Abstract
The field of bariatric surgery has been rapidly growing and evolving over the past several decades. During the period that obesity has become a worldwide epidemic, new interventions have been developed to combat this complex disorder. The development of new laparoscopic and minimally invasive treatments for medically-complicated obesity has made it essential that gastrointestinal physicians obtain a thorough understanding of past developments and possible future directions in bariatrics. New laparoscopic advancements provide patients and practitioners with a variety of options that have an improved safety profile and better efficacy without open, invasive surgery. The mechanisms of weight loss after bariatric surgery are complex and may in part be related to altered release of regulatory peptide hormones from the gut. Endoscopic techniques designed to mimic the effects of bariatric surgery and endolumenal interventions performed entirely through the gastrointestinal tract offer potential advantages. Several of these new techniques have demonstrated promising, preliminary results. We outline herein historical and current trends in the development of bariatric surgery and its transition to safer and more minimally invasive procedures designed to induce weight loss.
Keywords: Obesity, Bariatrics, Bariatric surgery, Weight loss, Endoscopy
Core tip: Obesity and its associated co-morbidities are on the rise worldwide and have reached epidemic proportions. Surgical procedures have been developed and refined to manage obesity. Bariatric surgery is now the preferred modality of therapy for medically-complicated obesity. Attempts to replace invasive bariatric techniques are the driving factors behind studies of newer, minimally invasive procedures. Our therapeutic armamentarium continues to expand for treatment of morbid obesity and its medical complication as new research is completed and novel minimally invasive techniques are assessed. Preliminary results in several of these areas are promising and provide practitioners with a potential future array of options and modes of therapy.
INTRODUCTION
Over the past several decades, obesity has increasingly become a major health care concern. From 1980 to 2008, the global trend has shown a rapid increase in the mean body mass index (BMI) by a rate of 0.4 kg/m2 per decade[1]. The highest mean BMI among high-income countries is found in the United States[1]. According to the Centers for Disease Control and Prevention National Center for Health Statistics, 35.7% of adults in the US were obese in 2009 and 2010[2]. Although the overall trend continues to rise, the prevalence of obesity may be reaching a plateau as it did not significantly change in 2009 and 2010 when compared with 2003 to 2008[3]. While the increasing prevalence of obesity may be slowing, morbid obesity continues to rise at a brisk rate in the United States[4].
The rise in obesity is a major contributing factor to the growing prevalence of type 2 diabetes mellitus[5,6]. The risk of developing type 2 diabetes mellitus increases proportionally with increasing BMI as each unit of increased BMI correlates with an approximately 12% increase in risk[7]. Obesity has also been significantly associated with high blood pressure, high cholesterol, asthma, arthritis, and overall poor health status[8]. Both the United States Preventive Services Task Force and the National Institutes of Health (NIH) recommend treatment of obesity through improvement of diet or nutrition, increasing physical activity, and behavioral interventions[9,10]. However, treatment of morbid obesity with bariatric surgical procedures has been shown to be significantly superior to intensive medical programs and has demonstrated improvement in type 2 diabetes mellitus indices, sustained weight loss, improvement in quality of life, and amelioration of several cardiovascular risk factors[11-15].
Guidelines set forth by the NIH recommend bariatric surgery as a treatment option for obesity in patients with a BMI ≥ 40 kg/m2 or for patients with BMI ≥ 35 kg/m2 and co-morbidities, for whom other therapies have failed[9]. Some experts have advocated for less stringent guidelines for bariatric surgery as a treatment modality. Although there is data to suggest patients with BMI ≤ 35 kg/m2 would benefit from bariatric surgery[16,17] more extensive and robust data is needed before such widespread recommendations can be made[18] .
Bariatric surgical options are numerous and continue to expand with advancing technology due to the availability of less invasive procedures. In patients who undergo bariatric surgery, the utilization of minimally invasive, laparoscopic surgery provides multiple advantages over the older, open surgical methods. Laparoscopic bariatric surgery provides earlier ambulation after surgery, less postoperative abdominal pain, lower postoperative risks of pneumonia and deep venous thrombosis, decreased length of hospital stay, improved cosmetic appearance, lower risk of postoperative wound complications (including those of infection and hernia development), and an earlier return to societal activities, including their job activities.
