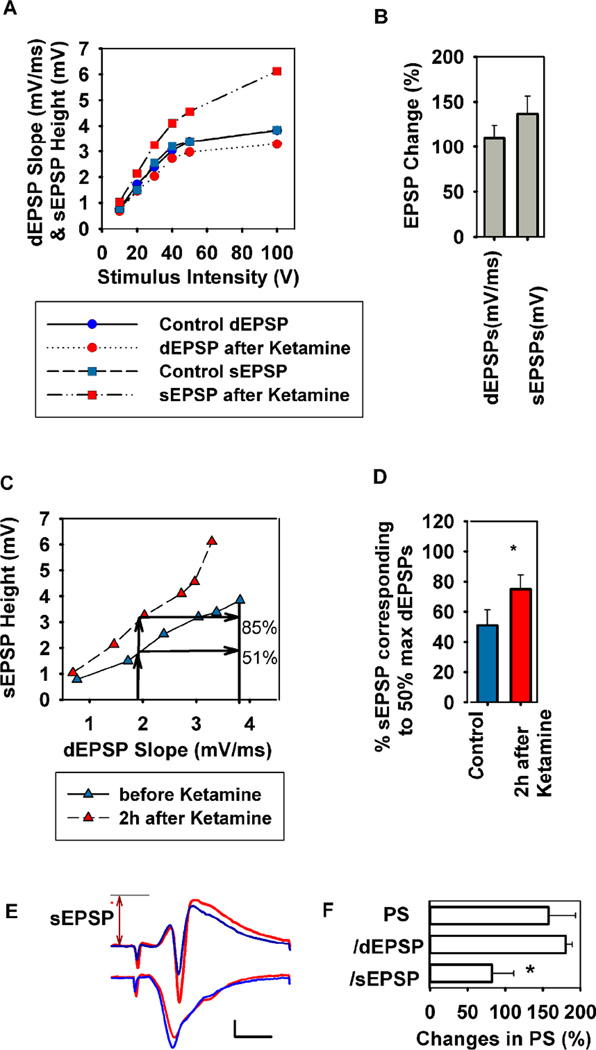
The effects of low micromolar ketamine on somatic EPSPs without effect on dendritic EPSPs suggest that ketamine may alter CA1 dendrosomatic processing without directly modulating synaptic inputs in the Schaffer collateral pathway. We examined this by analyzing the relationship between dendritic and somatic EPSPs and PSs. At 1 µM, ketamine produced a leftward shift and increase in amplitude of maximal responses in the input-output curve for somatic EPSP amplitude (sEPSPs) with no effect on dendritic EPSP slopes (dEPSPs) (Figure 7A,B). Furthermore, ketamine caused a leftward shift and enhanced maximal sEPSP amplitudes relative to dEPSP inputs (Figure 7C–E). Consistent with the increase in sEPSPs, ketamine also enhanced CA1 population spike (PS) firing (Figure 7F). Relative to the dEPSP required to evoke a 50% maximal PS, PS amplitudes were augmented by 180.6 ± 8.7% of control following ketamine (N=5). This increase in PS amplitude, however, is accounted for by the enhanced sEPSPs following ketamine. Thus, when adjusted for increases in sEPSP height, PS amplitude is not augmented (82.3 ± 8.8% of control). Taken together these findings indicate that ketamine produces a form of E-S (EPSP-Spike) dissociation resulting from changes in dendrosomatic charge transfer but not involving direct changes in synaptic inputs (as measured by dEPSPs) or somatic excitability (as measured by PS amplitude relative to sEPSPs).
Figure 7.
Low ketamine alters the dendritic-somatic EPSP relationship in the CA1 area. To determine changes in the relationship between dendritic (dEPSPs) and somatic EPSPs (sEPSPs), we analyzed input-output (IO) curves to find somatic EPSP changes at 50% maximal dendritic EPSPs (N = 6 slices). A. Representative IO curves of dendritic field EPSP slope (mV/ms, circles) and somatic EPSP height (mV, squares) elicited by 6 different stimuli before (blue) and 2 hours after 30 min administration of 1 µM ketamine (red). B. A summary of changes in dendritic EPSP slope and somatic EPSP height after ketamine administration compared to baseline at the 50% maximal point on the IO curves. C. From the IO curves for dendritic EPSP slope and somatic EPSP height (A), the somatic EPSP height induced by a half maximal dendritic EPSP stimulus was determined. The upward arrows show the somatic EPSP height corresponding to the dendritic EPSPs at the original half maximal stimulus. In this case, the original somatic EPSP corresponding to the half maximal dendritic EPSP was 51% maximal; this value was increased to 85% maximal after ketamine. D. A summary of changes in somatic EPSP height after ketamine administration based on analyses shown in C. * P < 0.05 by paired t-test. E. Representative EPSPs and PSs obtained from the same slice shown in A and C before (blue) and 2 hours after ketamine (red). In this case, in spite of a subtle decrease in dendritic EPSPs after ketamine, somatic EPSPs and PSs were augmented. The arrow depicts the height of somatic EPSP after ketamine. Calibration: 1 mV, 5 msec. F. The graph shows changes in 50% maximal PS amplitudes following ketamine uncorrected for changes in EPSPs (top bar) and corrected for changes dendritic (d) EPSP slopes (middle bar) and somatic (s) EPSP heights (bottom); *P < 0.05 by paired t-test.
