Abstract
Zhu Sa, Rezvani Mb, Harbell Jc, Mattis ANb,d,e, Wolfe ARf, Benet LZf, Willenbring Hb,c,d, Ding Sa,g. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature. 2014;508(7494):93–97.
aGladstone Institute of Cardiovascular Disease, bEli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research, University of California San Francisco, cDepartment of Surgery, Division of Transplantation, University of California San Francisco, dLiver Center, University of California San Francisco, eDepartment of Pathology, University of California San Francisco, fDepartment of Bioengineering and Therapeutic Sciences, University of California, gDepartment of Pharmaceutical Chemistry, University of California San Francisco, San Francisco, California, USA.
Transplantation of human adult hepatocyte cells have been used for treatment of both genetic and non genetic human liver diseases but with limited success because of several reasons such as poor quality of hepatocytes, inability to proliferation upon transplantation, chance of immunorejection, patient unmatched hepatocytes, difficult to cryopreservation, needed immunosuppressant drugs. Comparatively, human fetal hepatocyte cell has significantly higher proliferation potential than primary adult hepatocyte cells upon transplantation. Due to ethical issue, the human fetal hepatocyte option does not look realistic solution for liver patients. Very recently, induced pluripotent stem cell (iPS) technology together with chemical biology brings new golden opportunities to generate the human hepatocyte like cells from skin cell. Since introduction of iPS technology in 2006, it was thought that one has to make pluripotency state and then differentiate towards hepatocyte cells. But now researchers proved that it doesnot need to go to back to pluripotent state for generation of hepatocyte cells. The intermediate endodermal state is sufficient and appropriate step for generation of hepatocyte cells. They developed a short cut route via endodermal state and created hepatocytes cells. The clinical potential of these created hepatocyte cells were evaluated the functional and proliferation ability of in injured humanized mouse model. After nine month transplantation, they observed that the functional and proliferation ability of transplanted hepatocyte cells. In fact, most of conventional iPSC-derived hepatocyte cells unable to proliferate satisfactorily upon transplantation like adult human hepatocytes. Transplanted hepatocyte should be functional and ability for proliferate quickly, are both main prerequisites for therapeutic applications. This short cut route is not only for generation of hepatocyte cells but also for creation of other human body cells. Such shortcut routes for generation of functional hepatocyte cells allogenically from human skin raise a new hope for regeneration of acute or chronic liver who are risk of developing liver failure.
Comments
Since 2006, induced pluripotent stem cells (iPS) technology1 has been revolutionizing preclinical research and clinical practice rapidly across the globe. The Nobel strategy of iPS technology is that patient's specific functional cells can be generating from their skin cells for their treatment of incurable diseases. Although the iPS technology is very initial stage in human clinical trial but it may eliminate the need of organ transplantation in future. In early 2013, the world first clinical trial of iPS technology for treatment of nearly blind peoples has been started in Japan. The generation of functional hepatocytes from human skin cells by utilizing iPS technology is unprecedented opportunities for surgeons as well as liver patients for transplantation to get allogenic functional hepatocyte cells to regenerate their liver without waiting for donor liver. The clinical applicability of such iPS derived hepatocytes has not been tested in human clinical trials because of the oncogenic concerns of iPS cell and other limitations. However, potential of iPS derived hepatocytes have been tested in various animal models of injured livers and found fascinating results about recovery of liver disorders. Previously, all hepatic differentiation methods utilize iPS cells for conversion to hepatocytes cells. Currently, it was thought that skin cell must go back to pluripotency state in order to make hepatocytes cells. Pluripotent state of iPS cell is associated with tumor formations. Very recently, Zhu et al2 discovered the breakthrough invention that generation of functional hepatocytes is possible and advisable from endodermal state without going back to pluripotent state unlike current protocols. These researchers compared pluripotent state derived hepatocyte with endodermal state derived hepatocytes and found the endodermal state derived hepatocytes