In addition to the increasing number of surgical options, endoscopic techniques that have had varying degrees of success in the treatment of morbid obesity are becoming more widely studied. An array of approaches has been explored ranging from duodenal and intralumenal intestinal electrical stimulation to natural orifice translumenal endoscopic surgery (NOTES). In this article, we review the hypothesized pathophysiology, surgical procedures, and endoscopic procedures currently available as well as future directions in the field of bariatrics.
TYPES OF BARIATRIC SURGERY AND THEIR PROPOSED EFFECTS
The first surgical procedure for the purpose of inducing weight loss was described by Dr. Viktor Henrikson in a 1952 case report discussing resection of the small intestine[19]. Bariatric surgery for the treatment of obesity was studied by Kremen et al[20] in 1954 using dogs as an animal model. They subsequently performed a jejuno-ileal bypass on a human subject. The early forms of bariatric surgical procedures had high rates of morbidity and mortality. With evolution of the field, bariatric surgeries have been altered to increase safety and to allow for fewer adverse effects. The annual number of bariatric surgical procedures performed worldwide has surged since 1998[21], and has now become a widely available and accepted modality for the treatment of morbid obesity. Approximately 146000 bariatric surgeries were performed worldwide in 2003[22]. The upward trend has continued with greater than 340000 annual bariatric surgeries worldwide in 2008 and 2011[23].
The most common bariatric surgical procedures are outlined in Figure 1. Roux-en-Y gastric bypass (RYGB) is the most commonly performed bariatric surgery worldwide, including in the United States/Canada, Europe, and Latin/South America. Adjustable gastric banding (AGB) is currently the third most common bariatric surgical procedure worldwide, and by 2011 AGB was less commonly utilized (17.8% internationally) compared to 2003 (24.4% internationally)[23]. The major reason for the decline in utilization of AGB has been the pronounced upward trend in the percentage of vertical sleeve gastrectomy (SG) surgeries performed between 2003 (0% internationally) and 2011 (27.89% internationally)[23]. This shift in the choice of laparoscopic surgical procedures may be due to a more rapid and more substantial weight loss after SG; it may be due to the removal of the risk related to the chronic implantation of an adjustable band; it may be due to the removal of requirement of follow up adjustments in individuals with adjustable bands; it may be related to an absence of urgent return visits following the overfilling of adjustable bands.
Figure 1.
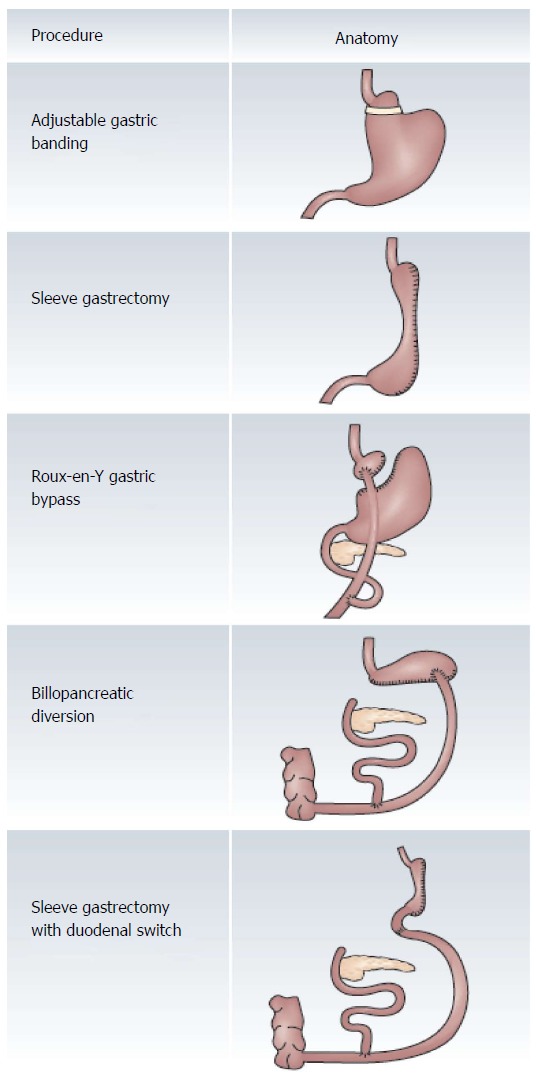
Comparison of bariatric surgical procedures. The major restrictive bariatric procedures depicted at the top of the figure include the adjustable gastric banding and sleeve gastrectomy. The restrictive and malabsorptive procedures are depicted in the bottom of the figure. There are elements of both food restriction as well as malabsorption in patients who have undergone either Roux-en-Y gastric bypass or sleeve gastrectomy with duodenal switch. The major malabsorptive bariatric procedure is the biliopancreatic diversion. Reprinted by permission from Ref [34].