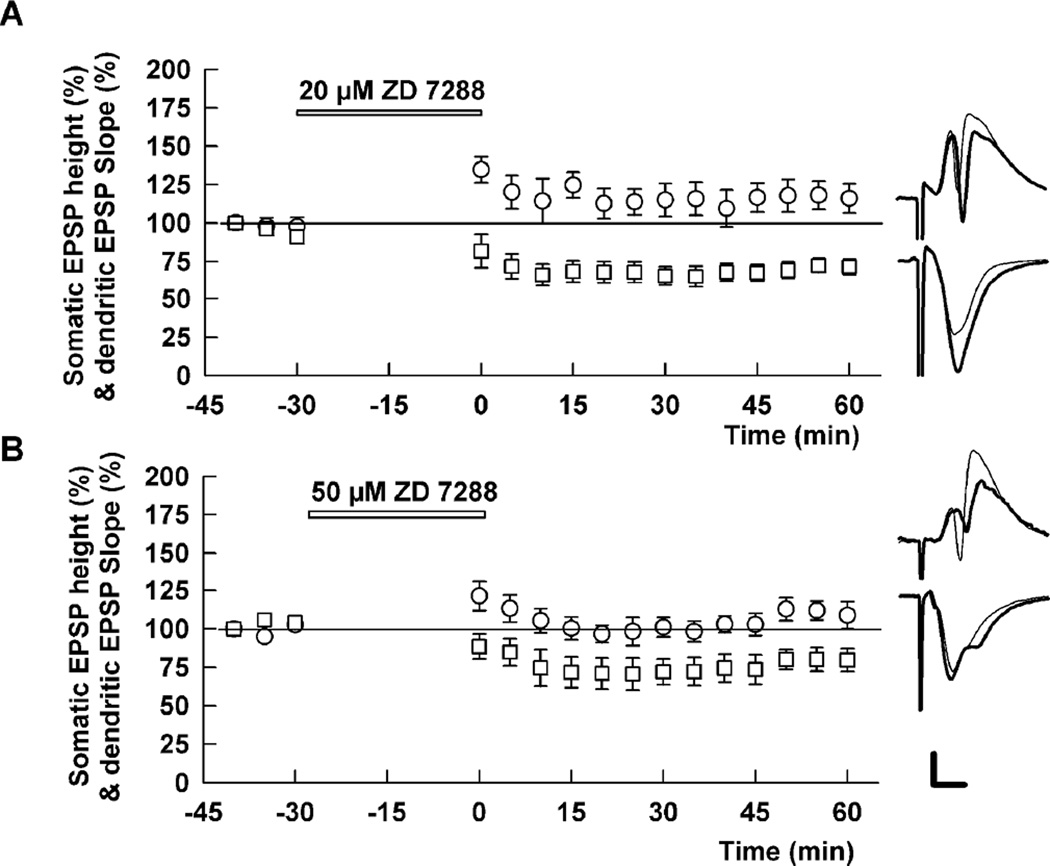
In addition to effects on NMDARs, prior studies indicate that low micromolar ketamine inhibits hyperpolarization activated cation channel1 (HCN1) and that these effects contribute to anesthetic actions (Chen et al., 2009). Because HCN channels are expressed on pyramidal neuron dendrites, blockade of HCN channels could alter the propagation of synaptic inputs from dendrites to the cell body layer, accounting for changes in somatic EPSPs without influence on dendritic EPSPs (Magee, 2000). To examine the possible role of HCN channels, we used the HCN inhibitor, ZD7288 (Harris and Constanti, 1995). The effects of ketamine on CA1 EPSPs were not mimicked by 20 µM or 50 µM ZD7288. In contrast, 20 µM ZD7288 produced a depression of somatic EPSPs (71.4 ± 5.1% of control, N=5) with augmented dendritic EPSPs (116.0 ± 9.5% of control) (Figure 8). Somatic EPSPs corresponding to 50% maximal dendritic EPSPs were originally 52.3 ± 5.5% but declined to 32.0 ± 5.4% following ZD7288 (p < 0.05), indicating that effects on EPSP ratios also differed from ketamine. Similar effects on somatic EPSPs were observed with 50 µM ZD7288 (79.7 ± 7.5% of control, N=5), although effects on dendritic EPSPs were not statistically significant (108.8 ± 8.9% of control).