is much more superior than pluripotent state derived hepatocyte in term of proliferation capacity upon transplantation and hepatic functions. Since pluripotency is associated with tumor formation, it is a clinically innovative idea not to go back to pluripotent state of iPS cell in order to avoid the tumor formation. They reprogrammed skin cells to induced multipotent progenitor cell, which is much faster process than conventional reprogramming methods. Polygonal hepatocytes were generated from induced multipotent progenitor cell. These differentiated hepatocytes were occasionally binucleate but express hepatocyte markers such as HNF4-alfa, albumin synthesis, cytokeratin 18 and alpha-1 antitrypsin. Interestingly, glycogen storage, lipid uptake and urea production were observed in these differentiated hepatocytes. Zhu et al2 reported that the gene expression analysis showed that induced multipotent progenitor cell derived hepatocyte cells were more similar with human fetal primary hepatocyte than mature adult primary hepatocytes. Virtually, trasnplated hepatocytes cell do not proliferate rapidly and survive for short time during hepatocyte transplantation. Hepatocyte cells are originally obtained from dead or donor tissue. The survival time of these hepatocyte average of week and negligible ability to multiply. Proliferation of transplanted cells in transplanted site and long term survival are main prerequisites for successful therapeutic applications for cell transplantation. Primary human fetal hepatocyte has extensive potential to meet the both prerequisites, proliferation of transplanted cells in transplanted site and long-term survival for successful therapeutic applications. Successful treatment of fulminant hepatic failure and clinical end-stage chronic liver failure by transplantation of human fetal hepatocyte was reported in human cases.3,4 Gridelli and coauthors3 reported the strong proliferative potential of human fetal cells. Successful treatment was done by transplantation of immortalized human fetal hepatocytes in a mouse model of acute liver failure induced by 90% hepatectomy5 and rat acute liver model.6 Although the human fetal hepatocyte has strong potential to cure liver diseases but ethical concern is an ongoing issue. But Zhu et al2 discovered another shot cut way to create human fetal hepatocyte cells using skin cells without need of human fetal donor which is valuable milestone for cell therapies of liver diseases. First they transduced 10,000 cells human fibroblast cells with three pluripotent genes (OCT4, SOX2 and KLF4) for three days and then transferred these transduced cells into endodermal state using several growth factors and small molecule CHIR99021. Interestly, they observed the endodermal specific genes after 2 weeks. The idea behind that was whether it is essential to back to pluripotent state or endodermal state. The human liver itself is endogermal origin. After their exciting experiment, it seems that it is not essential to go back to pluripotent state to generate functional hepatocyte cells. After getting the endodermal state of transduced cells, Zhu et al2 directed these cells towards hepatic differentiation by inhibiting biliary differentiation using some small molecules. Biopotential embryonic liver progenitor cells under goes biliary fate by transforming growth factors and Notch signaling molecules. Zhu et al2 used very innovative approach to promote hepatic differentiation using two small molecules A83 and Notch inhibitors compound which inhibit biliary differentiation. They used a humanized Fah−/−/Rag2−/−/Il2rg−/− (FRG) mice7, an immune-deficient mouse model of human tyrosinemia type 1 to evaluate the clinical potential and transplanted 10,000,000 induced multipotent progenitors derived hepatocyte cells and adult primary hepatocyte cells into this mice. They found human serum albumin of induced multipotent progenitors derived hepatocyte cells is tenfold higher than adult primary hepatocyte cells in transplanted mouse model. The human serum albumin was monitored more than nine months after transplantation and found appropriate level of human serum albumin. They noticed that induced multipotent progenitor derived hepatocyte cells are still proliferating after nine months of transplantation. Zhu et al2 observed a nodule size of 4000 multipotent progenitor derived hepatocytes cells which could be 2% of liver populations. Finally, multipotent progenitor derived hepatocytes cells transplantation improved the survival of mice with chronic liver failure. Zhu et al2 experimental is giving a take home message to stem cell researchers how to create a short way for generation of functional hepatocyte cells from human skin allogenically for autologous treatment of chronic liver diseases.
