Abstract
Nonalcoholic fatty liver disease (NAFLD), the most common of chronic liver disease in Western Country, is closely related to insulin resistance and oxidative stress and includes a wide spectrum of liver diseases ranging from steatosis alone, usually a benign and non-progressive condition, to nonalcoholic steatohepatitis (NASH), which may progress to liver fibrosis and cirrhosis. NAFLD is considered the hepatic manifestation of the metabolic syndrome with which shares several characteristics, however recent data suggest that NAFLD is linked to increased cardiovascular risk independently of the broad spectrum of risk factors of metabolic syndrome. Accumulating evidence suggests that the clinical burden of NAFLD is not restricted to liver-related morbidity and mortality, with the majority of deaths in NAFLD patients related to cardiovascular disease and cancer and not to the progression of liver disease. Retrospective and prospective studies provide evidence of a strong association between NAFLD and subclinical manifestation of atherosclerosis (increased intima-media thickness, endothelial dysfunction, arterial stiffness, impaired left ventricular function and coronary calcification). A general agreement emerging from these studies indicates that patients with NASH are at higher risk of cardiovascular diseases than those with simple steatosis, emphasizing the role of chronic inflammation in the pathogenesis of atherosclerosis of these patients. It is very likely that the different mechanisms involved in the pathogenesis of atherosclerosis in patients with NAFLD have a different relevance in the patients according to individual genetic background. In conclusion, in the presence of NAFLD patients should undergo a complete cardiovascular evaluation to prevent future atherosclerotic complications. Specific life-style modification and aggressive pharmaceutical modification will not only reduce the progression of liver disease, but also reduce morbidity for cardiovascular disease improving overall prognosis and survival.
Keywords: Intima-media thickness, Steatosis, Nonalcoholic fatty liver disease, Non-alcoholic steatohepatitis, Early atherosclerosis, Cardiovascular risk, Inflammation, Epicardic fat
Core tip: Nonalcoholic fatty liver disease (NAFLD) is emerging as an independent risk factor for the occurrence and progression of cardiovascular disease. In this review we have systematically analyzed the correlation between NAFLD and cardiovascular diseases (CVD) focusing on the different aspects of CVD (increased carotid intima media thickness, increased arterial stiffness, endhotelial dysfunction, impaired left ventricular function, coronary calcification, epicardic fat), on the clinical manifestations (obstructive sleep apnea syndrome, clinical consequences of microangiopathy), and on the underlying physiopathogenic mechanisms (insulin resistance, atherogenic dyslipidemia, inflammation, oxidative stress, adipokynes imbalance, coagulation imbalance, increased assumption of fructose). Furthermore, we emphasized that NAFLD, by itself, is probably an independent risk factor for the occurrence of CVD.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) represents an emerging public health problem worldwide, has reached epidemic proportions and, currently, is the most common of chronic liver disease in Western Country. NAFLD includes a wide spectrum of liver diseases ranging from steatosis alone, usually a benign and non-progressive condition, to non-alcoholic steatohepatitis (NASH), which may progress to liver fibrosis and cirrhosis. NAFLD is defined by the accumulation of liver fat > 5% per liver weight, in the presence of < 20 g/d for women and < 30 g/d for men of daily alcohol intake; viral or other causes of liver disease must be excluded[1-4]. Patients with NAFLD usually present no manifestation of liver disease and are often identified by elevation of liver enzymes or ultrasonographic evidence of liver steatosis[5].
NAFLD affects about 20%-30% of the general population and is closely related to insulin resistance and oxidative stress[4,6,7]. The reported prevalence of NAFLD varies widely depending on the population studied and definition used and ranges around 20%-25% in the general population, reaching values of 50% and 75% in obese subjects and 65%-90% in those affected by type 2 diabetes[8]. The prevalence was even higher (96%) in obese undergoing bariatric surgery[9]. Interestingly, children are also known to be at risk of developing NAFLD: the prevalence was about 3%-10% in lean subjects and about 53% in obese pediatric population. In the United States, 9-15 millions of people are affected by NASH (prevalence of 3%-5%) of whom 15%-20% may evolve to cirrhosis[10].
In addition to be at risk for NASH, cirrhosis and its complications, NAFLD patients are also at higher risk of systemic and cardiovascular diseases (CVD), including coronary heart disease and stroke[7]. NAFLD is considered the hepatic manifestation of the metabolic syndrome (MS), with which shares several characteristics, and despite the relationship between NAFLD and MS is now increasingly recognized, recent data suggest that NAFLD is linked to increased cardiovascular risk independently of the broad spectrum of risk factors of MS. Indeed, it is hypothesized that NAFLD is not merely a marker of cardiovascular disease but may also be involved in its pathogenesis[7,11-13] (Figure 1).
Figure 1.

Common features in nonalcoholic fatty liver disease and cardiovascular disease. NAFLD: Nonalcoholic fatty liver disease; CVD: Cardiovascular disease.
NAFLD, diagnosed either by ultrasonography or liver biopsy, has been associated with a higher prevalence of early asymptomatic cardiovascular alterations such as increased carotid intima-media thickness (IMT) and carotid atherosclerosis[13-15], coronary calcification[13,16], altered left ventricular geometry and early left ventricular diastolic dysfunction[17], and low coronary flow reserve[18]. In a recent study a higher prevalence of NAFLD was also shown in patients with atrial fibrillation[19]. These alterations have been partly associated with the severity of liver damage, defined by both lobular inflammation and fibrosis.
Thus, accumulating evidence suggests that the clinical burden of NAFLD is not restricted to liver-related morbidity and mortality, with the majority of deaths in NAFLD patients related to cardiovascular disease and cancer and not to the progression of liver disease.
In the present review we present an overview on the state of the art of the latest discoveries about the correlation between NAFLD and CVD focusing on the different aspects of CVD (increased carotid intima media thickness, increased arterial stiffness, endothelial dysfunction, impaired left ventricular function, coronary calcification, epicardic fat), on the clinical manifestations [obstructive sleep apnea syndrome (OSAS), clinical consequences of microangiopathy], and on the underlying physiopathogenic mechanism (insulin resistance, atherogenic dyslipidemia, inflammation, oxidative stress, adipokynes imbalance, coagulation imbalance, increased assumption of fructose).
SURVIVAL STUDIES
Recent evidence suggests that NAFLD patients have a decreased survival as compared with general population, and cardiovascular disease dictates the outcome in NAFLD patients more frequently and to a greater extent than does the progression of liver disease[11]. Over 6-28 years of follow-up, several hospital-based studies have reported that patients with NAFLD (biopsy-proven or diagnosed by ultrasound) have a significantly higher all-cause and CVD-related than general population. Adams et al[20] in a population-based cohort study, found that 25% of related NAFLD death was linked to acute myocardial infarction and only 13% to liver disease. In a study published in 2006, which included 212 consecutive patients with biopsy-proven NAFLD, it was found an increased mortality in NASH but not in simple steatosis. Moreover, this study showed that the higher mortality of NASH patients was primarily a result of CVD and, to a lesser extent, of liver-related causes[5]. Similarly, in a study of patients with biopsy-proven NAFLD with 21 years follow-up the main causes of death were CVD and malignancy[21]. Rafiq et al[22] in a long-term follow-up evaluation showed that death from coronary artery disease was the most common cause of death followed by malignancy; the liver-related death, including death from hepatocellular carcinoma, was the third most common cause of death. Furthermore, Söderberg et al[23] recently confirmed that NASH, but not simple steatosis, was associated with increased mortality from all causes during a 28 years follow-up; cardiovascular disease was the most frequent cause of death followed by extrahepatic cancer and, at third place, by liver-related causes. It should be noted, however, that these studies had a retrospective design and small cohort sizes and the adjustment for potential confounding metabolic factors was not performed[24]. Stepanova et al[25] in a combined large cohort of patients with biopsy-proven NAFLD confirmed that cardiovascular (CV) death remains the top cause of death in the NAFLD cohort. However, this retrospective study did not show any difference in CV mortality between NASH and non-NASH subtypes of NAFLD.
A series of prospective studies demonstrated that NAFLD was independently associated with increased risk either of non-fatal CVD events or CVD mortality (Table 1[26]). Targher et al[27] using age and sexed matched controls from a diabetes outpatient department, reported that the presence of NAFLD, diagnosed by ultrasound, was independently associated with an increased risk of incident non-fatal CVD events and CVD mortality over 6.5 years of follow-up. Large community and population-based studies showed similar results not only in European but also in Eastern countries[12,28-31]. Lazo et al[32] in 2011 and Stepanova et al[33] in 2012, using a database of over 11000 US participants found that NAFLD was independently associated with the risk of CVD, but, in contrast with the previous studies, not with increased risk in all-cause mortality. This study, however, was limited by the inclusion of mild hepatic steatosis within the control arm and by using only ultrasonography to exclude hepatic steatosis. Similarly, Kim et al[34] in the third NHANES study population found that ultrasonography - defined NAFLD was not associated with overall mortality but, interestingly, NAFLD with advanced fibrosis, was associated with the risk of overall mortality, of which the majority of deaths were due to CVD, even after adjustment for other common risk factors. Also this study had the same limitation of the two above (same database).
Table 1.
Prospective and retrospective studies of the risk of cardiovascular mortality in patients with nonalcoholic fatty liver disease diagnosed by liver biopsy and/or by ultrasonography
| Ref. | Study design | NAFLD patients | Diagnosis of NAFLD | Mean duration follow-up (yr) | Result |
| Matteoni et al[26], 1999 | Retrospective, Hospital-based | 132 | Histology | 18.0 | No adjustment made. Higher all-cause, liver-related mortality but no difference in CVD mortality (NASH vs simple steatosis) |
| Dam-Larsen et al[21], 2004 | Retrospective, Hospital-based | 109 | Histology | 16.7 | Analysis performed by gender. No difference in all-cause and liver-related or CVD mortality (simple steatosis vs general population) |
| Adams et al[20], 2005 | Retrospective community-based | 420 | US and/or histology | 7.6 | Matched for gender, age and country Higher all-causae, liver-related and CVD mortality (CVD is the second cause of death by frequency) in NAFLD patients (especially cirrhosis) |
| Targher et al[12], 2005 | Prospective case-control, Hospital-based | 248/21031 | US | 5.0 | NAFLD was independently associated with increased nonfatal CVD and CVD mortality |
| Ekstedt et al[5], 2006 | Retrospective, Hospital-based | 129 | Histology | 13.7 | Matched for gender, age and country Higher all-cause, liver-related and CVD mortality (NASH but no simple steatosis vs general population) |
| Hamaguchi et al[28], 2007 | Prospective, community-based | 312/16372 | US | 5.0 | NAFLD was independently associated with increased risk of nonfatal CVD events |
| Targher et al[27], 2007 | Prospective, Hospital-based | 1421/21031 | US | 6.5 | NAFLD was independently associated with increased risk of nonfatal CVD events and CVD mortality |
| Haring et al[29], 2009 | Prospective, community-based | 2490/41603 | US and altered GGT | 7.3 | NAFLD was independently associated with increased risk of all-cause and CVD mortality in men |
| Rafiq et al[22], 2009 | Retrospective, Hospital-based | 173 | Histology | 13.0 | No adjustments made Higher liver-related mortality, but no difference in overall mortality (NASH vs simple steatosis) |
| Söderberg et al[23], 2010 | Retrospective, Hospital-based | 118 | Histology | 18.0 | Matched for gender, age and year Higher all-cause, liver-related and CVD mortality (NASH but no simple steatosis vs general population) |
| Lazo et al[32], 2011 | Prospective, population-based (third NHANES study population 1988-1994) | 2089/113714 | US | 14.3 | Independent increased risk of CVD but no increased risk in all-cause, liver-related and CVD mortality |
| Stepanova et al[33], 2012 | Prospective, population-based (third NHANES study population 1988-1994) | 2492/113714 | US | 14.3 | Independent increased risk of CVD but no increased risk in all-cause, liver-related and CVD mortality |
| Treeprasertsuk et al[31], 2012 | Prospective, community-based | 309 | US, CT, MRI, and or Histology | 11.5 | Higher 10-Year CVD risk (NAFLD vs general population) |
| Kim et al[34], 2013 | Prospective, population-based (third NHANES study population 1988-1994) | 4083/111544 | US and NAFLD Fibrosis Score | 14.5 | US-Defined NAFLD was not associated with overall mortality. NAFLD with advanced fibrosis was independently associated with overall mortality (majority of deaths were due to CVD) |
| Stepanova et al[25], 2013 | Retrospective, Population-based | 289 | Histology | 6.25 | No adjustments made Higher risk of liver-related mortality in NASH than non-NASH. NAFLD and type II diabetes highest risk for overall and liver-related mortality |
Patients with type 2 diabetes;
Participants of health checkup program;
West Pomerania population;
United Stated adult population. US: Ultrasonography; CT: Computed tomography; MRI: Magnetic resonance imaging; CVD: Cardiovascular disease; NAFLD: Nonalcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis.