Figure 8.
An HCN blocker does not mimic ketamine’s effects on dendritic and somatic EPSPs. A. Administration of 20 µM ZD7288 slightly enhanced dendritic EPSPs (circles) but depressed somatic EPSPs (squares) (N=5). B. Similarly, administration of 50 µM ZD7288 depressed somatic EPSPs (squares). Dendritic EPSPs were not significantly enhanced. Traces show representative waveforms 60 min after washout of ZD 7288 with the initial control traces as thin lines. Calibration: 1 mV, 5 msec.
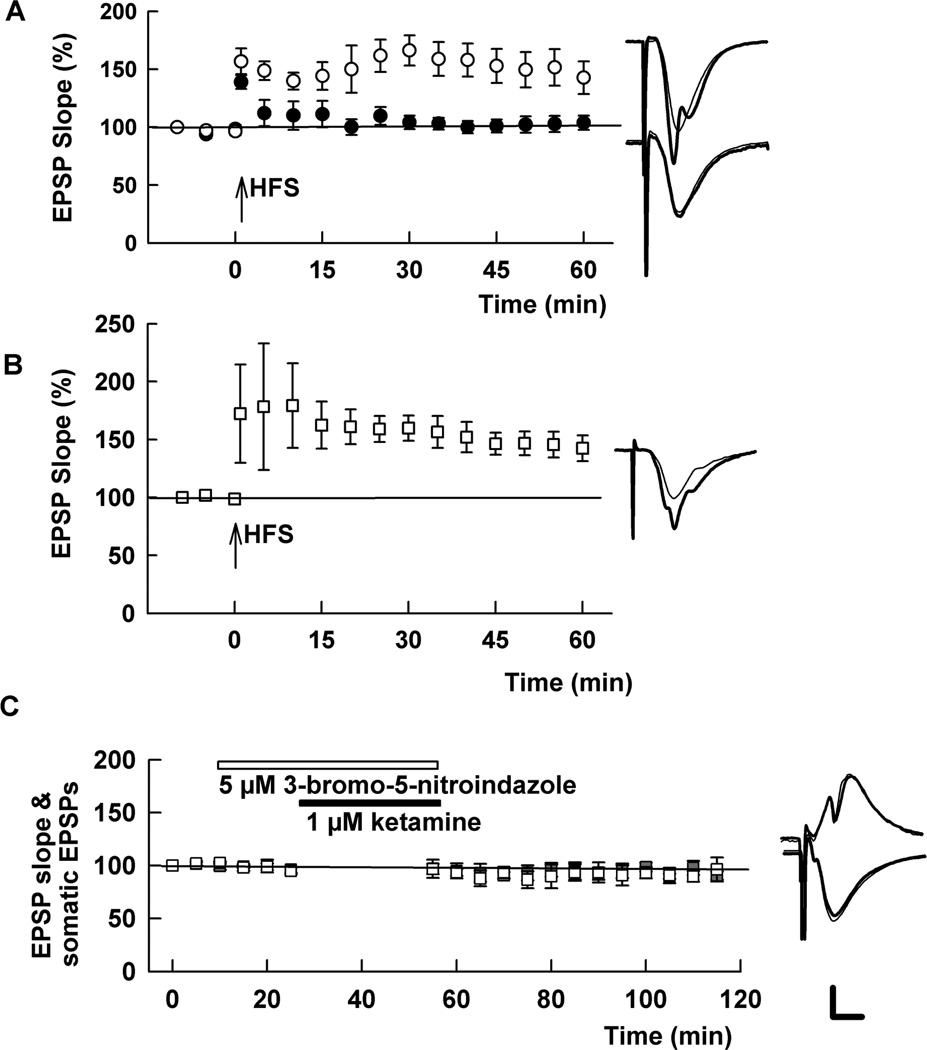
In a final set of experiments, we explored possible mechanisms contributing to ketamine-mediated modulation. Based on prior work showing that mTOR contributes to synaptic effects of ketamine in cortex (Li et al., 2010), we examined the effects of pretreatment of slices with ketamine plus rapamycin, an inhibitor of mTOR. At 200 nM, rapamycin had no effect on 1 µM ketamine’s ability to block LTP (102.7 ± 9.9%, N=5; not shown). We also found no effect of pretreatment with 1 µM finasteride (109.1 ± 5.1%, N=5; not shown), an inhibitor of neurosteroid synthesis that overcomes the metaplastic effects of ethanol on LTP (Tokuda et al., 2011). In contrast, 100 µM L-N-monomethylarginine (L-NMMA), a competitive nitric oxide synthase (NOS) antagonist, reversed the effects of 1 µM ketamine pretreatment on LTP (EPSP slopes: 145 ± 9.5% of baseline 60 min following HFS, N=5), Figure 9A), consistent with the previously described role of NO in acute NMDAR-mediated LTP inhibition (Izumi et al., 1992b; 2008). The effects of L-NMMA were reversed by co-treatment with 1 mM L-arginine, the natural substrate for NOS (104.4 ± 2.8%, N=5; p < 0.01). Similarly, a potent inhibitor of neuronal NOS, 3-bromo-7-nitroindazole (5 µM), overcame the effects of ketamine on both LTP (133.8 ± 7.0%, N=5, Figure 9B) and somatic EPSPs (96.2 ± 11.4% of baseline 60 min following drug administration, N=5, Figure 9C), while having no effect on dendritic EPSPs (90.4 ± 3.8% of baseline).