The initial versions of AGB were introduced in the 1980s and then further refined to insertion with a laparoscopic technique in 1993. The advantage of AGB is that it does not involve reduction or stapling of any portion of the stomach, and it does not involve alteration of the anatomy with the creation of a bypass of segments of the small intestine. It is a relatively safe procedure with low morbidity and mortality, and offers patients the potential for minimally invasive adjustments of the band or even a reversal of the procedure. Although the efficacy is not as dramatic as other bariatric surgical procedures, the majority of patients experience greater than 40% of excess weight loss (EWL) within one year[24,25]. The mechanisms associated with weight loss in AGB are not well understood. Weight loss was initially thought to be related to a restrictive mechanism; however, several studies indicated that appetite reduction plays an important role[26,27]. The reasons for this suppression of appetite remain unclear.
SG is gaining popularity worldwide and is becoming one of the most commonly performed bariatric surgical procedures. The gastric fundus and body are surgically excised, leaving a narrow, tubular stomach along the lesser curvature of the stomach. It allows for an immediate restriction of caloric intake without placement of a foreign body, or the need for adjustments. Although SG is irreversible and has a relatively increased operative risk compared with other bariatric surgeries, the procedure is often less time consuming and requires less time under general anesthesia. The long-term data on the efficacy and durability of this procedure is lacking. Patients experience 65% of EWL within one year and maintain approximately 50% EWL within 6 years[28-30]. Similarly to AGB, weight loss after SG was first theorized to be due to a restrictive mechanism; however, recent studies have indicated that hormonal changes play a critical role[31-33].
RYGB continues to be the most commonly performed bariatric surgery performed worldwide and comprises nearly 50% of all procedures performed. Despite its popularity, there is a recent downward trend in the number of RYGB procedures being performed annually worldwide[23], possibly due to the increasing favor gained by SG. In this procedure, a small gastric pouch is created and directly connected to the jejunum, thus bypassing a large portion of the stomach and duodenum. Three channels are formed: the continuous digestive tract (termed the Roux limb), the biliopancreatic limb, and the common channel (between the jejunojejunostomy and the ileocecal valve). Patients with a short common channel (< 120 cm from the ileocecal valve) are at greater risk of developing severe malabsorption than those with a longer common channel[34]. RYGB effectively reduces the gastric reservoir to provide a restrictive component for weight loss, and also creates a malabsorptive component by excluding a variable portion of the duodenum and jejunum to inhibit fat absorption[35]. Hormonal effects may also provide a significant contribution to the efficacy of RYGB related weight loss[36]. In an examination of the potential benefit of probiotics, Woodard et al[37] randomized 44 individuals after Roux-en-Y gastric bypass to receive either placebo control or 2.4 billion Lactobacillus species daily. At 6 mo, there were no differences in weight loss between these two groups. There was reduced breath hydrogen levels in subjects who received daily Lactobacillus, supporting suppression of small intestinal bacterial overgrowth. The importance of gastric bypass surgery in improving patients’ quality of life was supported by improvements in gastrointestinal quality of life surveys in both of the groups. Scopinaro et al[38] initially developed biliopancreatic diversion (BPD) in the mid-1970s. This procedure involves a distal gastrectomy with closure of the duodenal stump. The jejunum is divided and the distal limb (termed the roux limb) is anastomosed to the proximal stomach, while the proximal limb (termed the biliopancreatic limb) is anastomosed to the ileum. Initial versions of this procedure had high morbidity with patients often experiencing dumping syndrome. To resolve this problem, a variation of the procedure, termed biliopancreatic diversion with duodenal switch (BPD/DS), was developed to preserve the pylorus and control gastric emptying. To help maintain the restrictive component of BPD/DS, a partial gastrectomy of 70%-80% of the greater curve of the stomach is performed in a gastric sleeve configuration[39].