Additional long-term prospective and case-control studies are needed to demonstrate the independent risk of premature CVD and decreased survival in patients with well-defined NAFLD/NASH. In particular, understanding how modification of the progression of liver disease could affect and modify CVD progression and outcome is essential.
CAROTID INTIMA MEDIA THICKNESS
Increased IMT of carotid artery, considered a validated parameter of subclinical atherosclerosis and an independent predictor of stroke and myocardial infarction[35-38], and the presence of plaques as index of late atherosclerosis damage, have been used to characterize CVD risk of patients with NAFLD.
Retrospective studies
Several papers reported a strong association between increased IMT and NAFLD. One of the first studies was performed by Targher et al[39] in 2004. In this case-control study emerged that in NAFLD patients, all non-obese volunteers, with normal or moderately increased body weight, abdominal visceral fat accumulation was the key mediator for the relationship linking nonalcoholic hepatic steatosis to increased IMT.
Interestingly, many of these studies, conducted among different ethnic populations, showed that NAFLD predicted an increased carotid IMT independently from traditional risk factors, including insulin-resistance[15,40-48]. Fracanzani et al[15] in a paired-sample case-control study (125 patients with nonalcoholic fatty liver disease and 250 controls), without a prior diagnosis of diabetes, hypertension, and cardiovascular disease, matched for sex, age, and body mass index found a significant difference in mean values of IMT (0.89 ± 0.26 mm and 0.64 ± 0.14 mm, P = 0.0001) and prevalence of plaques [26 (21%) and 15 (6%), P < 0.001] between the two groups. In this study independent risk predictors of IMT higher than 0.64 mm (the median value of normal subjects) were presence of steatosis (OR = 6.9), age (OR = 6.0), and systolic blood pressure (OR = 2.3). Aygun et al[49] in a population of 40 age-matched-control and biopsy proven NAFLD patients reported that metabolic syndrome and increased IMT were more prevalent in the NAFLD cohort. Kim et al[42] in a case-control study stratified by gender concluded that NAFLD was independently associated with carotid atherosclerosis in patients with metabolic syndrome.
Salvi et al[50] in the Cardio-GOOSE (Cardio-Gambettola ObservatOry liver Steatosis Estimation) study, a population-based cohort study finalized to evaluate the relationship between NAFLD and subclinical vascular damage, showed that NAFLD was associated with the metabolic syndrome in 48% of cases. IMT values were strongly related to the number of metabolic syndrome factors. In fact no significant differences in IMT were found between controls and patients with NAFLD and no manifestation of metabolic syndrome (0.77 +/- 0.15 mm vs 0.76 +/- 0.14 mm). Conversely, in patients with NAFLD and associated metabolic syndrome, IMT values were significantly higher than in patients with NAFLD alone (0.85 +/- 0.16 mm, P < 0.005)[50]. From a large population based survey of over 8500 middle aged and elderly Chinese population, Huang et al[46] reported that NAFLD was associated with elevated IMT independently of the conventional risk factors. Thakur et al[51] in a case-control study in non-diabetic population reported that NAFLD seemed to be an independent predictor of having high average IMT, independently of obesity and metabolic syndrome. Notably, this is the first study in Asian Indians demonstrating the association.
The association between NAFLD and increased carotid IMT was independent of the metabolic syndrome in at least four studies[44-47].
Increased IMT and liver damage
It has been reported that subclinical cardiovascular disease was significantly associated with the severity of liver disease. Targher et al[52] compared carotid IMT in 85 consecutive patients with biopsy-proven NAFLD and 160 age-, sex-, and BMI-matched healthy control subjects and found that the severity of liver histopathology in NAFLD patients was strongly associated with early carotid atherosclerosis, independently of classical risk factors, insulin resistance, and the presence of metabolic syndrome, suggesting that the severity of liver damage could play a role in atherosclerosis development. Băloşeanu et al[53] reported that the severity of liver histopathological lesions among NAFLD patients was strongly associated with increased IMT. This study, however, had a limitation because it was performed in a small sample of NAFLD subjects. Also Kucukazman et al[54] in a recent study found that the mean carotid IMT was significantly increased in patients with NAFLD and showed a significant positive cancel weak correlation between ultrasonographic steatosis grade and mean IMT (r = 0.376, P < 0.001).
IMT and type 2 diabetes
At least four studies examined the link between NAFLD, type 2 diabetes and subclinical atherosclerosis damage. It is well known that NAFLD is extremely common in people with type 2 diabetes and is mainly associated with uncontrolled diabetes. Targher et al[55] studied a population of 200 diet-controlled type 2 diabetic subjects, reported that NAFLD patients had a markedly greater carotid IMT and that the increase of carotid IMT was largely explained by HOMA-estimated insulin resistance. In contrast IMT values were found to be significantly higher in diabetic patients regardless of the degree of steatosis by Cakir et al[56]. Similarly other two studies performed in diabetic population reported that hepatic steatosis was not associated with carotid atherosclerosis and suggested that the association of hepatic steatosis and cardiovascular disease might be just an epiphenomenon[57,58].
Pediatric population
One of the first evidence of the association between fatty liver and atherosclerosis were the autoptic findings in 817 children who died of external causes showing that fatty liver was present in 15% of the children, mild atherosclerosis in 21% and moderate to severe atherosclerosis in 2%. Atherosclerosis was found more common in children with fatty liver (30%) than those without fatty liver (19%). Interestingly, growing evidence suggests a strong relationship between early carotid atherosclerosis and NAFLD, although longitudinal studies are needed to clarify the extent to which pediatric NAFLD and its severity influence long-term cardiovascular outcomes in the general population[59-65].
In conclusion, considering the large number of publications on the relationship between IMT and vascular damage, carotid screening for NAFLD might be beneficial for assessment of future atherosclerotic complications.
ARTERIAL STIFFNESS
Measurement of biomechanical properties of arteries has become an important surrogate outcome used in epidemiological and interventional cardiovascular research. A loss of arterial elastic properties results in a range of linked pathophysiological changes in the circulation including increased pulse pressure, left ventricular hypertrophy, subendocardial ischaemia, vessel endothelial dysfunction and cardiac fibrosis[66]. Arterial stiffness, determined by measurement of pulse wave velocity, has been shown to be an independent indicator of symptomatic cardiovascular disease[67]. Carotid and femoral pulse wave velocity has been considered the noninvasive gold standard measure of arterial stiffness[50]. Brachial-ankle pulse wave velocity is also widely used to screen for atherosclerotic vascular damage[13,67]. Several recent studies reported a strong association between NAFLD and impaired arterial elastic properties. In the GOOSE study Salvi et al[50] showed that NAFLD may play an independent role in determining arterial stiffness. Vlachopoulos et al[67] in a case-control study with biopsy-proven NAFLD reached similar results. Huang et al[46] in the study including 8632 Chinese men aged ≥ 40 years showed that brachial-ankle pulse wave velocity was increased in NAFLD independently of conventional cardiovascular disease risk factors and metabolic syndrome. In another study of the same author performed in adolescents it was found that NAFLD was only associated with increased arterial stiffness in the presence of the “high risk” metabolic cluster, suggesting that arterial stiffness related to the presence of NAFLD was predicted in adolescents by the presence of an adverse metabolic profile[68]. Kim et al[69] in a case-control study indicated that the presence and the degree of NAFLD were associated with arterial stiffness, measured by brachial-ankle pulse wave velocity, in non-hypertensive, non-diabetic individuals, especially in those without metabolic syndrome. Similarly, Lee et al[70] showed that arterial stiffness, was independently associated with the risk for NAFLD regardless of classical CVD risk factors.
Işilak et al[71] and Fotbolcu et al[72] reported that elastic properties of aorta were abnormally changed in patients with NAFLD, probably related to or associated with insulin resistance[71,72]. In contrast, Catena et al[73] revealed that in essential hypertensive patients without additional cardiovascular risk factors, NAFLD was associated with insulin resistance but not with increased arterial stiffness.
ENDOTHELIAL DYSFUNCTION
Endothelial dysfunction is one of the earliest markers of atherosclerosis. Recent studies showed an association between NAFLD and endothelial dysfunction, assessed by brachial artery flow mediated dilatation (FMD). In 2005 Villanova et al[74] reported that FMD was significantly reduced in NAFLD population; in this case-control study NAFLD, diagnosed by liver biopsy or by ultrasound, predicted a reduced FMD after adjusting for age, sex, BMI and insulin resistance. Senturk et al[75] also reported that endothelial dysfunction was worse in NASH compared to simple steatosis and controls suggesting that inflammation in the liver plays a role.
Mohammadi et al[44] in a study performed in 2011, reported that pure NAFLD without metabolic syndrome in middle-aged subjects was strongly associated with morphological (increased carotid IMT) and physiological flow-mediated changes.
In a cohort of never treated uncomplicated hypertensive outpatient subjects the presence of NAFLD was associated with physiological FMD changes and insulin-resistance[76]. Thakur et al[51] in a case-control study reported that in a cohort of Asian Indians subjects NAFLD is significantly associated with subclinical atherosclerosis and endothelial dysfunction. Interestingly, these results were independent from the classical cardiovascular risk factors, i.e., obesity and metabolic syndrome. In another observational case-control study the measurement of brachial artery FDM was significantly lower in patients with NAFLD group compared to control group suggesting that NAFLD, and in particular NASH, is associated with endothelial dysfunction, independently from metabolic syndrome[48]. Similar results were reported by Kucukazman et al[54]. In addition plasma levels of fetuin-A, which is known to inhibit insulin signalling and recently emerged as a biomarker for diabetes risk, was found independently associated with endothelial dysfunction and subclinical atherosclerosis in NAFLD patients[77].
IMPAIRED LEFT VENTRICULAR FUNCTION
At least seven studies reported that NAFLD was associated with impaired left ventricular diastolic dysfunction, alteration in energy metabolism and disturbance of cardiac rhythm (Table 2). Goland et al[78] found that patients with NAFLD, in the absence of morbid obesity, hypertension, and diabetes had early features of left ventricular diastolic dysfunction and mildly altered left ventricular (LV) geometry. Lautamäki et al[79] found that in patients with type 2 diabetes and coronary artery disease, liver fat content was a novel independent indicator of myocardial insulin resistance and reduced coronary functional capacity. Fallo et al[80] in a study conducted in a cohort of never-treated essential hypertensive patients with nonalcoholic fatty liver disease, reported that NAFLD was significantly associated with insulin resistance and abnormalities of left ventricular diastolic function, suggesting a concomitant increase of metabolic and cardiac risk. On the contrary, Perseghin et al[81] reported that in newly diagnosed individuals with fatty liver no impaired LV morphological features and systolic and diastolic functions were present but they had abnormal LV energy metabolism that correlated with higher mediastinal visceral fat. In a recent study in which 50 consecutive patients with type 2 diabetes were enrolled it was observed that in patients with NAFLD LV morphology and systolic function were preserved but early features of LV diastolic dysfunction were present[17].
Table 2.