Figure 9.
Nitric oxide synthase (NOS) inhibitors overcome the effects of low ketamine on LTP and somatic EPSPs. A. Co-administration of 100 µM L-NMMA, a broad-spectrum and competitive NOS inhibitor, with 1 µM ketamine for 30 min followed by 2–4 hour drug washout overcame the inhibitory effects of ketamine on LTP (white circles). A single 100 Hz × 1 s HFS was administered at the arrow. The effects of L-NMMA were reversed by 1 mM L-arginine, the natural substrate for NOS (black circles). B. Pre-incubation of slices in 1 µM ketamine plus 5 µM 3-bromo-7-nitroindazole, a potent neuronal NOS inhibitor, ketamine on LTP. C. 3-Bromo-7-nitroindazole (5 µM) also blocked the acute effects of ketamine of somatic EPSPs (gray squares), and had no effect on dendritic EPSPs (white squares). Traces depict representative EPSPs with baseline traces shown as thin lines. Calibration: 1 mV, 5 msec.
4. DISCUSSION
The present results demonstrate that low micromolar concentrations of ketamine, similar to those likely achieved in brain during ketamine infusions for the treatment of depression or induction of psychosis (Zhao et al., 2012; Hartvig et al., 1995; Doyle et al., 2013), have significant effects on CA1 hippocampal function in juvenile rats, resulting in changes in dendrosomatic processing and negatively modulating LTD and LTP, two forms of synaptic plasticity thought to underlie memory. LTD inhibition and enhancement of somatic EPSPs are observed shortly following ketamine administration, while modulation of LTP develops more slowly, being absent immediately and one hour post-ketamine, but present two hours or more after exposure. How or whether these effects relate to psychotomimetic and antidepressant actions of ketamine remains to be determined, although these effects could contribute to ketamine-induced cognitive dysfunction. The time course of the effects has some correlation with observations in humans. When 0.5 mg/kg ketamine is infused into humans over 40 min, there is an early phase of disinhibition that includes persecutory thinking, sensory misinterpretation and memory impairment (Krystal et al., 1994; Zarate et al., 2006). These symptoms dampen a few hours after ketamine at a time when antidepressant effects become manifest (about two hours after ketamine) (Zarate et al., 2006). In our studies, somatic EPSP changes and acute LTD inhibition occur early, while altered LTP induction becomes manifest hours later.