Vertical banded gastroplasty (VBG) is a procedure that was developed in the 1970s and 1980s by surgeons who were attempting to develop a bariatric surgical procedure without significant morbidity. Designed to be a purely restrictive procedure, the gastric anatomy is altered to restrict the daily caloric intake and cause early satiety. The fundus of the stomach is separated with a stapled partition, creating a vertical pouch along the proximal lesser curvature. A narrow band of polypropylene mesh is used to reinforce the outlet of the pouch. This procedure is not technically challenging for most surgeons and has less morbidity than gastric bypass. Despite the excellent short-term outcomes with EWL of 60% or more at one year, patients often do not maintain this level of weight loss and have only 40% of EWL ten years after their surgery. The failure to maintain long-term weight loss is often attributed to dehiscence of the staple line, allowing food to re-enter the main body of the stomach and thus negating the effects of surgical restriction[19]. Because of the aforementioned reasons, this procedure has largely fallen out of favor and accounted for only 0.7% of bariatric procedures worldwide in 2011[23].
GASTROINTESTINAL PHYSIOLOGICAL BASIS FOR WEIGHT LOSS PROCEDURES
Weight loss observed in patients with malabsorptive and/or restrictive bariatric procedures was initially believed to be the result of readily apparent mechanisms. The procedures that have a restrictive component (AGB, VBG, SG, and RYGB) were designed to drastically decrease the size of the stomach. According to this theory, patients experience early satiety with smaller meal portions due to the reduced gastric capacity. If restriction of the gastric capacity was the only mechanism involved in weight loss after bariatric surgery, the body would likely compensate by increasing the intake of calorie-dense food and meal frequency. Paradoxically, after RYGB and vertical gastroplasty, patients were noted to have lower calorie food preferences and consumed significantly smaller meal portions[40,41]. Individuals with VBG do not experience the same degree of effects as they tend to consume a slightly higher proportion of dietary fat and calorie-dense foods when compared to patients after RYGB[42]. There is an abundance of data showing that RYGB causes more significant amounts of weight loss and is more effective at reducing appetite than VBG[13,43-46]. These findings are suggestive of an alternative, underlying mechanism which contributes to weight loss, rather than merely being induced by simple restriction of the gastric reservoir.
Evidence has been mounting in support of these less well defined physiological changes that, in addition to causing weight loss, also help improve co-morbidities such as type 2 diabetes mellitus. In a large meta-analysis including 22094 patients after bariatric surgery, Buchwald et al[43] found that 1417 of 1846 patients had complete resolution of type 2 diabetes mellitus. Further categorization by type of bariatric surgery shows drastic differences in efficacy. Of those with AGB and VBG, 47.8% and 68.2% had resolution of type 2 diabetes mellitus respectively, contrasted with 83.8% of patients with RYGB, and 97.9% with BPD or BPD/DS. A plausible theory to explain this observation is that RYGB, BPD, and BPD/DS affect glucose homeostasis by a weight loss-independent mechanism.
Time course studies have further supported the likelihood of physiological and hormonal changes contributing to the efficacy of bariatric surgery. Wickremesekera et al[47] assessed 71 obese patients undergoing RYGB who were categorized into three groups: diabetics, impaired glucose tolerance, and normal glucose tolerance. All three groups had insulin resistance prior to surgery (diabetic patients had greatest insulin resistance), and they all showed improvement of insulin resistance within six days of RYGB. Of the 31 diabetic patients included in the study, only three individuals required medications for treatment of diabetes mellitus at the time of their discharge. Given the lack of any appreciable weight loss six days after RYGB, this finding is consistent with a humoral mechanism for control of hyperglycemia. Similarly, Tsoli et al[48] studied 12 patients undergoing BPD. All of the patients discontinued their anti-diabetic medications postoperatively, and at one month, only one patient still had diabetes. To aid our consideration of the potential mechanisms to explain these clinical findings, the proposed physiological roles of regulatory peptide hormones in the gastrointestinal tract are summarized in Table 1.
Table 1.