Studies of the impaired left ventricular function and disturbance of rhythm in patients with nonalcoholic fatty liver disease
| Ref. | Study design | Study population | Study size | Diagnosis of NAFLD | Cardiac parameters examined | Results |
| Left ventricular function | ||||||
| Goland et al[78], 2006 | Retrospective, Case-control study, Hospital-based | NAFLD vs control | 38 NAFLD 25 non-NAFLD | US or histology | Complete echocardiographic study, TDI, peak velocities of E and A diastolic filling, E/A ratio, Vp, E', and S' of mitral annulus | NAFLD patients had increased thickening of the intraventricular septum and posterior, lower E diastolic filling velocity, lower E/A ratio, longer, lower Vp and lower E' No differences were found according to LV systolic function |
| Lautamaki et al[79] 2006 | Retrospective, Case-control study, Hospital-based | Patients with type 2 diabetes and CVD | 28 low liver fat 27 high liver fat Matched for age, BMI, fasting glucose | H-MRS | Positron emission tomography. Myocardial perfusion was measured with[15O]H2O and myocardial and skeletal muscle glucose uptake with 2-deoxy-2-[18F]fluoro-D-glucose during hyperinsulinemic euglycemia | Liver fat content is independently associated with impaired myocardial metabolism |
| Fallo et al[80] 2009 | Retrospective, Case-control study, Hospital-based | Never-treated essential hypertensive patients | 48 NAFLD 38 non-NAFLD Matched for sex, age and blood pressure levels | US | Complete echocardiographic study: TDI, peak velocities of E and A diastolic filling, E/A ratio, Vp, E', and S' of mitral annulus | NAFLD patients had increased prevalence of left ventricular hypertrophy , diastolic dysfunction that increased according to the degree of NAFLD |
| Perseghin et al[81] 2008 | Retrospective, Case-control study, Hospital-based | Young non-diabetic men | 21 fatty liver 21 non fatty liver Matched for age, BMI, blood pressure levels, lipid values | H-MRS | MRI and MRS | No difference in morphological parameters of the LV, systolic and diastolic functions NAFLD patients had reduced values of PCr/ATP ratio |
| Bonapace et al[17] 2012 | Retrospective, Case-control study, Hospital-based | Patients with type 2 diabetes | 32 NAFLD 18 non-NAFLD Matched for age, sex, BMI, waist circumference, hypertension, smoking, diabetes duration, microvascular complication status, medication use | US | Complete echocardiographic study, TDI, peak velocities of E and A diastolic filling, E/A ratio, Vp, E', and S' of mitral annulus | NAFLD patients had lower E', tissue velocity, higher E-to-e' ratio, higher time constant of isovolumic relaxation, higher LV-end diastolic pressure (EDP), higher LV EDP/end diastolic volume No difference in morphological parameters of the LV, systolic functions |
| Hallsworth et al[82] 2013 | Retrospective, Case-control study, Hospital-based | NAFLD and healthy controls | 19 NAFLD 19 non-NAFLD Matched for age, sex, BMI, weight, and body surface area vs control | H-MRS | MRI and MRS | NAFLD patients had thicker left ventricular walls at systole and diastole, decreased longitudinal shortening, higher concentric remodelling. No difference in PCr/ATP ratio |
| Disturbance of cardiac rhythm | ||||||
| Targher et al[19] 2013 | Prospective, Hospital-based | Type 2 diabetes | 281 NAFLD 119 non-NAFLD Adjustments for age, sex, hypertension and electrocardiographic features | US | 12-lead electrocardiogram | NAFLD was associated with an increased risk of incident AF |
A: Late; BMI: Body mass index; E: Early; E': Early diastolic velocity; H-MRS: Proton magnetic resonance spectroscopy; cardiac MRI: Magnetic resonance imaging; MRS: 31P-MR spectroscopy; NAFLD: Nonalcoholic fatty liver disease; PCr/ATP ratio: Phosphocreatine/adenosine triphosphate ratio; S': Systolic velocity; TDI: Tissue Doppler imaging; US: Ultrasonography; Vp: Flow propagation velocity; AF: Atrial fibrillation.
Hallsworth et al[82] in a cohort of nineteen adults with NAFLD age-, sex-, and BMI-matched to healthy controls without liver or metabolic diseases, reported that adults with NAFLD had significantly thicker left ventricular wall at systole (14 ± 3 mm vs 12 ± 2 mm, P < 0.01) and diastole (8 ± 1 mm vs 7 ± 1 mm, P < 0.01) than those without fatty liver and showed decreased longitudinal shortening (14% ± 3% vs 17% ± 3%, P < 0.01). This case-control study concluded that significant changes in cardiac structure and function are evident in adults with NAFLD in the apparent absence of metabolic changes or overt cardiac disease.
Interestingly, a recent study demonstrated that NAFLD was associated with an increased risk of incident atrial fibrillation (AF), (OR = 4.49, 95%CI: 1.6-12.9, P < 0.005). Adjustments for age, sex, hypertension and electrocardiographic features (left ventricular hypertrophy and PR interval) did not attenuate the association between NAFLD and incident AF (adjusted OR = 6.38, 95%CI: 1.7-24.2, P = 0.005)[19].
CORONARY CALCIFICATION
Computed tomography examination of the coronary arteries can detect calcification indicative of arterial disease in asymptomatic people which has been identified as a surrogate marker of subclinical atherosclerosis[83]. Coronary artery calcification (CAC) was considered an independent marker of CVD risk[84]. Akabame et al[85] in a case-control study, evaluating vulnerable coronary plaques and steatosis, by multi-slice computed tomography, showed that liver fat was significantly correlated with lipid core plaques. Adjusted odds ratio of NAFLD for remodeling lesions was 2.41 (95%CI: 1.24-4.67; P = 0.009), and those for lipid core lesions 2.29 (95%CI: 1.15-4.56; P = 0.018)[85]. A population study performed in 2007, evaluating 371 non-diabetic, asymptomatic middle-aged men free of known coronary heart disease, showed that the presence of hepatic steatosis was associated with a greater prevalence of risk factors for atherosclerosis, with a higher 10-year total coronary heart disease risk (calculating by Framingham Risk Score) and was independently associated with CAC in subjects with metabolic syndrome[86].
Three studies published in 2010 reported similar results. Assy et al[87] found that patients with NAFLD, even without metabolic syndrome, are at high risk for atherosclerosis and had a higher prevalence of coronary calcification. In a retrospective study, Chen et al[88] reported that besides traditional risk factors, such as male gender, increased age, and DM, NAFLD was also associated with moderate to high risk of coronary disease and with the severity of CAC. Jung et al[89] found, in a cohort of 1218 Korean subjects, that NAFLD predicted coronary calcification. Individuals with combined elevated ALT (> 30 U/L) and ultrasound diagnosed hepatic steatosis had higher odds of a CAC score > 100 compared to controls and individuals with elevated ALT or steatosis alone. However it is not possible to understand from the paper whether the patients with increased ALT had NASH. Kim et al[16] in the largest population-based study published in 2012 showed a strong relationship between NAFLD and CAC; 4123 cases and controls from Seoul were enrolled. The study reported that NAFLD predicted a CAC score > 10; computed tomography (CT) examination revealed that visceral adiposity attenuated but did not eliminate the relationship between NAFLD and CAC (adjusted OR = 1.32 (95%CI: 1.12-1.57)[16]. These recent evidences suggested that detection of NAFLD could signal the existence of an increased coronary artery disease risk independent of visceral adiposity.
Only two studies showed no significant association between NAFLD and coronary calcification, probably one for the different selection of patients[57] and the other for the small series of patients (70 subjects)[90].
OBSTRUCTIVE SLEEP APNEA SYNDROME
OSAS is a common medical condition that affects about 4% of general population with higher prevalence among obese subjects (incidence 25%-35%)[91]. Besides obesity, age, male, sex, smoke and alcohol intake are risk factors for OSA. OSAS is a form of sleep disordered breathing, which is characterized by repetitive complete and/or partial collapses (apnea and hypopnea respectively) of the upper airways. OSAS is characterized by episodes of chronic intermittent hypoxia and sleep fragmentation, with increase in sympathetic activity and promotion of oxidative stress, pro-inflammatory cytokine production, platelet aggregation, endothelial dysfunction and metabolic dysregulation. All these mechanisms provide the pathophysiological basis for the increased risk of CVD observed in these patients[92].
OSAS has been identified as an independent comorbid factor in cardiovascular and cerebrovascular diseases, and physiopathological links may exist with onset and progression of heart failure. It has also been found an association between OSAS and the manifestations of the metabolic syndrome, in particular with obesity[91], and with arterial stiffness, endothelial dysfunction and carotid IMT[93]. The disease is classified as mild, moderate and severe based on the number of apneas and/or hypopneas per hour of sleep, known as the apnea-hypopnea index. This is assessed by polysomnography (PSG) or other forms of sleep monitoring[94]. Both OSAS and metabolic syndrome, of which NAFLD represents the hepatic manifestation, may exert negative synergistic effects on the cardiovascular system through multiple mechanisms (e.g., hypoxemia, sleep disruption, activation of the sympathetic nervous system, and inflammatory activation). It has been found that continuous positive airway pressure therapy for OSAS provides an objective improvement in symptoms and cardiac function, decreases cardiovascular risk, improves insulin sensitivity and adiponectin level, reduces adipocytokines (leptin and resistin) levels, and normalized biomarkers[95].
Recent experimental evidence connected chronic intermittent hypoxia with insulin-resistance and reported that intermittent hypoxia concurred to the pathogenesis of NAFLD. Moreover, in NAFLD patients, the presence of OSAS resulted linked to the progression of NAFLD to NASH[91].
NAFLD AND MICROANGIOPATHY
It has been reported that NAFLD may also be associated with micro-vascular changes in other organs that have direct or indirect influence on CVD and may accelerate the presentation of CVD. For example, patients with NAFLD, in a type 2 diabetic population, had significant higher age- and sex-adjusted prevalence rates of both non-proliferative (39% vs 34%) and proliferative/laser treated retinopathy (11% vs 5%) and of chronic kidney disease (15% vs 9%) than counterparts without NAFLD. Furthermore, in this study, NAFLD was associated with an increased incidence of chronic kidney disease, independent of classical risk factors[96]. At least three studies showed an association between retinal vascular changes and NAFLD. Băloşeanu et al[53] in 10 biopsy-proven NAFLD patients, found a connection between the degree of NAFLD and the retinal vascular changes in those with carotid atherosclerosis. Josef et al[97] analyzing a cohort of non-diabetic and non-hypertensive patients, found that fatty liver (OR = 2.5; P < 0.01), IMT (OR = 2.3; P < 0.001), and retinal changes (OR = 1.5; P < 0.01) were strongly associated with CAD independent of metabolic syndrome (OR = 1.2; P < 0.05). Also Targher et al[98] suggested that patients with NAFLD have an increased prevalence of chronic kidney disease, known risk factor for CVD. An increasing number of studies showed that NAFLD may be an independent risk factor for chronic kidney disease[96,98-108]. In several retrospective studies the prevalence of chronic kidney disease defined as eGFR < 60 mL/min/1.73 m2 or proteinuria- and/or microalbuminuria were significantly higher among NAFLD patients compared with those without[100,101,103]. Interestingly, the majority of these studies reported that NAFLD, and in particular NASH, was independently associated with chronic kidney disease /microalbuminuria even after adjusting for traditional risk factors, i.e., age, sex, BMI, hypertension, diabetes, smoking and hyperlipidemia[100,102,104,109]. However some studies defined NAFLD according to elevated of GGT or ALT, and thus they need to be interpreted with caution due to the relative lack of diagnostic accuracy. In addition Neri et al[110] reported that, independently related with the traditional risk factors for cardiovascular disease, nonspecific inflammation and oxido-reductive imbalance may play an important role in the progression of NAFLD and atherosclerotic disease in hemodialysis patients.
In conclusion NAFLD, in particular biopsy proven NASH, was associated with a greater prevalence of microvessel damage, in particular chronic kidney disease.
EPICARDIC FAT
Ectopic fat accumulation within and around organs such as the heart and the liver are linked to an increased cardiovascular risk[111,112]. The epicardial fat, is considered the true visceral fat and the best representation of fatty heart[113]. Epicardial fat displays peculiar biomolecular and anatomic characteristics, and its direct contiguity to myocardium reflects the intra-myocardial fat accumulation. Epicardial adipose tissue might secrete vasoactive products that regulate coronary arterial tone, in addition various studies have highlighted the potential importance of adipose tissue in relation to inflammatory burden in cardiovascular diseases. In fact epicardial fat has been demonstrated to be a source of several pro-inflammatory and proatherogenic cytokines that could influence the cardiac function[114-116]. It has been reported that epicardial fat also correlates with several cardiac comorbidities, including coronary artery disease, left ventricular dysfunction, atrial fibrillation; moreover a pivotal role of body fat has been suggested in the development and progression of both diastolic and systolic heart failure[117,118].