At low micromolar concentrations, ketamine partially inhibits synaptic NMDARs, blocking NMDAR EPSPs by about 50% at 10 µM. Although ketamine is not NMDAR subtype selective, it has some preference for GluN2C–expressing receptors in the presence of extracellular magnesium (Kotermanski and Johnson, 2009). Furthermore, the EC50 for inhibiting GluN1/GluN2A and GluN1/GluN2B receptors in the presence of magnesium is similar to what we observed (Kotermanski and Johnson, 2009), and GluN2A and GluN2B are the predominant GluN2 subunits expressed at synapses in hippocampus (Gray et al., 2011; Tovar and Westbrook, 1999; Tovar et al., 2013). We found that addition of 10 µM ifenprodil to 10 µM ketamine produces no further synaptic NMDAR inhibition, in contrast to nearly complete block with addition of low micromolar APV. At low micromolar concentrations, ifenprodil is a GluN2B antagonist (Priestley et al., 1995) and a prior study found that the NMDA channel blockers with properties similar to ketamine have some preference for GluN1/GluN2B over GluN1/GluN2A receptors (Bettini et al., 2010). Differences in the kinetics of GluN2A and GluN2B NMDARs could contribute to greater apparent effects of low ketamine on ifenprodil-sensitive NMDARs (Paoletti et al., 2013), and our experiments do not address possible contributions of presynaptic or extrasynaptic NMDARs (Kato et al., 1999). Previous studies also suggest that GluN2B–expressing receptors contribute significantly to LTD induction (Liu et al., 2004; Massey et al., 2004; Berberich et al., 2005; Izumi et al., 2005; 2006; Bartlett et al., 2007) and we found that acute administration of low micromolar ketamine was effective against LTD but not LTP. NMDARs appear to contribute to LTD induction by both metabotropic (Navabi et al., 2013) and ionotropic (Babiec et al., 2014) signaling. While we did not directly examine this issue, we found that the effects of acute low ketamine on LTD correlate with block of a subtype of synaptic NMDARs.
Prior studies have found that ketamine can enhance CA1 dendritic EPSPs following 30 min acute administration at 20 µM in the absence of synaptic stimulation (Autry et al., 2011; Nosyreva et al., 2013), and that ketamine enhances synaptogenesis in neocortex (Li et al., 2010), possibly via effects on spontaneous neurotransmission (Autry et al., 2011). We found that low micromolar ketamine did not enhance dendritic EPSPs even in the absence of stimulation, but augmented somatic EPSPs, resulting in a form of E-S dissociation. These effects are observed soon after ketamine and are blocked by co-treatment with low APV to inhibit NMDARs completely during ketamine exposure. Because ketamine also blocks HCN1 channels (Chen et al., 2009) and these channels are expressed on pyramidal neuron dendrites where they regulate dendrosomatic charge transfer (Magee, 2000) and temporal synchronization (Vaidya and Johnston, 2013), we examined whether the effects of ketamine are mimicked by an HCN inhibitor. In contrast to ketamine, however, the HCN inhibitor enhanced dendritic EPSPs and depressed somatic EPSPs. The E-S dissociation observed with ketamine could involve changes in GABA-mediated inhibtion, given that NMDAR activation can depress GABA responses (Chisari et al., 2012). In prior studies, however, we found that picrotoxin, a GABAA receptor antagonist, can facilitate population spike firing (Nagashima et al., 2005), but does not alter dendritic or somatic EPSPs (Izumi et al., 2007). Interestingly, the effects of low ketamine on somatic EPSPs were not mimicked by a concentration of ketamine that blocks NMDARs completely, suggesting that full NMDAR block prevents this effect. A prior study in rodents found that anesthetic doses of ketamine, associated with greater NMDAR block, do not produce antidepressant effects (Li et al., 2010).
We also examined ketamine on LTP and found that acute LTP inhibition requires high concentrations (above 30 µM) (Emnett et al., 2013). Low micromolar ketamine also negatively modulates LTP, but does so more slowly, two or more hours following drug washout. The slowly developing LTP inhibition does not result from slowly and progressively developing NMDAR inhibition because it is not mimicked by 100 µM ketamine (which completely blocks NMDARs acutely), and intact NMDAR responses can be recorded at a time when LTP is inhibited following low ketamine. Furthermore, the slowly developing effects of ketamine are overcome by full NMDAR block during ketamine administration. For these reasons, it appears that LTP modulation by low ketamine likely involves a form of drug-induced metaplasticity (Abraham, 2008; Hulme et al., 2013), resulting from activation of unblocked NMDARs during ketamine exposure. Prior studies have shown that tonic low-level NMDAR activation before tetanic stimulation results in persistent LTP block and that NMDAR inhibition by low concentrations of APV during the period of tonic NMDAR activation overcomes LTP inhibition (Izumi et al., 1992a; Zorumski and Izumi, 2012). How unblocked NMDARs are activated during ketamine exposure is uncertain, although prior studies found that ketamine promotes release of glutamate in the hippocampus (Schobel et al., 2013; Gass et al., 2014), possibly via NADPH oxidase (Behrens et al., 2007; Sorce et al., 2013).