Proposed physiological roles of gastrointestinal peptide hormones
| Hormone | Localization | Proposed physiological roles |
| Peptide YY | Ileum and colon | Inhibits gastric emptying/intestinal transit; possible blockade of hunger |
| Glucagon-like | Ileum and colon | Delays gastric emptying; |
| Peptide 1 | Incretin: Augments insulin secretion | |
| Glucose-dependent insulinotropic peptide | Duodenum and proximal jejunum | Incretin: Augments insulin secretion |
| Oxyntomodulin | Ileum and colon | Blockade of hunger; inhibits gastric secretion and emptying |
| Ghrelin | Gastric fundus | Appetite stimulant; increases gastric motility |
| Pancreatic polypeptide | Pancreas | Suppresses food intake; inhibits gastric emptying |
| Cholecystokinin | Duodenum and proximal jejunum | Suppresses food intake; initiates satiety; inhibits gastric emptying |
Two major hypotheses have been proposed to explain the immediate beneficial effects on type 2 diabetes mellitus seen in patients after duodeno-jejunal bypass (as performed with RYGB, BPD, and BPD/DS) in humans. The “foregut hypothesis” suggests that bypass of the proximal intestines exerts an anti-diabetic effect by disallowing calories from being exposed to the foregut, and thus, preventing release of a diabetogenic, hormonal mediator. An alternative hypothesis is termed the “hindgut hypothesis.” This theory proposes that an increased delivery of calories and nutrients to the distal jejunum and ileum increases the secretion of enteroendocrine hormones, including peptide YY and glucagon-like peptide-1 (GLP-1)[36]. Although the proper mechanism(s) remain unknown, an animal model created to study the differences between these theories supports the foregut hypothesis. This study showed that duodeno-jejunal bypass directly ameliorates type 2 diabetes mellitus independently of food intake, weight, malabsorption, or nutrient delivery to the hindgut. Therefore, unknown or undiscovered factors from the proximal portions of the bowel may contribute to the pathophysiology of type 2 diabetes mellitus[49]. The proposed effects of bariatric surgery on peptide hormones in the gastrointestinal tract are summarized in Table 2.
Table 2.
Gastrointestinal peptides hormones after bariatric surgery
| Hormone | Bariatric surgery | Blood hormone levels |
| Peptide YY | RYGB | Increased postprandially |
| Glucagon-like peptide 1 | RYGB | Increased postprandially |
| Glucose-dependent insulinotropic peptide | RYGB and AGB | Increased postprandially |
| Oxyntomodulin | RYGB | Increased postprandially |
| Ghrelin | RYGB and SG | Low Fasting and Postprandially |
| Pancreatic polypeptide | RYGB | Decreased |
| Cholecystokinin | VBG | Increased |
RYGB: Roux Y gastric bypass surgery; AGB: Adjustable gastric band; SG: Vertical sleeve gastrectomy; VBG: Vertical banded gastroplasty.
Peptide YY
The hypothalamus regulates food intake and satiety with neuropeptides. Peptide YY is a member of the neuropeptide Y family of biologically active peptides and is an important hormone in gut-brain communication[50]. Peptide YY is almost exclusively expressed in the gastrointestinal tract and is primarily secreted from mucosal enteroendocrine L-cells[51]. As shown by our group, Peptide YY is mainly concentrated in the ileum and colon, with the highest concentrations found in the distal colon[52]. Exercise and food intake stimulate the release of Peptide YY causing levels to peak within two hours and remain elevated for several hours[53]. Peptide YY interacts with Y receptors and has an inhibitory effect on gastric emptying and intestinal transit. Expression of Peptide YY has been demonstrated in the hypothalamus and pituitary tissues of human brains, which may suggest a role for Peptide YY as a neurotransmitter involved in the regulation of appetite and energy expenditure[54].
Lower fasting plasma Peptide YY concentrations have been exhibited in obese individuals when compared to their lean counterparts[55,56]. After VBG, patients have continually increasing levels of basal Peptide YY concentrations at 6 and 12 mo that correlate with their weight loss and approach the control levels. However, these patients do not have significant postprandial spikes one hour after meals as would be expected with a normal physiological response. This implies that the cause of increases in the basal Peptide YY concentrations may be related to their weight loss rather than the surgical procedure. Although basal Peptide YY concentrations are highest in lean controls, there is no statistical difference compared to patients with RYGB. In response to a meal, RYGB patients have an exaggerated spike in Peptide YY levels. Postprandial Peptide YY concentrations start to rise almost immediately and have been demonstrated to increase as early as two days after RYGB[57] and one week after BPD[58]. At 90 min after a meal, they have approximately two to three fold higher concentrations compared with lean and matched controls[59].