Epicardial fat has been found clinically related to abdominal visceral adiposity, coronary artery disease, subclinical atherosclerosis and metabolic syndrome[119-121]. In particular Lai et al[122] reported graded increases in fasting glucose, insulin resistance, and alanine transaminase levels across higher tertiles of epicardial fat thickness. In addition the measurement, by magnetic resonance imaging, of epicardial fat, as well as the degree of hepatic steatosis, correlates with abdominal adiposity and hypertriglyceridemia. A good relation between epicardial fat thickness and the degree of fatty liver severity was described in adult and pediatric patients with NAFLD and obesity[65,123], and with early atherosclerotic damage[45].
The imaging tools used to study and quantify epicardial fat were cardiac CT and magnetic resonance imaging (MRI). Recently, Lai et al[122] showed that ultrasonographic measurement of EAT thickness highly correlated with that measured with CT.
GENETIC
In the last few years, growing evidence indicates that in all diseases, including cardiovascular diseases, environment and genetic factors cooperate in inducing disease manifestation. Data arising from genome-wide (GWAS) or candidate association studies identified single nucleotide polymorphisms (SNPs) in genes involved in metabolic homeostasis, inflammation, oxidative stress and fibrogenesis, as associated with NAFLD and its severity. Patatin-like phospholipase-3 (PNPLA3)/adiponutrin, rs738409 C.G SNP, remains the most validated risk gene in this setting[124]. A recent study by Valenti et al[125] showed that PNPLA3 variant was directly related to hepatic apoptosis in NAFLD. According to these data, it is plausible to hypothesize that PNPLA3 genotype, by regulating apoptotic activity, may be also involved in the pathogenesis of atherosclerosis and might modulate vascular damage. Recently, Petta et al[126] showed that PNPLA3 GG genotype was associated with higher severity of carotid atherosclerosis in younger patients with NAFLD. PNPLA3 gene variants might increase lipid storage in the arterial vessels and could also induce release of ICAM-1, an endothelium-derived inflammatory marker that has been associated with myocardial infarction and stroke[127].
It was also hypothesized that the same pathways generating liver disease might also be involved in vascular damage.
RELATION BETWEEN NAFLD AND ATHEROSCLEROSIS INDEPENDENTLY OF KNOWN RISK FACTORS: POSSIBLE MECHANISMS
Growing evidence suggests that patients with NAFLD are at higher risk of cardiovascular disease than those without NAFLD, even when their primary disease (diabetes, hypertension¡) has a strong potential for atherosclerosis[9,11,13].
Several mechanisms can be involved in atherosclerosis acceleration in patients with NAFLD, including genetic predisposition, insulin resistance and atherogenic dyslipidemia, oxidative stress, chronic inflammation, reduced levels of the adiponectin (an adipocytochine with antiatherogenic and antidiabetic properties), and altered production of pro and anticoagulant factors. All these mechanisms are present at the same time (Figure 2)[11,128].
Figure 2.
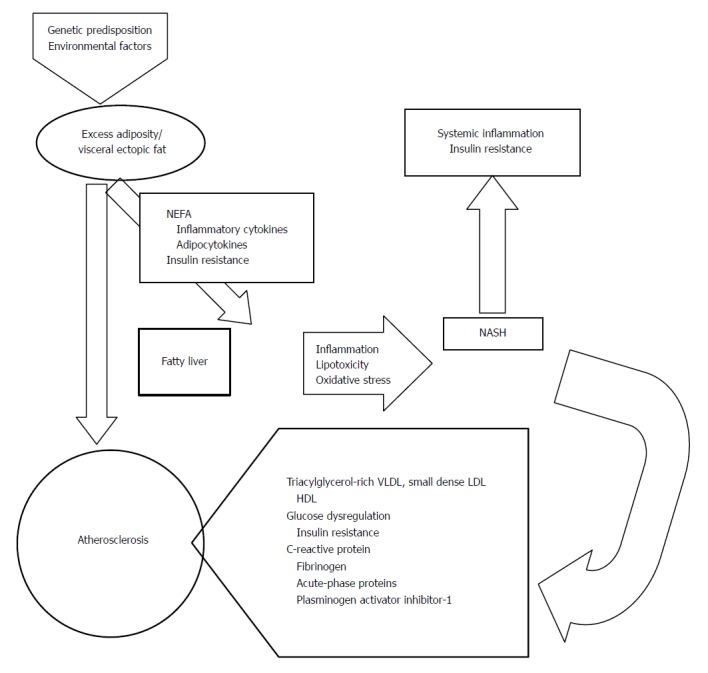
Mechanisms potentially responsible for atherosclerosis in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. NASH: Non-alcoholic steatohepatitis.
The association between early atherosclerosis and NAFLD has been extensively described and summarized in Table 3 in which are reported variables independently associated with IMT in adult subjects[7,13,15,25,41-44,46,47,49-52,55,58,110,125,129-133].
Table 3.
Studies on the association between non-alcoholic fatty liver disease and carotid intima-media thickness in adults: evaluation of the independent predictors for intima-media thickness increase
| Ref. | cases n | Cohort | NAFLD diagnosis by | Independent predictors for IMT |
| Brea et al[40] 2005 | 80 | NALFD and matched population controls | US | NAFLD, age, serum ferritin |
| Volzke et al[41] 2005 | 1261 + 2961 | German population | US | Hepatic steatosis |
| Lonardo et al[129] 2006 | 449 | Population/NAFLD | US | Age |
| Targher et al[52] 2006 | 145 | NAFLD and matched healthy controls | Biopsy | Severity of histological feature of NAFLD |
| Targher et al[55] 2006 | 200 | Type2 diabetes +/- NAFLD | US | HOMA-IR |
| Aygun et al[49] 2008 | 80 | NAFLD and matched healthy controls | US | NAFLD and BMI |
| Sookoian et al[7] 2008 (Meta-regression analysis) | 3497 | Subjects | US | ALT, GGT, NAFLD |
| Fracanzani et al[15] 2008 | 375 | NAFLD and matched population controls | US and biopsy | Steatosis, age and blood pressure |
| Kim et al[42] 2009 | 1021 | Cross sectional | US | NAFLD with MS |
| Petit et al[58] 2009 | 101 | Type 2 diabetes | MRS | Age, no association with steatosis |
| Ramilli et al[43] 2009 | 154 | Referred subjects for abdominal US | US | NAFLD |
| Wang et al[130] 2009 | 170 | Healthy subjects | US | ALT levels |
| Salvi et al[50] 2010 | 220 | NAFLD, healthy controls | NAFLD and MS | |
| Mohammadi et al[44] 2011 | 335 | Male NAFLD controls | US | NAFLD with or without MS |
| Neri et al[110] 2011 | 90 | Chronic hemodialysis plus steatosis and healthy controls | US, biopsy | Histological steatosis |
| Valenti et al[131] 2011 | 506 | NAFLD | US | Systolic blood pressure, glucose, LDL, abdominal circumference, age, ferritin |
| Huang et al[46] 2012 | 2590 + 6042 | Chinese population +/- NAFLD | US | NAFLD |
| Kang et al[47] 2012 | 633 | Non-diabetic +/- NAFLD | US | NAFLD with or without MS |
| Thakur et al[51] 2012 | 80 | Non-diabetic NAFLD, healthy controls | US | NAFLD |
| Kim et al[132] 2013 | 769 | Healthy subjects | US | Upper normal range of ALT in women with NAFLD |
| Oni et al[13] 2013 Systematic review | 16 studies | Population, cross- sectional, case-control | US, biopsy | Positive association between IMT and NAFLD |
| Petta et al[126] 2013 | 162 + 267 | NAFLD and validation cohort | Biopsy | PNPLA3 polymorphism in younger patients |
| Kim et al[133] 2014 | 4437 | Type 2 diabetes | US | NAFDL with insulin resistance |
US: Ultrasonography; MS: Metabolic syndrome; IMT: Intima-media thickness; NAFLD: Nonalcoholic fatty liver disease; MRS: Magnetic resonance spectroscopy.
INSULIN RESISTANCE
Normally insulin inhibits adipose tissue lipolysis and hepatic VLDL secretion by the liver, and on the contrary, insulin resistance promotes fatty acid accumulation in the liver which in turn causes an increase in insulin resistance responsible for a reduced suppression of endogenous liver glucose production. Given the very close relationship between NAFLD and insulin resistance[134,135], which is present in more than 90% of patients with NAFLD, it is still debated which one of the two is the earlier event. In the presence of overweight/obesity and of increased circulating insulin, due to insulin resistance, there is increased lipolysis of adipose tissue which leads to increased flux of free fatty acid (FFA) to the liver from all lipid depots, including ectopic fat. In addition hepatic lipid accumulation occurs also because of dietary chilomicrons and de novo intrahepatic lipogenesis. Further accumulation of lipids in the liver is, in turn, responsible for insulin resistance worsening with a consequent vicious circle. In addition, besides the augment of FFA, the increased release of different molecules from visceral adipose tissue, including inflammatory cytokines, leads to adipose tissue inflammation which aggravates insulin resistance and facilitates atherosclerosis development and cardiovascular disease. Experimental evidences indicate that activation of proinflammatory pathways occurs mainly through the nuclear factor κB (NF-κB) and the c-Jun N-terminal kinase (JNK) pathways which cooperate in inducing insulin resistance which, in association with several factors, including chronic inflammation, atherogenic dyslipidemia, hypercoagulation and hypofibrinolysis, and liver involvement either as target of the metabolic abnormalities or as producer of proatherogenic molecules, triggers arterial damage leading to accelerated atherosclerosis. It is possible that in lean subjects, which are roughly 10%-15% of patients with NAFLD, the insulin resistance and chronic inflammation of visceral adiposity is at least initially replaced by insulin resistance in skeletal muscle[11].
ATHEROGENIC DYSLIPIDEMIA
Typical atherogenic dyslipidemia is frequently detected in NAFLD, characterized by high triglycerides and low HDL-C which, as above reported, together with insulin resistance and visceral adiposity play a major role in accelerated atherosclerosis[136-138]. A high level of low density lipoprotein (LDL) cholesterol, in particular of small dense LDL which are more atherogenic than type A LDL particles, is frequently detected in patients with NAFLD as well as increased levels of oxidized LDL, which are highly atherogenic. Moreover, in a recent large, multi-ethnic, gender balanced cohort, NAFLD was associated with the atherogenic dyslipidemia phenotype (higher fasting serum triglycerides and lower serum HDL) in a dose dependent fashion, suggesting a possible independent pathophysiologic role between NAFLD and dyslipidemia[139]. The main alteration believed to play a key role in atherosclerosis is the overproduction of larger TG-rich VLDL particles from the liver. Furthermore, as described in the insulin resistance paragraph, in the presence of increased adipose tissue there is an increased flux of FFA from adipose tissue to the liver where are re-esterified into triglycerides with consequent increased VLDL secretion into the blood stream. In addition, increased lipid availability within the liver and insulin resistance inhibit apolipoprotein B (APO-B) degradation with formation of more VLDL. The increased level of APO-B, a large protein involved in the transport of triglycerides and cholesterol from the liver to peripheral tissues, which is the primary protein component of the atherogenic LDL particles, is also responsible for accelerated atherosclerosis. The altered ratio between APO-B and apolipoprotein A-1 (APO-A1), the protein component of the anti-atherogenic HDL particles, has been suggested to be a good predictor of cardiovascular risk, even better than HDL/LDL ratio, with more cholesterol deposited in the arterial wall when the ratio is higher[140-142].