There are some alternative short routes to generate hepatocytes using transcription factors only. Japanese research teams8 were screening the effects of twelve transcription factors for conversion of mouse embryonic and adult fibroblasts into hepatocyte like cells, finally they found three specific combinations of two transcription factors, comprising Hnf4α plus Foxa1, Foxa2 or Foxa3 is sufficient for generation of hepatocyte like cells. In the same time, Chinese research teams9 reported in independently that new cocktails of transcription factors of Gata4, HNF1alpha, Foxa3, and RNAi-mediated inactivation of p19Arf for direct induction of functional hepatocyte like from mouse tail-tip fibroblasts. Yu et al,10 brilliantly decided to explore two liver organogenesis transcription factors Hnf1β and Foxa3, to reprogram mouse embryonic fibroblasts into induced hepatic stem cells and successfully converted to induced hepatic stem cells from mouse embryonic fibroblasts. They proved that these induced hepatic stem cells differentiate into both hepatocytes and cholangiocytes in vivo as well as in vitro. Huang et al11 reprogrammed human fibroblast by three transcription factors (FOXA3, HNF1A, and HNF4A) and converted to mature hepatocytes which display both cytochrome P450 enzyme activity and biliary drug clearance in vitro and improved the liver functions in acute liver model of mouse. Du and teams12 designed to use hepatic fate transcription factors HNF1A, HNF4A, and HNF6 first and then maturation factors ATF5, PROX1, and CEBPA during reprogramming of fibroblast cells to hepatocyte cells. They showed mature functional human induced hepatocytes display the phase I and II drug-metabolizing enzymes and phase III drug transporters. When they transplanted differentiated hepatocytes into Tet-uPA/Rag2?/?/γc?/? mice, transplanted differentiated hepatocytes repopulated up to 30% of the livers and able to secrete more than 300 μg/ml human albumin in vivo. Large scales of functional hepatocytes are needed for treatment of liver diseases. Shan and teams found two interesting molecules called FPH1 FPH2 after a high-throughput screening of 12,480 small molecules on primary human hepatocytes.13 They found that FPH1 and FPH2 small molecules are highly useful for proliferation of mature human primary hepatocytes and stimulate the maturation of iPS derived cell types.13
Human liver has high regenerative potential to replace injured or aged cells. The average life span of human is around 70 years and the life span of in vivo human hepatocyte is 150 days. So during our lifetime, the liver replaces the aged hepatocytes cells by generation of functional hepatocytes in nature liver microenvironment via a regenerative mechanism using transcription factors. Recently, researchers used such liver specific transcription factors for generation of hepatocyte cells from skin fibroblast cells in vitro.8–12 The ultimate aim of generation of hepatocytes from skin cells to treat liver diseases. If we know the transcription factors and small molecules which have potential to convert hepatocyte from fibroblast, why not to explore transcription factors and small molecules in vivo directly to repair or regenerating the tissue or organs. Conventional iPS derived hepatocyte method and induced pluripotent derived hepatocytes method and a hypothetical the shortest route for liver regeneration is shown in Fig. 1. This strategy seems more clinical sense to do experiment in vivo using explores transcription factors and small molecules. Qian and colleagues reprogrammed cardiac fibroblasts to into cardiomyocyte-like cells in vivo.14 They reprogrammed cardiac fibroblasts to into cardiomyocyte-like cells by local delivery of transcription factors Gata4, Mef2c, Tbx5 (GMT) in murine heart. They generated new cardiomyocytes in vivo and observed that bi-nucleated and assembled sarcomeres in vivo in induced cardiomyocytes. These new cardiomyocytes generated from in vivo fibroblast improve cardiac function after myocardial infarction. This outstanding experiment open platform to how to regenerate organ or tissue in native environment using transcription factors. Thereafter, a series of in vivo reprogramming has been reported in different models. For an example, Spinal cord injury has been cured in living adult mammal mice model by utilizing vivo reprogramming methods.15 They reprogrammed resident astrocytes by a single transcription factor, SOX2 for conversion to mature synapse-forming neurons in vivo. Additionally, Su and colleagues used a valproic acid small molecule to promote the neuronal maturation in vivo. They conducted a mice mode of spinal cord injury and concluded that in vivo repair or regeneration of spinal cord by in vivo reprogramming which is more direct and shortest route for organ or tissue regeneration. Spinal cord injury remains an unsolved clinical problem, could be solved in human in near future in vivo reprogramming strategy. It is the time to jump on in vivo reprogramming by skipping in vitro reprogramming in order to establish to in vivo hepatocyte conversion method for liver regeneration in a native microenvironment of human liver, which could be useful affordable autologous therapy to treat the millions of chronic and acute liver patients.
Figure 1.