GLP-1
Incretins are gastrointestinal hormones that augment insulin secretion from the beta cells of the islets of Langerhan in the pancreas, even prior to elevations in blood glucose. There are currently two hormones that are classified as incretins: glucagon-like peptide or GLP-1 and glucose-dependent insulinotropic polypeptide (GIP). Similar to Peptide YY, GLP-1 is produced by enteroendocrine L-cells primarily located in the distal ileum and colon[60]. There is biphasic secretion of GLP-1 from the gut in response to food intake. The early phase occurs within 10-15 min after ingestion of food and the late phase peaks at approximately 30-60 min[60]. GLP-1 receptors have been identified in a wide range of tissues including the lung, heart, kidney, stomach, intestine, pancreatic islets, and the peripheral and central nervous systems[61]. Similar to Peptide YY, GLP-1 has been implicated in the regulation of food intake and energy homeostasis. GLP-1 delays gastric emptying and acts as a physiological regulator of food intake and appetite, increases satiety, reduces hunger, and plays a role in glucose homeostasis[62-65]. Although there are GLP-1 receptors in the intestines and in numerous areas of the brain, the exact roles of this peptide have not yet been delineated. Several studies have supported the theory that GLP-1 exerts its effects on delaying gastric emptying through the vagus nerve[66-68]. However, animal studies have supported the notion that the effects of GLP-1 may not be solely dependent on vagal afferent signaling, but rather may also involve receptors within the central nervous system[60].
Although AGB also induces satiety with optimal adjustment[26], it has not been shown to be associated with the exaggerated postprandial hormonal responses seen after RYGB[69]. As seen with Peptide YY, patients have an increased and exaggerated postprandial GLP-1 response after RYGB when compared to lean and obese controls. These hormonal effects, however, were not observed in patients that have lost an equivalent amount of weight from AGB[70]. Elevations of both GLP-1 and peptide YY have been associated with an attenuated appetite after RYGB. Subsequent inhibition of the release of these hormones with octreotide (a somatostatin analogue) causes increases in food intake in patients after RYGB[57]. This finding suggests that these gut hormones play a crucial role in the reduced food intake after RYGB, but not after AGB. The enhanced hormone level response is sustained two years after surgery and may help to explain the maintained weight loss seen with RYGB[71].
GIP
GIP is synthesized and secreted from intestinal K-cells, a majority of which are located in the duodenum and proximal jejunum[72]. It is secreted in response to ingestion of nutrients, especially glucose and fat, and is significantly higher after ingestion of large meals compared to small meals[73]. The rate of GIP release is related directly to nutrient absorption, rather than the mere presence of intralumenal food and nutrients. Therefore, malabsorption and decreased nutrient absorption reduces the secretion of GIP[61]. In the pancreas, GIP inhibits β-cell apoptosis and stimulates proliferation of β-cells and glucose-dependent insulin secretion[74]. Although one of the main functions of GIP is its effect on the pancreas, GIP receptors have been identified in the stomach, small intestine, heart, lung and brain[72].
Patients with type 2 diabetes mellitus have a severely reduced incretin effect, which is likely multi-factorial in origin. Despite normal or even increased basal and postprandial GIP concentrations[75], its insulinotropic effect is greatly diminished[76,77]. GIP has a sharp postprandial trajectory after RYGB compared to matched controls with caloric restriction. The increment in GIP from baseline to its peak levels were larger and occur much more swiftly in patients after RYGB than with caloric restriction[78]. Despite the sharp increases seen after RYGB, comparisons with AGB reveal even higher concentrations of postprandial GIP after AGB than RYGB[69]. One study compared patients with AGB and SG to patients with RYGB and BPD/DS (defined in that study as restrictive procedures vs malabsorptive procedures, respectively). Although patients with malabsorptive procedures experienced increased concentrations of GIP, it was not statistically significant. Those with restrictive procedures had much larger longitudinal increases at one month and three months after surgery[79]. These findings may be due to bypass of the K cells in the duodenum and proximal jejunum with procedures such as RYGB and BPD/DS[79].