INFLAMMATION
Several results obtained in animal models have shown that lipid accumulation in the liver leads to sub-acute hepatic inflammation followed by cytokine production via the NF-κB pathway, in turn followed by increase in insulin resistance. Activation of the NF-kB pathway in the liver of patients with NASH leads to increased transcription of several pro-inflammatory gene that mediate the progression of systemic, low-grade inflammation. In line with these results a growing body of evidence indicates the atherosclerosis is proportional to the severity of liver damage, with CVD accelerated in patients with NASH but to a much less extent in those with simple steatosis[143]. Although the relation between hepatic inflammation and atherosclerosis is still matter of debate, these results are very suggestive for a role played by inflammation in atherosclerosis acceleration. In addition, the evidence that serum and liver tissue proinflammatory markers, such as C reactive protein, IL6, TNF and TGF-β and procoagulant factors, fibrinogen, and plasminogen activator inhibitors are increased in patients with accelerated atherosclerosis reinforces this hypothesis[11]. Furthermore, high-sensitivity C-reactive protein, biomarker of sub-clinical inflammation and of cardiovascular and cerebrovascular disease was found the strongest independent variable associated with NAFLD development in a case-control study[144].
OXIDATIVE STRESS
Several studies have shown that in patients with NAFLD, and in particular in those with NASH, there is evidence of systemic inflammation and increased oxidative stress, relevant factors contributing to metabolic alterations and cardiovascular damage. Altered oxidant/antioxidant status and subclinical inflammation are believed to play a key role in the pathogenesis of atherosclerosis. Hyperglycemia and inflammation increase the production of reactive oxygen species (ROS) triggering oxidative stress which is responsible for oxidation of carbohydrates, lipids and protein. Oxidation of polyunsaturated fatty acids gives raise to the production of malonylaldehyde (MDA), 4-hydroxy-nonenal (HNE) and 4-oxy-2-nonenal (ONE). Increases of these markers, have been detected in patients with NAFLD and correlated to vascular damage as well as in animal models and in vitro studies[145]. Furthermore, it has been found that specific localization in liver tissues cells of oxidized phosphatidylcholine (oxPC, a lipid peroxide that serves as a ligand for scavenger receptors), suggests that neutrophil myeloperoxidase-derived oxidative stress may be crucial in the progression of steatotic liver disease[146].
In addition inflammation and oxide-reductive imbalance may play an important role in the progression of NAFLD and atherosclerotic disease as documented in patients in chronic hemodialysis in which a progressive chronic liver and vascular damage developed faster than in patients without renal failure[110].
The “oxidative stress theory” implies that the initial phase of atherosclerosis is sustained by oxidation of LDL which accumulate in the arterial wall and are taken up by macrophages which, in turn, transform into foam cells. The increased serum levels of LDL and several other pathways of oxidative stress present in patients with NAFLD will accelerate atherosclerosis process[147]. Interestingly the “iron theory” of atherosclerosis, suggested by Sullivan[148], emphasizes the role of iron as a free radical inducer through oxidative stress could find consistency in patients with NAFLD. In fact the high prevalence of increased ferritin, detected in roughly 30% of the patients, could partly account for the accelerated atherosclerosis of these patients. Recent data point out that the relation between increased ferritin and early atherosclerosis, as evaluated by increased IMT, is valid only in patients negative for HFE mutations, suggesting that patients positive for these mutations with lower level of serum hepcidin may be protected from atherosclerosis[131]. This could explain the apparent paradox of the role of iron in atherosclerosis despite the lack of evidence of increased CVD in patients with hereditary hemochromatosis.
Thus, results from experimental and clinical studies suggest that oxidative stress is increased in patients with NAFLD/NASH and that adipose tissue is very likely the major source of ROS which in turn will cooperate in accelerating atherosclerosis.
ADIPOKYNES IMBALANCE
The increase in adipose tissue and chronic inflammation cause an imbalance in adipokines secretion, in particular a reduction of adiponectin and an increase in leptin, two adipokines with opposite function. Adiponectin has been shown to have anti-inflammatory and anti-fibrotic capability and it is believed that its low level may facilitate the progression of CVD and of steatosis to NASH, in addition has been reported to have specifically antiatherogenic properties, whereas leptin, found increased in NAFLD, has been shown in animal models to exert opposite effect. Furthermore, leptin has a multifunctional role in inflammation, that is fundamental in the atherogenic pathway, acting as a proinflammatory stimulus. Among other factors released within adipose tissue, TNF-α promotes lipolysis and increases FFAs; both TNF-α and IL-6 are related to mitochondrial and endothelial dysfunction, to atherosclerosis and cardiovascular disease; at the same time the reduction of protective adipokynes as adiponectin improves vascular damage[149-151].
COAGULATION IMBALANCE
Dyslipidemia, lipid-based oxidative injury and necroapoptosis underpin a multifactorial disorder which may result in a hypercoagulable state. This was suggested on the basis of the evidence of long-lasting ongoing inflammatory state associated with this condition and on epidemiological studies, although someone argues that despite an increased CVD risk, no strong evidence has been reported that patients with NAFLD are at increased risk of thrombosis.
Although a direct link between NAFLD and coagulation has not yet established, several cross-sectional studies indicate that NAFLD and NASH are associated with a systemic prothrombotic state possibly through the release of procoagulant factors from steatotic liver (C-reactive protein, plasminogen activator inhibitor-1, fibrinogen), in addition high levels of factors VIII, IX, XII, VII, von Willebrand, soluble tissue factor, platelet hyperaggregability, procoagulant microparticles are found in NAFLD independently of age, BMI waist circumference, plasma triglycerides and insulin resistance[152-155] as well as in cirrhosis[156]. A possible explanation for the failure to ascertain a causal effect between NAFLD and an impairment of coagulation is the lack of appropriate tests. In fact, coagulation evaluated by traditional global tests such as the prothrombin and activated partial thromboplastin times (PT/APTT) or through the measurement of the individual pro- or anti-coagulants does not allow to detect the complex interplay between the procoagulants (i.e., thrombin-generation drivers) and their naturally-occurring anticoagulant counterparts (i.e., thrombin-neutralization drivers) operating in vivo. However despite the lack of conclusive data, prelimary results seem to suggest the existence a coagulation unbalance between production and/or half-life of both pro- and anticoagulant proteins[157] as shown in Figure 3.
Figure 3.
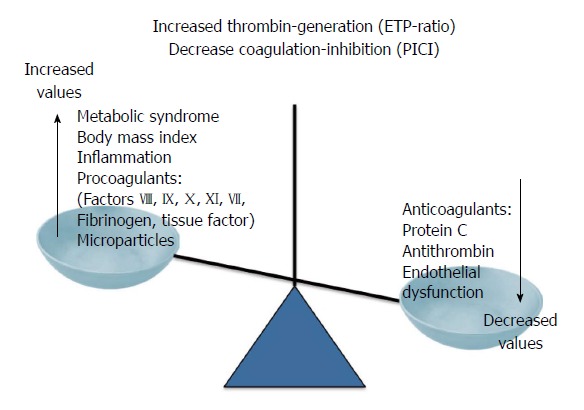
Causes and consequences of the procoagulant imbalance in patients with nonalcoholic fatty liver disease. ETP-ratio: Ratio of the endogenous thrombin potential measured in the presence or absence of thrombomodulin; PICI: Protac®-induced coagulation inhibition.
INCREASED ASSUMPTION OF FRUCTOSE
Patients with NAFLD frequently consume a large amount of fructose, a highly lipogenic sugar which is a common component in all major caloric sweeteners (sucrose, high-fructose corn syrup, honey and fruit juice concentrates) and soft drinks[128,158]. Fructose consumption promotes hepatic de novo lipogenesis and intrahepatic lipids deposition, inhibition of mitochondrial beta-oxidation of long-chain fatty acids , triglycerides formation and steatosis, increased plasma levels of fasting small dense LDL-C, oxidized LDL-C, and activation of inflammatory pathways. All these fructose-induced effects synergize with the metabolic alterations typical of NAFLD playing a key role in atherosclerosis acceleration. This mechanism is particularly important in lean subjects who very often consume a large amount of fructose.
CONCLUSION
All data reported in this review provide evidence of a strong association between NAFLD and subclinical atherosclerosis. Furthermore, current evidence suggests that NAFLD, although considered the hepatic manifestation of the metabolic syndrome, is emerging as an independent risk factor for the occurrence and progression of CVD. The findings and conclusions are similar in retrospective and prospective studies, which evaluated different manifestations of subclinical atherosclerosis (increased IMT, endothelial dysfunction, arterial stiffness, impaired left ventricular function and coronary calcification).
A general agreement emerging from these studies indicates that patients with NASH are at higher risk of CVD than those with simple steatosis, emphasizing the role of chronic inflammation in the pathogenesis of atherosclerosis of these patients.
It is very likely that the different mechanisms involved in the pathogenesis of atherosclerosis in patients with NAFLD have a different relevance in the different patients according to individual genetic background.
In conclusion, in the presence of NAFLD greater emphasis should be placed on the cardiovascular risk with patients undergoing a complete cardiovascular evaluation to prevent future atherosclerotic complications. Specific life-style modification and aggressive pharmaceutical modification will not only reduce the progression of liver disease, but also reduce morbidity for cardiovascular disease improving over-all prognosis and survival.