In prior work, we found that ethanol, another partial NMDAR antagonist with acute preference for GluN2B receptors in P30 rat hippocampus (Izumi et al., 2005), also causes metaplastic LTP inhibition in the CA1 region (Tokuda et al., 2011). Ethanol-mediated LTP inhibition, like low ketamine, can be overcome by full NMDAR block during drug exposure. Unlike ketamine, however, ethanol’s effects occur soon after (and even during) ethanol exposure. LTP inhibition by ethanol also involves GABA-enhancing 5α-reduced neurosteroids and are overcome by agents that inhibit neurosteroid synthesis or that block neurosteroid effects (Izumi et al., 2005; Tokuda et al., 2011). In our studies, we found no effect of finasteride, a 5α-reductase inhibitor, on ketamine-induced metaplasticity. Thus, different mechanisms likely contribute to the effects of ethanol and ketamine. Interestingly, mechanisms shared between ethanol and ketamine may be relevant to clinical effects because prior studies in humans have shown that ketamine has ethanol-like effects on subjects with histories of alcohol abuse (Krystal et al., 1998; 2003) and individuals with family histories of alcohol dependence have better antidepressant responses to ketamine than those without such family histories (Phelps et al., 2009).
At the minimum, the effects of low micromolar ketamine on LTD and LTP provide potential explanations for the cognitive dysfunction associated with ketamine and perhaps for its psychotomimetic actions. A prior study found that another trapping channel blocker, MK-801, has persistent effects on learning and synaptic plasticity, lasting days after a single exposure (Manahan-Vaughan et al., 2008), effects that are unlikely to result from ongoing drug-induced channel block.
Our studies also provide some mechanistic insights into ketamine’s modulation of LTP at low micromolar concentrations. While we found no role for either mTOR or 5α-reduced GABAergic neurosteroids, we did find that NOS inhibitors co-administered with ketamine overcame both the acute effects on somatic EPSPs and delayed effects on LTP. These results suggest that although there is a temporal delay between ketamine administration and LTP inhibition, ketamine-induced metaplasticity shares at least some mechanisms with the acute LTP inhibition observed following other treatments that activate NMDARs (Zorumski and Izumi, 2012). Interestingly, NOS inhibitors have also been found to overcome memory impairment (Boultadakis and Pitsikas, 2010; 2011; Wass et al., 2006) and information processing defects (Palsson et al., 2010) by ketamine and other NMDAR channel blockers, and have antidepressant effects in rodent models (Doucet et al., 2012). Whether ketamine-induced metaplasticity shares other mechanisms with acute NMDA-mediated LTP inhibition, including calcium influx, serine phosphatases and p38 mitogen activated protein kinase (Izumi et al., 1992b; Izumi et al., 2008) remains to be determined. Similarly, other studies indicate that signaling pathways leading to LTP inhibition by behavioral stressors involve NMDAR activation and effectors associated with ketamine, including mTOR and BDNF (Yang et al., 2008). Other possible contributors include efk2 (Autry et al., 2011).
In summary, we find that low micromolar ketamine has complex metaplastic effects on CA1 hippocampal function involving stimulation of unblocked NMDARs during ketamine exposure. These actions could contribute to neuropsychiatric effects of this novel and potentially therapeutic agent.
HIGHLIGHTS.
Low micromolar ketamine acutely inhibits hippocampal long-term depression but not long-term potentiation
Low micromolar ketamine enhances somatic but not dendritic synaptic potentials
Low micromolar ketamine produces a slowly developing block of long-term potentiation more than two hours following washout
The effects of low micromolar ketamine involve activation of unblocked NMDA receptors and nitric oxide
Acknowledgements
This work was supported by NIH grants MH077791 and AA017413 and the Bantly Foundation. The authors thank Kazuko O’Dell for technical assistance.
Abbreviations
- APV
2-amino-5-phosphonovalerate
- BDNF
brain derived neurotrophic factor
- efk2
elongation factor kinase 2
- EPSP
excitatory postsynaptic potential
- GABA
gamma-aminobutyric acid
- HCN
hyperpolarization activated cation channel
- HFS
high frequency stimulus
- LFS
low frequency stimulus
- L-NMMA
L-N-monomethylarginine
- LTP
long-term potentiation
- LTD
long-term depression
- mTOR
mammalian target of rapamycin
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- PS
population spike
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
CFZ is a member of the Scientific Advisory Board of Sage Therapeutics. There are no other competing financial interests. Sage Therapeutics did not fund this research and was not involved in the conduct of this research.