Oxyntomodulin
Oxyntomodulin is another product of the L-cells found in the distal portions of the gastrointestinal tract and is secreted in response to food ingestion[80]. It binds to the same receptors as GLP-1, but with lower affinity. Oxyntomodulin is an anorectic hormone. After a meal, its circulation increases in proportion to the caloric intake and peaks within 30 min, remaining elevated for several hours[53]. It can inhibit gastric acid production and pancreatic secretion, and it delays gastric emptying[81]. Administration of oxyntomodulin causes reductions in food intake in lean and obese individuals and promotes a reduction in body weight[82,83]. The changes in oxyntomodulin concentrations correlate with the changes seen in GLP-1 and peptide YY, which makes it difficult to distinguish its effects from those of other peptides. Various studies have supported the possibility that these three hormones work in synergy and act as a powerful hormonal triad contributing to post-surgical weight loss[80]. After RYGB, the reported results are similar to that reported with GLP-1 and Peptide YY. Patients experience an exaggerated rise in oxyntomodulin levels, more than two-fold higher than controls, in response to an oral glucose load[84].
Ghrelin
Ghrelin is an orexigenic (appetite stimulant) hormone that is secreted from the X/A-like cells within the gastric oxyntic cells[85]. It appears to increase gastric motility, stimulates the hypothalamo-pituitary-adrenal axis, and enhances cardiac contractility[86]. Ghrelin is principally secreted from X/A-like cells within the fundus of the stomach, but may be found in smaller amounts in the duodenum, jejunum, and ileum[80]. Ghrelin also functions as a neurotransmitter and is expressed within the arcuate nucleus of the hypothalamus and periventricular regions[87].
In humans, ghrelin demonstrates a diurnal rhythm by rising throughout the day to a zenith at 0100, but then falling overnight to a nadir at 0900. Secretion of ghrelin is stimulated by fasting, by cholecystokinin (CCK), and by gastrin, and is inhibited by food ingestion, by somatostatin, and by growth hormone[80]. There is a clear rise in preprandial ghrelin concentrations followed by a postprandial decline[88]. Patients with anorexia nervosa have high fasting plasma ghrelin concentrations with normalization of levels after weight gain[89]. Similarly, diet-induced weight loss also leads to increases in plasma ghrelin concentrations[90]. Although ghrelin levels decrease postprandially in normal-weight subjects, obese individuals demonstrate a much smaller drop in ghrelin levels after meals[91,92]. Circulating ghrelin concentrations have been found to be negatively correlated with BMI[93]. These findings suggested that ghrelin may be a strong contributor in the regulation of and pathophysiology of obesity.
The role of ghrelin post-bariatric surgery is complex and difficult to assess. The findings are dependent on several variables including the different types of bariatric surgeries, various levels of weight loss, and different intervals after surgery. One of the first reports of decreased ghrelin concentrations after RYGB was by Cummings et al[90] and similar results have since been replicated by several other studies[94-96]. They compared patients after RYGB to lean volunteers and matched obese patients that lost weight through diet. RYGB resulted in lower fasting, postprandial, and interprandial plasma ghrelin levels compared with obese and lean patients. The decline in ghrelin is almost immediate after RYGB with levels demonstrated to be significantly lower as early as one day after surgery. This decline is maintained for at least two years[97].
Similarly, ghrelin plasma levels are noted to decrease immediately with SG as early as one day after surgery, but not with AGB. The ghrelin levels remain low for at least six months after SG[98], but in patients with AGB, levels may actually increase after the surgery[26,98,99]. Plasma concentrations of ghrelin are also increased after BPD[100]. However, when BPD/DS is performed, which includes resection of the gastric fundus, a decrease in ghrelin concentration is observed[101]. A series of studies have demonstrated that bariatric procedures preserving the gastric fundus do not result in decreases in plasma ghrelin, whereas procedures that resect the gastric fundus lead to lower fasting plasma ghrelin concentrations[59,96,102-104]. The exact role and overall effect of ghrelin on weight loss and obesity remains unclear. Despite findings that procedures causing decreases in levels of ghrelin (RYGB and SG) result in better weight loss, effective weight loss is still demonstrated in procedures in which ghrelin increases[80].