Footnotes
P- Reviewer: Al-Gayyar MMH, Ikura Y S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Serfaty L, Lemoine M. Definition and natural history of metabolic steatosis: clinical aspects of NAFLD, NASH and cirrhosis. Diabetes Metab. 2008;34:634–637. doi: 10.1016/S1262-3636(08)74597-X. [DOI] [PubMed] [Google Scholar]
- 2.Petta S, Muratore C, Craxì A. Non-alcoholic fatty liver disease pathogenesis: the present and the future. Dig Liver Dis. 2009;41:615–625. doi: 10.1016/j.dld.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Cheung O, Sanyal AJ. Recent advances in nonalcoholic fatty liver disease. Curr Opin Gastroenterol. 2009;25:230–237. doi: 10.1097/mog.0b013e3283294a18. [DOI] [PubMed] [Google Scholar]
- 4.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Hernández H, Gonzalez JL, Márquez-Ramirez MD, Flores-Hernandez M, Rodríguez-Morán M, Guerrero-Romero F. Risk factors associated with nonalcoholic fatty liver disease and its relationship with the hepatic histological changes. Eur J Gastroenterol Hepatol. 2008;20:399–403. doi: 10.1097/MEG.0b013e3282f448af. [DOI] [PubMed] [Google Scholar]
- 7.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600–607. doi: 10.1016/j.jhep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Kars HZ, Hekimoglu B, Cepoglu C. Spinal epidural hydatid cyst: radiological and ultrasonographical workup of a case. Eur J Radiol. 1990;11:212–214. doi: 10.1016/0720-048x(90)90059-k. [DOI] [PubMed] [Google Scholar]
- 9.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600–606. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Boyer TD, Manns MP, Sanyal AJ. Zakim and Boyer’s Hepatology, a textbook of liver disease. 6th ed. USA: Saunders; 2011. pp. 941–968. [Google Scholar]
- 11.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 12.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 13.Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258–267. doi: 10.1016/j.atherosclerosis.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 14.Brea A, Puzo J. Non-alcoholic fatty liver disease and cardiovascular risk. Int J Cardiol. 2013;167:1109–1117. doi: 10.1016/j.ijcard.2012.09.085. [DOI] [PubMed] [Google Scholar]
- 15.Fracanzani AL, Burdick L, Raselli S, Pedotti P, Grigore L, Santorelli G, Valenti L, Maraschi A, Catapano A, Fargion S. Carotid artery intima-media thickness in nonalcoholic fatty liver disease. Am J Med. 2008;121:72–78. doi: 10.1016/j.amjmed.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 16.Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, Kim YJ, Yoon JH, Jeong SH, Lee DH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–613. doi: 10.1002/hep.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonapace S, Perseghin G, Molon G, Canali G, Bertolini L, Zoppini G, Barbieri E, Targher G. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35:389–395. doi: 10.2337/dc11-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yilmaz Y, Kurt R, Yonal O, Polat N, Celikel CA, Gurdal A, Oflaz H, Ozdogan O, Imeryuz N, Kalayci C, et al. Coronary flow reserve is impaired in patients with nonalcoholic fatty liver disease: association with liver fibrosis. Atherosclerosis. 2010;211:182–186. doi: 10.1016/j.atherosclerosis.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 19.Targher G, Valbusa F, Bonapace S, Bertolini L, Zenari L, Rodella S, Zoppini G, Mantovani W, Barbieri E, Byrne CD. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One. 2013;8:e57183. doi: 10.1371/journal.pone.0057183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44:1236–1243. doi: 10.1080/00365520903171284. [DOI] [PubMed] [Google Scholar]
- 22.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong MJ, Adams LA, Canbay A, Syn WK. Extra-hepatic complications of Nonalcoholic fatty liver disease. Hepatology. 2013:In press. doi: 10.1002/hep.26717. [DOI] [PubMed] [Google Scholar]
- 25.Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, McCullough A, Goodman Z, Younossi ZM. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD) Dig Dis Sci. 2013;58:3017–3023. doi: 10.1007/s10620-013-2743-5. [DOI] [PubMed] [Google Scholar]
- 26.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 27.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haring R, Wallaschofski H, Nauck M, Dörr M, Baumeister SE, Völzke H. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyl transpeptidase levels. Hepatology. 2009;50:1403–1411. doi: 10.1002/hep.23135. [DOI] [PubMed] [Google Scholar]
- 30.Zhou YJ, Li YY, Nie YQ, Huang CM, Cao CY. Natural course of nonalcoholic fatty liver disease in southern China: a prospective cohort study. J Dig Dis. 2012;13:153–160. doi: 10.1111/j.1751-2980.2011.00571.x. [DOI] [PubMed] [Google Scholar]
- 31.Treeprasertsuk S, Leverage S, Adams LA, Lindor KD, St Sauver J, Angulo P. The Framingham risk score and heart disease in nonalcoholic fatty liver disease. Liver Int. 2012;32:945–950. doi: 10.1111/j.1478-3231.2011.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646–650. doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 34.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cobble M, Bale B. Carotid intima-media thickness: knowledge and application to everyday practice. Postgrad Med. 2010;122:10–18. doi: 10.3810/pgm.2010.01.2091. [DOI] [PubMed] [Google Scholar]
- 36.Homma S, Hirose N, Ishida H, Ishii T, Araki G. Carotid plaque and intima-media thickness assessed by b-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke. 2001;32:830–835. doi: 10.1161/01.str.32.4.830. [DOI] [PubMed] [Google Scholar]
- 37.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 38.Engström G, Melander O, Hedblad B. Carotid intima-media thickness, systemic inflammation, and incidence of heart failure hospitalizations. Arterioscler Thromb Vasc Biol. 2009;29:1691–1695. doi: 10.1161/ATVBAHA.109.193490. [DOI] [PubMed] [Google Scholar]
- 39.Targher G, Bertolini L, Padovani R, Zenari L, Zoppini G, Falezza G. Relation of nonalcoholic hepatic steatosis to early carotid atherosclerosis in healthy men: role of visceral fat accumulation. Diabetes Care. 2004;27:2498–2500. doi: 10.2337/diacare.27.10.2498. [DOI] [PubMed] [Google Scholar]
- 40.Brea A, Mosquera D, Martín E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol. 2005;25:1045–1050. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- 41.Volzke H, Robinson DM, Kleine V, Deutscher R, Hoffmann W, Ludemann J, Schminke U, Kessler C, John U. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol. 2005;11:1848–1853. doi: 10.3748/wjg.v11.i12.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HC, Kim DJ, Huh KB. Association between nonalcoholic fatty liver disease and carotid intima-media thickness according to the presence of metabolic syndrome. Atherosclerosis. 2009;204:521–525. doi: 10.1016/j.atherosclerosis.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Ramilli S, Pretolani S, Muscari A, Pacelli B, Arienti V. Carotid lesions in outpatients with nonalcoholic fatty liver disease. World J Gastroenterol. 2009;15:4770–4774. doi: 10.3748/wjg.15.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohammadi A, Sedani HH, Ghasemi-Rad M. Evaluation of carotid intima-media thickness and flow-mediated dilatation in middle-aged patients with nonalcoholic fatty liver disease. Vasc Health Risk Manag. 2011;7:661–665. doi: 10.2147/VHRM.S26011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colak Y, Karabay CY, Tuncer I, Kocabay G, Kalayci A, Senates E, Ozturk O, Doganay HL, Enc FY, Ulasoglu C, et al. Relation of epicardial adipose tissue and carotid intima-media thickness in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2012;24:613–618. doi: 10.1097/MEG.0b013e3283513f19. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Bi Y, Xu M, Ma Z, Xu Y, Wang T, Li M, Liu Y, Lu J, Chen Y, et al. Nonalcoholic fatty liver disease is associated with atherosclerosis in middle-aged and elderly Chinese. Arterioscler Thromb Vasc Biol. 2012;32:2321–2326. doi: 10.1161/ATVBAHA.112.252957. [DOI] [PubMed] [Google Scholar]
- 47.Kang JH, Cho KI, Kim SM, Lee JY, Kim JJ, Goo JJ, Kim KN, Jhi JH, Kim DJ, Lee HG, et al. Relationship between Nonalcoholic Fatty Liver Disease and Carotid Artery Atherosclerosis Beyond Metabolic Disorders in Non-Diabetic Patients. J Cardiovasc Ultrasound. 2012;20:126–133. doi: 10.4250/jcu.2012.20.3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colak Y, Senates E, Yesil A, Yilmaz Y, Ozturk O, Doganay L, Coskunpinar E, Kahraman OT, Mesci B, Ulasoglu C, et al. Assessment of endothelial function in patients with nonalcoholic fatty liver disease. Endocrine. 2013;43:100–107. doi: 10.1007/s12020-012-9712-1. [DOI] [PubMed] [Google Scholar]
- 49.Aygun C, Kocaman O, Sahin T, Uraz S, Eminler AT, Celebi A, Senturk O, Hulagu S. Evaluation of metabolic syndrome frequency and carotid artery intima-media thickness as risk factors for atherosclerosis in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2008;53:1352–1357. doi: 10.1007/s10620-007-9998-7. [DOI] [PubMed] [Google Scholar]
- 50.Salvi P, Ruffini R, Agnoletti D, Magnani E, Pagliarani G, Comandini G, Praticò A, Borghi C, Benetos A, Pazzi P. Increased arterial stiffness in nonalcoholic fatty liver disease: the Cardio-GOOSE study. J Hypertens. 2010;28:1699–1707. doi: 10.1097/HJH.0b013e32833a7de6. [DOI] [PubMed] [Google Scholar]
- 51.Thakur ML, Sharma S, Kumar A, Bhatt SP, Luthra K, Guleria R, Pandey RM, Vikram NK. Nonalcoholic fatty liver disease is associated with subclinical atherosclerosis independent of obesity and metabolic syndrome in Asian Indians. Atherosclerosis. 2012;223:507–511. doi: 10.1016/j.atherosclerosis.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, Cigolini M, Falezza G, Arcaro G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29:1325–1330. doi: 10.2337/dc06-0135. [DOI] [PubMed] [Google Scholar]
- 53.Băloşeanu CL, Streba CT, Vere CC, Comănescu V, Rogoveanu I. Association between liver histology, carotid ultrasonography and retinal vascular changes in patients with nonalcoholic fatty liver disease (NAFLD) Rom J Morphol Embryol. 2012;53:609–614. [PubMed] [Google Scholar]
- 54.Kucukazman M, Ata N, Yavuz B, Dal K, Sen O, Deveci OS, Agladioglu K, Yeniova AO, Nazligul Y, Ertugrul DT. Evaluation of early atherosclerosis markers in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2013;25:147–151. doi: 10.1097/MEG.0b013e32835a58b1. [DOI] [PubMed] [Google Scholar]
- 55.Targher G, Bertolini L, Padovani R, Poli F, Scala L, Zenari L, Zoppini G, Falezza G. Non-alcoholic fatty liver disease is associated with carotid artery wall thickness in diet-controlled type 2 diabetic patients. J Endocrinol Invest. 2006;29:55–60. doi: 10.1007/BF03349177. [DOI] [PubMed] [Google Scholar]
- 56.Cakır E, Ozbek M, Colak N, Cakal E, Delıbaşi T. Is NAFLD an independent risk factor for increased IMT in T2DM? Minerva Endocrinol. 2012;37:187–193. [PubMed] [Google Scholar]
- 57.McKimmie RL, Daniel KR, Carr JJ, Bowden DW, Freedman BI, Register TC, Hsu FC, Lohman KK, Weinberg RB, Wagenknecht LE. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol. 2008;103:3029–3035. doi: 10.1111/j.1572-0241.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petit JM, Guiu B, Terriat B, Loffroy R, Robin I, Petit V, Bouillet B, Brindisi MC, Duvillard L, Hillon P, et al. Nonalcoholic fatty liver is not associated with carotid intima-media thickness in type 2 diabetic patients. J Clin Endocrinol Metab. 2009;94:4103–4106. doi: 10.1210/jc.2009-0541. [DOI] [PubMed] [Google Scholar]
- 59.Pacifico L, Cantisani V, Ricci P, Osborn JF, Schiavo E, Anania C, Ferrara E, Dvisic G, Chiesa C. Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res. 2008;63:423–427. doi: 10.1203/PDR.0b013e318165b8e7. [DOI] [PubMed] [Google Scholar]
- 60.Abdou AS, Magour GM, Mahmoud MM. Evaluation of some markers of subclinical atherosclerosis in Egyptian young adult males with abdominal obesity. Br J Biomed Sci. 2009;66:143–147. doi: 10.1080/09674845.2009.11730261. [DOI] [PubMed] [Google Scholar]
- 61.Kelishadi R, Cook SR, Amra B, Adibi A. Factors associated with insulin resistance and non-alcoholic fatty liver disease among youths. Atherosclerosis. 2009;204:538–543. doi: 10.1016/j.atherosclerosis.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 62.Caserta CA, Pendino GM, Amante A, Vacalebre C, Fiorillo MT, Surace P, Messineo A, Surace M, Alicante S, Cotichini R, et al. Cardiovascular risk factors, nonalcoholic fatty liver disease, and carotid artery intima-media thickness in an adolescent population in southern Italy. Am J Epidemiol. 2010;171:1195–1202. doi: 10.1093/aje/kwq073. [DOI] [PubMed] [Google Scholar]