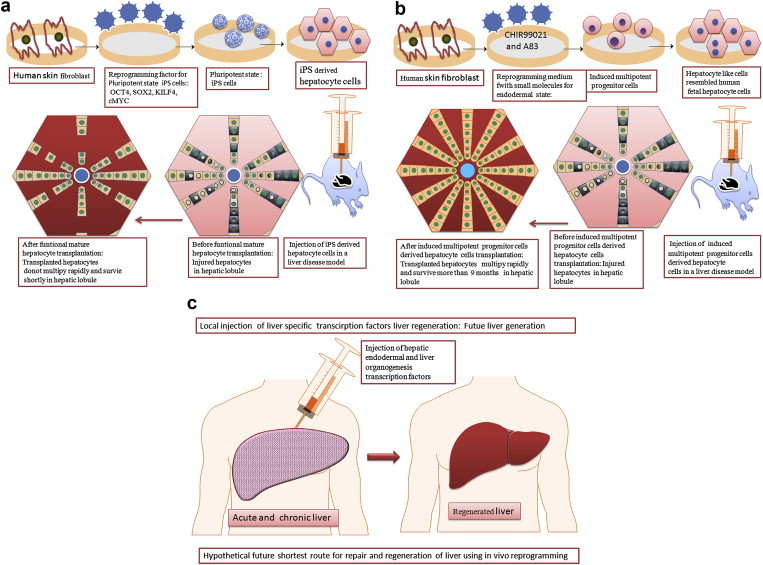
(a) Conventional route reprogramming methods for pluripotent state and thereafter hepatic differentiation, (b) Shortcut route reprogramming methods for endodermal state and thereafter hepatic differentiation, (c) Hypothetical future shortest route for liver regeneration of acute and chronic liver by local delivery of hepatic endodermal and liver organogenesis transcription factors. The hypothesis is that some local remaining local resident hepatic stem cells or hepatic fibroblast could be converting to hepatocyte by local delivery of hepatic endodermal and liver organogenesis transcription factors. Further research is needed.
References
- 1.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Zhu S., Rezvani M., Harbell J. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature. 2014;508(7494):93–97. doi: 10.1038/nature13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gridelli B., Vizzini G., Pietrosi G. Efficient human fetal liver cell isolation protocol based on vascular perfusion for liver cell-based therapy and case report on cell transplantation. Liver Transpl. 2012;18(2):226–237. doi: 10.1002/lt.22322. [DOI] [PubMed] [Google Scholar]
- 4.Habibullah C.M., Syed I.H., Qamar A., Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation. 1994;58(8):951–952. doi: 10.1097/00007890-199410270-00016. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y., Li J., Liu X., Zhao W., Wang Y., Wang X. Transplantation of immortalized human fetal hepatocytes prevents acute liver failure in 90% hepatectomized mice. Transpl Proc. 2010;42(5):1907–1914. doi: 10.1016/j.transproceed.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 6.Hu X., Yang T., Li C. Human fetal hepatocyte line, L-02, exhibits good liver function in vitro and in an acute liver failure model. Transpl Proc. 2013;45(2):695–700. doi: 10.1016/j.transproceed.2012.09.121. [DOI] [PubMed] [Google Scholar]
- 7.Azuma H., Paulk N., Ranade A. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol. 2007;25(8):903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekiya S., Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475(7356):390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 9.Huang P., He Z., Ji S. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475(7356):386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 10.Yu B., He Z.Y., You P. Reprogramming fibroblasts into bipotential hepatic stem cells by defined factors. Cell Stem Cell. 2013;13(3):328–340. doi: 10.1016/j.stem.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Huang P., Zhang L., Gao Y. Direct reprogramming of human fibroblasts to functional and Expandable hepatocytes. Cell Stem Cell. 2014;14(3):370–384. doi: 10.1016/j.stem.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Du Y., Wang J., Jia J. Human hepatocytes with drug Metabolic function induced from fibroblasts by Lineage reprogramming. Cell Stem Cell. 2014;14(3):394–403. doi: 10.1016/j.stem.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Shan J., Schwartz R.E., Ross N.T. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nat Chem Biol. 2013;9(8):514–520. doi: 10.1038/nchembio.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian L., Huang Y., Spencer C.I. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su Z., Niu W., Liu M.L., Zou Y., Zhang C.L. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]