REFERENCES
- aan het Rot M, Zarate C, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biol. Psychiatry. 2012;72:537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nature Rev. Neurosci. 2008;9:387–399. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P-F, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioral antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiec WE, Guglietta R, Jami SA, Morishita W, Malenka RC, O’Dell TJ. Ionotropic NMDA receptor signaling is required for the induction of long-term depression in the mouse hippocampal CA1 region. J. Neurosci. 2014;34:5285–5290. doi: 10.1523/JNEUROSCI.5419-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B–containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacol. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quck KL, Dugan LL. Ketamine-induced loss of phenotype of fast spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J. Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bettini E, Sava A, Griffante C, Carignani C, Buson A, Capelli AM, Negri M, Andreetta F, Senar-Sancho SA, Guiral L, Cardullo F. Identification and characterization of novel NMDA receptor antagonists selective for NR2A- over NR2B–containing receptors. J. Pharmacol. Exp. Therap. 2010;335:636–644. doi: 10.1124/jpet.110.172544. [DOI] [PubMed] [Google Scholar]
- Boultadakis A, Pitsikas N. Effects of the nitric oxide synthase inhibitor L-NAME on recognition and spatial memory deficits produced by different NMDA receptor antagonists in the rat. Neuropsychopharmacol. 2010;35:2357–2366. doi: 10.1038/npp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boultadakis A, Pitsikas N. Anesthetic ketamine impairs rats’ recall of previous information: the nitric oxide inhibitor N-nitro-L-arginine methylester antagonizes this ketamine-induced recognition memory deficit. Anesthesiology. 2011;114:1345–1353. doi: 10.1097/ALN.0b013e318219524e. [DOI] [PubMed] [Google Scholar]
- Chen X, Shu S, Bayliss DA. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J. Neurosci. 2009;29:600–609. doi: 10.1523/JNEUROSCI.3481-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Zorumski CF, Mennerick S. Cross talk between synaptic receptors mediates NMDA-induced suppression of inhibition. J. Neurophysiol. 2012;107:2532–2540. doi: 10.1152/jn.01145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ML, Chan S-L, Way W, Trevor A. Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology. 1973;39:370–376. doi: 10.1097/00000542-197310000-00003. [DOI] [PubMed] [Google Scholar]
- Doucet MV, Harkin A, Dev KK. The PSD-95/nNOS complex: new drugs for depression? Pharmacol. Therap. 2013;133:218–229. doi: 10.1016/j.pharmthera.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Doyle OM, De Simoni S, Schwarz AJ, Brittain C, O’Daly OG, Williams SCR, Mehta MA. Quantifying the attenuation of the ketamine pharmacological magnetic resonance imaging response in humans: a validation using antipsychotic and glutamatergic agents. J. Pharmacol. Exp. Therap. 2013;345:151–160. doi: 10.1124/jpet.112.201665. [DOI] [PubMed] [Google Scholar]
- Emnett CM, Eisenman LN, Taylor AM, Izumi Y, Zorumski CF, Mennerick S. Indistinguishable synaptic pharmacodynamics of the N-methyl-D-aspartate receptor channel blockers memantine and ketamine. Mol. Pharmacol. 2013;84:935–947. doi: 10.1124/mol.113.089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass N, Schwarz AJ, Sartorius A, Schenker E, Risterucci C, Spedding M, Zheng L, Meyer-Lindenberg A, Weber-Fahr W. Sub-anesthetic ketamine modulates intrinsic BOLD connectivity within the hippocampal-prefrontal circuit in the rat. Neuropsychopharmacol. 2014;39:895–906. doi: 10.1038/npp.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71:1085–1101. doi: 10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NC, Constanti A. Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. J. Neurophysiology. 1995;74:2366–2378. doi: 10.1152/jn.1995.74.6.2366. [DOI] [PubMed] [Google Scholar]
- Hartvig P, Valtysson J, Lindner K-J, Kristensen J, Karlsten R, Gustafsson LL, Persson J, Svensson JO, Oye I, Antoni G, Westerberg G, Langstrom B. Central nervous system effects of subdissociative doses of (S)-ketamine are related to plasma and brain concentrations measured with positron emission tomography in healthy volunteers. Clin. Pharmacol. Therap. 1995;58:165–173. doi: 10.1016/0009-9236(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc. Natl. Acad. Sci. (USA) 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme SR, Jones OC, Abraham WC. Emerging roles of metaplasticity in behavior and disease. Trends Neurosci. 2013;36:353–362. doi: 10.1016/j.tins.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Auberson YP, Zorumski CF. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J. Neurosci. 2006;26:7181–7188. doi: 10.1523/JNEUROSCI.1258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Low concentrations of N-methyl-D-aspartate inhibit the induction of long-term potentiation in rat hippocampal slices. Neurosci. Lett. 1992a;137:245–248. doi: 10.1016/0304-3940(92)90414-3. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science. 1992b;257:1273–1276. doi: 10.1126/science.1519065. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Nagashima K, Murayama K, Zorumski CF. Acute effects of ethanol on hippocampal LTP and LTD are mediated by different mechanisms. Neuroscience. 2005;136:509–517. doi: 10.1016/j.neuroscience.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Tokuda K, O’Dell KA, Zorumski CF, Narahashi T. Neuroexcitatory actions of tamiflu and its carboxylate metabolite. Neurosci. Lett. 2007;426:54–58. doi: 10.1016/j.neulet.2007.