- 63.Hacihamdioğlu B, Okutan V, Yozgat Y, Yildirim D, Kocaoğlu M, Lenk MK, Ozcan O. Abdominal obesity is an independent risk factor for increased carotid intima- media thickness in obese children. Turk J Pediatr. 2011;53:48–54. [PubMed] [Google Scholar]
- 64.Sert A, Pirgon O, Aypar E, Yilmaz H, Odabas D. Relationship between left ventricular mass and carotid intima media thickness in obese adolescents with non-alcoholic fatty liver disease. J Pediatr Endocrinol Metab. 2012;25:927–934. doi: 10.1515/jpem-2012-0187. [DOI] [PubMed] [Google Scholar]
- 65.Alp H, Karaarslan S, Selver Eklioğlu B, Atabek ME, Altın H, Baysal T. Association between nonalcoholic fatty liver disease and cardiovascular risk in obese children and adolescents. Can J Cardiol. 2013;29:1118–1125. doi: 10.1016/j.cjca.2012.07.846. [DOI] [PubMed] [Google Scholar]
- 66.Quinn U, Tomlinson LA, Cockcroft JR. Arterial stiffness. JRSM Cardiovasc Dis. 2012;1 doi: 10.1258/cvd.2012.012024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vlachopoulos C, Manesis E, Baou K, Papatheodoridis G, Koskinas J, Tiniakos D, Aznaouridis K, Archimandritis A, Stefanadis C. Increased arterial stiffness and impaired endothelial function in nonalcoholic Fatty liver disease: a pilot study. Am J Hypertens. 2010;23:1183–1189. doi: 10.1038/ajh.2010.144. [DOI] [PubMed] [Google Scholar]
- 68.Huang RC, Beilin LJ, Ayonrinde O, Mori TA, Olynyk JK, Burrows S, Hands B, Adams LA. Importance of cardiometabolic risk factors in the association between nonalcoholic fatty liver disease and arterial stiffness in adolescents. Hepatology. 2013;58:1306–1314. doi: 10.1002/hep.26495. [DOI] [PubMed] [Google Scholar]
- 69.Kim BJ, Kim NH, Kim BS, Kang JH. The association between nonalcoholic fatty liver disease, metabolic syndrome and arterial stiffness in nondiabetic, nonhypertensive individuals. Cardiology. 2012;123:54–61. doi: 10.1159/000341248. [DOI] [PubMed] [Google Scholar]
- 70.Lee YJ, Shim JY, Moon BS, Shin YH, Jung DH, Lee JH, Lee HR. The relationship between arterial stiffness and nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:196–203. doi: 10.1007/s10620-011-1819-3. [DOI] [PubMed] [Google Scholar]
- 71.Işilak Z, Aparci M, Kardeşoğlu E, Yiğiner O, Uz O, Sildiroglu O, Ozmen N, Yalçin M, Cingözbay BY, Cebeci BS. Abnormal aortic elasticity in patients with liver steatosis. Diabetes Res Clin Pract. 2010;87:44–50. doi: 10.1016/j.diabres.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 72.Fotbolcu H, Yakar T, Duman D, Ozden K, Karaahmet T, Tigen K, Kurtoglu U, Dindar I. Aortic elastic properties in nonalcoholic fatty liver disease. Blood Press Monit. 2010;15:139–145. doi: 10.1097/MBP.0b013e328339e2c8. [DOI] [PubMed] [Google Scholar]
- 73.Catena C, Bernardi S, Sabato N, Grillo A, Ermani M, Sechi LA, Fabris B, Carretta R, Fallo F. Ambulatory arterial stiffness indices and non-alcoholic fatty liver disease in essential hypertension. Nutr Metab Cardiovasc Dis. 2013;23:389–393. doi: 10.1016/j.numecd.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 74.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 75.Senturk O, Kocaman O, Hulagu S, Sahin T, Aygun C, Konduk T, Celebi A. Endothelial dysfunction in Turkish patients with non-alcoholic fatty liver disease. Intern Med J. 2008;38:183–189. doi: 10.1111/j.1445-5994.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 76.Sciacqua A, Perticone M, Miceli S, Laino I, Tassone EJ, Grembiale RD, Andreozzi F, Sesti G, Perticone F. Endothelial dysfunction and non-alcoholic liver steatosis in hypertensive patients. Nutr Metab Cardiovasc Dis. 2011;21:485–491. doi: 10.1016/j.numecd.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 77.Dogru T, Genc H, Tapan S, Aslan F, Ercin CN, Ors F, Kara M, Sertoglu E, Karslioglu Y, Bagci S, et al. Plasma fetuin-A is associated with endothelial dysfunction and subclinical atherosclerosis in subjects with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf) 2013;78:712–717. doi: 10.1111/j.1365-2265.2012.04460.x. [DOI] [PubMed] [Google Scholar]
- 78.Goland S, Shimoni S, Zornitzki T, Knobler H, Azoulai O, Lutaty G, Melzer E, Orr A, Caspi A, Malnick S. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol. 2006;40:949–955. doi: 10.1097/01.mcg.0000225668.53673.e6. [DOI] [PubMed] [Google Scholar]
- 79.Lautamäki R, Borra R, Iozzo P, Komu M, Lehtimäki T, Salmi M, Jalkanen S, Airaksinen KE, Knuuti J, Parkkola R, et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2006;291:E282–E290. doi: 10.1152/ajpendo.00604.2005. [DOI] [PubMed] [Google Scholar]
- 80.Fallo F, Dalla Pozza A, Sonino N, Lupia M, Tona F, Federspil G, Ermani M, Catena C, Soardo G, Di Piazza L, et al. Non-alcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in essential hypertension. Nutr Metab Cardiovasc Dis. 2009;19:646–653. doi: 10.1016/j.numecd.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 81.Perseghin G, Lattuada G, De Cobelli F, Esposito A, Belloni E, Ntali G, Ragogna F, Canu T, Scifo P, Del Maschio A, et al. Increased mediastinal fat and impaired left ventricular energy metabolism in young men with newly found fatty liver. Hepatology. 2008;47:51–58. doi: 10.1002/hep.21983. [DOI] [PubMed] [Google Scholar]
- 82.Hallsworth K, Hollingsworth KG, Thoma C, Jakovljevic D, MacGowan GA, Anstee QM, Taylor R, Day CP, Trenell MI. Cardiac structure and function are altered in adults with non-alcoholic fatty liver disease. J Hepatol. 2013;58:757–762. doi: 10.1016/j.jhep.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 83.Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G. The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review. Health Technol Assess. 2006;10:iii–iv, ix-x, 1-41. doi: 10.3310/hta10390. [DOI] [PubMed] [Google Scholar]
- 84.Hamirani YS, Pandey S, Rivera JJ, Ndumele C, Budoff MJ, Blumenthal RS, Nasir K. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis. 2008;201:1–7. doi: 10.1016/j.atherosclerosis.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 85.Akabame S, Hamaguchi M, Tomiyasu K, Tanaka M, Kobayashi-Takenaka Y, Nakano K, Oda Y, Yoshikawa T. Evaluation of vulnerable coronary plaques and non-alcoholic fatty liver disease (NAFLD) by 64-detector multislice computed tomography (MSCT) Circ J. 2008;72:618–625. doi: 10.1253/circj.72.618. [DOI] [PubMed] [Google Scholar]
- 86.Santos RD, Nasir K, Orakzai R, Meneghelo RS, Carvalho JA, Blumenthal RS. Relation of uric acid levels to presence of coronary artery calcium detected by electron beam tomography in men free of symptomatic myocardial ischemia with versus without the metabolic syndrome. Am J Cardiol. 2007;99:42–45. doi: 10.1016/j.amjcard.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 87.Assy N, Djibre A, Farah R, Grosovski M, Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology. 2010;254:393–400. doi: 10.1148/radiol.09090769. [DOI] [PubMed] [Google Scholar]
- 88.Chen CH, Nien CK, Yang CC, Yeh YH. Association between nonalcoholic fatty liver disease and coronary artery calcification. Dig Dis Sci. 2010;55:1752–1760. doi: 10.1007/s10620-009-0935-9. [DOI] [PubMed] [Google Scholar]
- 89.Jung CH, Lee WY, Kim SY, Jung JH, Rhee EJ, Park CY, Mok JO, Oh KW, Kim CH, Park SW, et al. The relationship between coronary artery calcification score, plasma osteoprotegerin level and arterial stiffness in asymptomatic type 2 DM. Acta Diabetol. 2010;47 Suppl 1:145–152. doi: 10.1007/s00592-009-0154-z. [DOI] [PubMed] [Google Scholar]
- 90.Ballestri S, Meschiari E, Baldelli E, Musumeci FE, Romagnoli D, Trenti T, Zennaro RG, Lonardo A, Loria P. Relationship of serum fetuin-A levels with coronary atherosclerotic burden and NAFLD in patients undergoing elective coronary angiography. Metab Syndr Relat Disord. 2013;11:289–295. doi: 10.1089/met.2012.0149. [DOI] [PubMed] [Google Scholar]
- 91.Mirrakhimov AE, Polotsky VY. Obstructive sleep apnea and non-alcoholic Fatty liver disease: is the liver another target? Front Neurol. 2012;3:149. doi: 10.3389/fneur.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417–431. doi: 10.1111/obr.12020. [DOI] [PubMed] [Google Scholar]
- 93.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62:569–576. doi: 10.1016/j.jacc.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mirrakhimov AE, Sooronbaev T, Mirrakhimov EM. Prevalence of obstructive sleep apnea in Asian adults: a systematic review of the literature. BMC Pulm Med. 2013;13:10. doi: 10.1186/1471-2466-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zamarrón C, Valdés Cuadrado L, Alvarez-Sala R. Pathophysiologic mechanisms of cardiovascular disease in obstructive sleep apnea syndrome. Pulm Med. 2013;2013:521087. doi: 10.1155/2013/521087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, Muggeo M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51:444–450. doi: 10.1007/s00125-007-0897-4. [DOI] [PubMed] [Google Scholar]
- 97.Josef P, Ali I, Ariel P, Alon M, Nimer A. Relationship between retinal vascular caliber and coronary artery disease in patients with non-alcoholic fatty liver disease (NAFLD) Int J Environ Res Public Health. 2013;10:3409–3423. doi: 10.3390/ijerph10083409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Targher G, Chonchol M, Zoppini G, Abaterusso C, Bonora E. Risk of chronic kidney disease in patients with non-alcoholic fatty liver disease: is there a link? J Hepatol. 2011;54:1020–1029. doi: 10.1016/j.jhep.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 99.Yun KE, Shin CY, Yoon YS, Park HS. Elevated alanine aminotransferase levels predict mortality from cardiovascular disease and diabetes in Koreans. Atherosclerosis. 2009;205:533–537. doi: 10.1016/j.atherosclerosis.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 100.Yilmaz Y, Alahdab YO, Yonal O, Kurt R, Kedrah AE, Celikel CA, Ozdogan O, Duman D, Imeryuz N, Avsar E, et al. Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: association with liver fibrosis. Metabolism. 2010;59:1327–1330. doi: 10.1016/j.metabol.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 101.Targher G, Bertolini L, Chonchol M, Rodella S, Zoppini G, Lippi G, Zenari L, Bonora E. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and retinopathy in type 1 diabetic patients. Diabetologia. 2010;53:1341–1348. doi: 10.1007/s00125-010-1720-1. [DOI] [PubMed] [Google Scholar]
- 102.Hwang ST, Cho YK, Yun JW, Park JH, Kim HJ, Park DI, Sohn CI, Jeon WK, Kim BI, Rhee EJ, et al. Impact of non-alcoholic fatty liver disease on microalbuminuria in patients with prediabetes and diabetes. Intern Med J. 2010;40:437–442. doi: 10.1111/j.1445-5994.2009.01979.x. [DOI] [PubMed] [Google Scholar]
- 103.Yasui K, Sumida Y, Mori Y, Mitsuyoshi H, Minami M, Itoh Y, Kanemasa K, Matsubara H, Okanoue T, Yoshikawa T. Nonalcoholic steatohepatitis and increased risk of chronic kidney disease. Metabolism. 2011;60:735–739. doi: 10.1016/j.metabol.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 104.Sirota JC, McFann K, Targher G, Chonchol M, Jalal DI. Association between nonalcoholic liver disease and chronic kidney disease: an ultrasound analysis from NHANES 1988-1994. Am J Nephrol. 2012;36:466–471. doi: 10.1159/000343885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee DH, Jacobs DR, Gross M, Steffes M. Serum gamma-glutamyltransferase was differently associated with microalbuminuria by status of hypertension or diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem. 2005;51:1185–1191. doi: 10.1373/clinchem.2004.045872. [DOI] [PubMed] [Google Scholar]
- 106.Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, Wang TJ, Benjamin EJ, D’Agostino RB, Vasan RS. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27:127–133. doi: 10.1161/01.ATV.0000251993.20372.40. [DOI] [PubMed] [Google Scholar]
- 107.Ryu S, Chang Y, Kim DI, Kim WS, Suh BS. gamma-Glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem. 2007;53:71–77. doi: 10.1373/clinchem.2006.078980. [DOI] [PubMed] [Google Scholar]