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Tokuda K, Zorumski CF. Long-term potentiation inhibition by low-level N-methyl-D-aspartate receptor activation involves calcineurin, nitric oxide and p38 mitogen-activated protein kinase. Hippocampus. 2008;18:258–265. doi: 10.1002/hipo.20383. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. NMDA receptors, mGluR5 and endocannabinoids are involved in a cascade leading to hippocampal long-term depression. Neuropsychopharmacol. 2012;37:609–617. doi: 10.1038/npp.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Li ST, Zorumski CF. Modulation of long-term potentiation induction in the hippocampus by N-methyl-D-aspartate-mediated presynaptic inhibition. Neuroscience. 1999;92:1261–1272. doi: 10.1016/s0306-4522(99)00080-9. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am. J. Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J. Neurosci. 2009;29:2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorgueva R, D’Souza DC, Boutros NN, Trevisan L, Charney DS. Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacol. 2003;28:2020–2028. doi: 10.1038/sj.npp.1300252. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, Namanworth S, Stetson P, Trevisan LA, Charney DS. Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Arch. Gen. Psychiatry. 1998;55:354–360. doi: 10.1001/archpsyc.55.4.354. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, Li X-Y, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Miljkovic Z, Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J. Neurophysiology. 1987;58:251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA. Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol. Ther. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nat. Rev. Neurosci. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–12. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U. A single application of MK-801 causes symptoms of psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18:125–134. doi: 10.1002/hipo.20367. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B–containing NMDA receptors in cortical long-term potentiation and long-term depression. J. Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, Kessels HW, Alfonso S, Aow J, Fox R, Malinow R. Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc. Natl. Acad. Sci (USA) 2013;110:4027–4032. doi: 10.1073/pnas.1219454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, Zorumski CF, Izumi Y. Nitrous oxide (laughing gas) facilitates excitability in rat hippocampal slices through g-aminobutyric acid A receptor-mediated disinhibition. Anesthesiology. 2005;102:230–234. doi: 10.1097/00000542-200501000-00034. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, Olney JW. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacol. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali E. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J. Neurosci. 2013;33:6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson E, Lowry J, Klamer D. Information processing defects and nitric oxide signaling in the phencyclidine model of schizophrenia. Psychopharmacol. 2010;212:643–651. doi: 10.1007/s00213-010-1992-7. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nature Rev. Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji H, Zarate CA. Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol. Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Myers J, Le Bourdelles B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol. Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce S, Schiavone S, Tucci P, Colaianna M, Jaquet V, Cuomo V, Dubois-Dauphin M, Trabace L, Krause KH. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J. Neurosci. 2010;30:11317–11325. doi: 10.1523/JNEUROSCI.1491-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J. Neurosci. 2010;31:9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, O’Dell KA, Izumi Y, Zorumski CF. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J. Neurosci. 2010;30:16788–16795. doi: 10.1523/JNEUROSCI.4101-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, McGinley MJ, Westbrook GL. Triheteromeric NMDA receptors at hippocampal synapses. J. Neurosci. 2013;33:9150–9160. doi: 10.1523/JNEUROSCI.0829-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J. Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur. J. Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- Vaidya SP, Johnston D. Temporal synchrony and gamma to theta power conversion in the dendrites of CA1 pyramidal neurons. Nature Neurosci. 2013;16:1812–1820. doi: 10.1038/nn.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass C, Archer T, Palsson E, Fejgin K, Klamer D, Engel JA, Svensson L. Effects of phencyclidine on spatial learning and memory: nitric oxide-dependent mechanisms. Behav. Brain Res. 2006;171:147–153. doi: 10.1016/j.bbr.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Yang P-C, Yang C-H, Huang C-C, Hsu K-S. Phosphatidylinositol 3-kinase activation is required for stress protocol-induced modification of hippocampal synaptic plasticity. J. Biol. Chem. 2008;283:2631–2643. doi: 10.1074/jbc.M706954200. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhao X, Venkata SLV, Moaddel R, Luckenbaugh DA, Brutsche NE, Ibrahim L, Zarate CA, Mager DE, Wainer IW. Simultaneous population pharmacokinetic modeling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression. Br. J. Clin. Pharmacol. 2012;74:304–314. doi: 10.1111/j.1365-2125.2012.04198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Izumi Y. NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2012;36:989–1000. doi: 10.1016/j.neubiorev.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]