- 108.Chang Y, Ryu S, Sung E, Woo HY, Oh E, Cha K, Jung E, Kim WS. Nonalcoholic fatty liver disease predicts chronic kidney disease in nonhypertensive and nondiabetic Korean men. Metabolism. 2008;57:569–576. doi: 10.1016/j.metabol.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 109.Targher G, Kendrick J, Smits G, Chonchol M. Relationship between serum gamma-glutamyltransferase and chronic kidney disease in the United States adult population. Findings from the National Health and Nutrition Examination Survey 2001-2006. Nutr Metab Cardiovasc Dis. 2010;20:583–590. doi: 10.1016/j.numecd.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 110.Neri S, Signorelli SS, Scuderi R, Bruno M, Bertino G, Clementi A, Torrisi I, Fidone F, Pagano AB, Malaguarnera M, et al. Carotid intima-media thickness and liver histology in hemodialysis patients with nonalcoholic Fatty liver disease. Int J Angiol. 2011;20:149–156. doi: 10.1055/s-0031-1283218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–457. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Malavazos AE, Di Leo G, Secchi F, Lupo EN, Dogliotti G, Coman C, Morricone L, Corsi MM, Sardanelli F, Iacobellis G. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am J Cardiol. 2010;105:1831–1835. doi: 10.1016/j.amjcard.2010.01.368. [DOI] [PubMed] [Google Scholar]
- 113.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 114.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 115.Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z, Haluzikova D, Bosanska L, Vokurka M, Svacina S, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91:4620–4627. doi: 10.1210/jc.2006-1044. [DOI] [PubMed] [Google Scholar]
- 116.Baker AR, Harte AL, Howell N, Pritlove DC, Ranasinghe AM, da Silva NF, Youssef EM, Khunti K, Davies MJ, Bonser RS, et al. Epicardial adipose tissue as a source of nuclear factor-kappaB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease. J Clin Endocrinol Metab. 2009;94:261–267. doi: 10.1210/jc.2007-2579. [DOI] [PubMed] [Google Scholar]
- 117.Nakazato R, Dey D, Cheng VY, Gransar H, Slomka PJ, Hayes SW, Thomson LE, Friedman JD, Min JK, Berman DS. Epicardial fat volume and concurrent presence of both myocardial ischemia and obstructive coronary artery disease. Atherosclerosis. 2012;221:422–426. doi: 10.1016/j.atherosclerosis.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 118.Khawaja T, Greer C, Chokshi A, Chavarria N, Thadani S, Jones M, Schaefle K, Bhatia K, Collado JE, Shimbo D, et al. Epicardial fat volume in patients with left ventricular systolic dysfunction. Am J Cardiol. 2011;108:397–401. doi: 10.1016/j.amjcard.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 119.Granér M, Siren R, Nyman K, Lundbom J, Hakkarainen A, Pentikäinen MO, Lauerma K, Lundbom N, Adiels M, Nieminen MS, et al. Cardiac steatosis associates with visceral obesity in nondiabetic obese men. J Clin Endocrinol Metab. 2013;98:1189–1197. doi: 10.1210/jc.2012-3190. [DOI] [PubMed] [Google Scholar]
- 120.Nelson MR, Mookadam F, Thota V, Emani U, Al Harthi M, Lester SJ, Cha S, Stepanek J, Hurst RT. Epicardial fat: an additional measurement for subclinical atherosclerosis and cardiovascular risk stratification? J Am Soc Echocardiogr. 2011;24:339–345. doi: 10.1016/j.echo.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 121.Cikim AS, Topal E, Harputluoglu M, Keskin L, Zengin Z, Cikim K, Ozdemir R, Aladag M, Yologlu S. Epicardial adipose tissue, hepatic steatosis and obesity. J Endocrinol Invest. 2007;30:459–464. doi: 10.1007/BF03346328. [DOI] [PubMed] [Google Scholar]
- 122.Lai YH, Yun CH, Yang FS, Liu CC, Wu YJ, Kuo JY, Yeh HI, Lin TY, Bezerra HG, Shih SC, et al. Epicardial adipose tissue relating to anthropometrics, metabolic derangements and fatty liver disease independently contributes to serum high-sensitivity C-reactive protein beyond body fat composition: a study validated with computed tomography. J Am Soc Echocardiogr. 2012;25:234–241. doi: 10.1016/j.echo.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 123.Iacobellis G, Barbarini G, Letizia C, Barbaro G. Epicardial fat thickness and nonalcoholic fatty liver disease in obese subjects. Obesity (Silver Spring) 2014;22:332–336. doi: 10.1002/oby.20624. [DOI] [PubMed] [Google Scholar]
- 124.Valenti L, Alisi A, Nobili V. I148M PNPLA3 variant and progressive liver disease: a new paradigm in hepatology. Hepatology. 2012;56:1883–1889. [PubMed] [Google Scholar]
- 125.Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, Nobili V, Mozzi E, Roviaro G, Vanni E, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209–1217. doi: 10.1002/hep.23622. [DOI] [PubMed] [Google Scholar]
- 126.Petta S, Valenti L, Marchesini G, Di Marco V, Licata A, Cammà C, Barcellona MR, Cabibi D, Donati B, Fracanzani A, et al. PNPLA3 GG genotype and carotid atherosclerosis in patients with non-alcoholic fatty liver disease. PLoS One. 2013;8:e74089. doi: 10.1371/journal.pone.0074089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Paré G, Ridker PM, Rose L, Barbalic M, Dupuis J, Dehghan A, Bis JC, Benjamin EJ, Shiffman D, Parker AN, et al. Genome-wide association analysis of soluble ICAM-1 concentration reveals novel associations at the NFKBIK, PNPLA3, RELA, and SH2B3 loci. PLoS Genet. 2011;7:e1001374. doi: 10.1371/journal.pgen.1001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627–636. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 129.Lonardo A, Lombardini S, Scaglioni F, Ballestri S, Verrone AM, Bertolotti M, Carulli L, Ganazzi D, Carulli N, Loria P. Fatty liver, carotid disease and gallstones: a study of age-related associations. World J Gastroenterol. 2006;12:5826–5833. doi: 10.3748/wjg.v12.i36.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang CC, Lin SK, Tseng YF, Hsu CS, Tseng TC, Lin HH, Wang LY, Kao JH. Elevation of serum aminotransferase activity increases risk of carotid atherosclerosis in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24:1411–1416. doi: 10.1111/j.1440-1746.2009.05872.x. [DOI] [PubMed] [Google Scholar]
- 131.Valenti L, Swinkels DW, Burdick L, Dongiovanni P, Tjalsma H, Motta BM, Bertelli C, Fatta E, Bignamini D, Rametta R, et al. Serum ferritin levels are associated with vascular damage in patients with nonalcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2011;21:568–575. doi: 10.1016/j.numecd.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 132.Kim KS, Oh HJ, Kim DJ, Kim SK, Park SW, Cho YW, Huh KB. The association between non-alcoholic fatty liver disease and carotid atherosclerosis in subjects with within-reference range alanine aminotransferase levels. Endocr J. 2013;60:1295–1301. doi: 10.1507/endocrj.ej13-0269. [DOI] [PubMed] [Google Scholar]
- 133.Kim SK, Choi YJ, Huh BW, Park SW, Lee EJ, Cho YW, Huh KB. Nonalcoholic Fatty liver disease is associated with increased carotid intima-media thickness only in type 2 diabetic subjects with insulin resistance. J Clin Endocrinol Metab. 2014;99:1879–1884. doi: 10.1210/jc.2013-4133. [DOI] [PubMed] [Google Scholar]
- 134.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 135.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 136.Nobili V, Alkhouri N, Bartuli A, Manco M, Lopez R, Alisi A, Feldstein AE. Severity of liver injury and atherogenic lipid profile in children with nonalcoholic fatty liver disease. Pediatr Res. 2010;67:665–670. doi: 10.1203/PDR.0b013e3181da4798. [DOI] [PubMed] [Google Scholar]
- 137.Alkhouri N, Carter-Kent C, Elias M, Feldstein AE. Atherogenic dyslipidemia and cardiovascular risk in children with nonalcoholic fatty liver disease. Clin Lipidol. 2011;6:305–314. doi: 10.2217/clp.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.da Luz PL, Favarato D, Faria-Neto JR, Lemos P, Chagas AC. High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics (Sao Paulo) 2008;63:427–432. doi: 10.1590/S1807-59322008000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, Blumenthal RS, Budoff MJ. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;227:429–436. doi: 10.1016/j.atherosclerosis.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Walldius G, Jungner I. Apolipoprotein B and apolipoprotein A-I: risk indicators of coronary heart disease and targets for lipid-modifying therapy. J Intern Med. 2004;255:188–205. doi: 10.1046/j.1365-2796.2003.01276.x. [DOI] [PubMed] [Google Scholar]
- 141.Sierra-Johnson J, Somers VK, Kuniyoshi FH, Garza CA, Isley WL, Gami AS, Lopez-Jimenez F. Comparison of apolipoprotein-B/apolipoprotein-AI in subjects with versus without the metabolic syndrome. Am J Cardiol. 2006;98:1369–1373. doi: 10.1016/j.amjcard.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 142.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51:1947–1953. doi: 10.1007/s00125-008-1135-4. [DOI] [PubMed] [Google Scholar]
- 143.Alkhouri N, Tamimi TA, Yerian L, Lopez R, Zein NN, Feldstein AE. The inflamed liver and atherosclerosis: a link between histologic severity of nonalcoholic fatty liver disease and increased cardiovascular risk. Dig Dis Sci. 2010;55:2644–2650. doi: 10.1007/s10620-009-1075-y. [DOI] [PubMed] [Google Scholar]
- 144.Nigam P, Bhatt SP, Misra A, Vaidya M, Dasgupta J, Chadha DS. Non-alcoholic fatty liver disease is closely associated with sub-clinical inflammation: a case-control study on Asian Indians in North India. PLoS One. 2013;8:e49286. doi: 10.1371/journal.pone.0049286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kathirvel E, Chen P, Morgan K, French SW, Morgan TR. Oxidative stress and regulation of anti-oxidant enzymes in cytochrome P4502E1 transgenic mouse model of non-alcoholic fatty liver. J Gastroenterol Hepatol. 2010;25:1136–1143. doi: 10.1111/j.1440-1746.2009.06196.x. [DOI] [PubMed] [Google Scholar]
- 146.Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, Nishiguchi S, Inoue T, Naruko T, Ehara S, et al. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43:506–514. doi: 10.1002/hep.21070. [DOI] [PubMed] [Google Scholar]
- 147.Hopps E, Noto D, Caimi G, Averna MR. A novel component of the metabolic syndrome: the oxidative stress. Nutr Metab Cardiovasc Dis. 2010;20:72–77. doi: 10.1016/j.numecd.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 148.Sullivan JL. Iron in arterial plaque: modifiable risk factor for atherosclerosis. Biochim Biophys Acta. 2009;1790:718–723. doi: 10.1016/j.bbagen.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 149.Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14:72–81. doi: 10.1016/j.molmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 150.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 151.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 152.Targher G, Chonchol M, Miele L, Zoppini G, Pichiri I, Muggeo M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost. 2009;35:277–287. doi: 10.1055/s-0029-1222606. [DOI] [PubMed] [Google Scholar]
- 153.Kotronen A, Joutsi-Korhonen L, Sevastianova K, Bergholm R, Hakkarainen A, Pietiläinen KH, Lundbom N, Rissanen A, Lassila R, Yki-Järvinen H. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver Int. 2011;31:176–183. doi: 10.1111/j.1478-3231.2010.02375.x. [DOI] [PubMed] [Google Scholar]
- 154.Kornek M, Lynch M, Mehta SH, Lai M, Exley M, Afdhal NH, Schuppan D. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology. 2012;143:448–458. doi: 10.1053/j.gastro.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Northup PG, Argo CK, Shah N, Caldwell SH. Hypercoagulation and thrombophilia in nonalcoholic fatty liver disease: mechanisms, human evidence, therapeutic implications, and preventive implications. Semin Liver Dis. 2012;32:39–48. doi: 10.1055/s-0032-1306425. [DOI] [PubMed] [Google Scholar]
- 156.Tripodi A, Primignani M, Lemma L, Chantarangkul V, Dell’Era A, Iannuzzi F, Aghemo A, Mannucci PM. Detection of the imbalance of procoagulant versus anticoagulant factors in cirrhosis by a simple laboratory method. Hepatology. 2010;52:249–255. doi: 10.1002/hep.23653. [DOI] [PubMed] [Google Scholar]
- 157.Tripodi A, Fracanzani AL, Primignani M, Chantarangkul V, Clerici M, Mannucci PM, Peyvandi F, Bertelli C, Valenti L, Fargion S. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;61:148–154. doi: 10.1016/j.jhep.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 158.Pacifico L, Nobili V, Anania C, Verdecchia P, Chiesa C. Pediatric nonalcoholic fatty liver disease, metabolic syndrome and cardiovascular risk. World J Gastroenterol. 2011;17:3082–3091. doi: 10.3748/wjg.v17.i26.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]