Pancreatic polypeptide
There is mounting evidence delineating the role of the vagus nerve in the regulation of the physiology of ghrelin and pancreatic polypeptide (PP). The vagus nerve innervates much of the gastrointestinal tract and helps mediate several hormonal pathways. In humans, blockade of vagal impulses with the use of a non-specific cholinergic antagonist reduces ghrelin secretion[105,106]. Furthermore, administration of exogenous ghrelin to patients with truncal vagotomy after lower esophageal or gastric surgery does not stimulate increased food intake[107]. This suggests that the vagus nerve is involved in the physiological regulation of ghrelin.
PP has structural homology to peptide YY. It is produced in endocrine type F cells in the pancreatic islets and is released into circulation after food ingestion and exercise. PP serves as a regulatory hormone by inhibiting pancreatic exocrine secretion, stimulating glucocorticoid secretion, and modulating gastric acid and gastrointestinal motility. It is secreted at a low basal rate in fasting states and is markedly increased during all phases of digestion[108]. Intact vagal cholinergic reflex circuits are a major regulator of PP secretion. The release of PP is diminished by atropine (a cholinergic antagonist) in normal patients and is completely abolished in patients with a vagotomy[109,110].
PP may be intricately involved in the regulation of food ingestion. Administration of exogenous PP has demonstrated suppression of food intake and inhibition of gastric emptying. After its release, PP has been shown in animal models to have a negative feedback mechanism by acting on receptors in the dorsal vagal complex. Thus, PP has a direct effect on the regulation of vagal input to the stomach and pancreas[108]. Since PP concentrations decrease with inhibition of the vagal cholinergic reflex circuits, however, this suggests that the effect of PP on food intake may be indirect and also regulated through the vagus nerve. After RYGB, PP levels have been shown to decrease as early as one day post-operatively, with subsequent normalization to pre-operative levels one month after surgery. This observation may, in part, be due to vagal dysfunction immediately after surgery with an ensuing return to normal function[111]. A comparison of RYGB with vagal nerve preservation to RYGB with vagal nerve dissection does not reveal any significant differences in the overall long term weight loss after three years[112]. A small study identified higher PP levels after RYGB than in patients with SG, but this finding did not reach statistical significance[113].
CCK
CCK is produced in the I-cells, which are mainly located in the duodenum and proximal jejunum, with small numbers of cells present throughout the rest of the small intestine. It is secreted after food ingestion, especially after meals with high contents of fat and protein[114]. The main functions of CCK are stimulation of pancreatic exocrine secretion, gall bladder contraction, inhibition of gastric emptying, and decreasing food intake[115]. Serum concentrations of CCK rise within 15 min after meals, but have a short plasma half-life of only a few minutes. The anorectic effects of CCK are mediated by its receptors within the central nervous system, which may allow it to operate as a stimulus for satiety[116].
A small preload meal causes a normal physiological response of CCK release with subsequent reduction in food intake and decreased sensations of hunger[117,118]. Parenteral administration of CCK has similar effects by increasing fullness and reducing hunger and food intake, irrespective of whether the patient is lean or obese[119,120]. These findings suggest the possibility that obese patients could have a defect in the secretion of CCK. However, this theory has been refuted by studies showing similar postprandial plasma CCK levels in lean and obese individuals[121,122]. Patients undergoing RYGB do not have statistically significant changes in the post-operative CCK levels[123,124]. After VBG, there were no significant changes in the CCK concentrations in response to a meal on consecutive days before and after surgery[122]. However, when comparing CCK levels of morbidly obese patients three months after VBG to that of lean controls, the peak concentration was significantly higher and required a shortened time period to reach its zenith[125]. These data suggest a possible role of CCK in stimulating satiety and encouraging decreased food intake to help achieve greater weight loss after VBG.
Gut microbiota
The physiology and pathophysiology of the gut microbiota in obesity and in treatment of obesity warrants extensive investigation. There have been preliminary studies examining the role of the gut microbiota in outcomes after bariatric surgery. This is of course a complex area of clinical research due to differences in concentrations of aerobic and anaerobic bacteria in different regions of the gut and due to differences in the composition of bacteria present in different individuals.
In a study from the Mayo Clinic, Scottsdale[126], nine individuals (3 normal weight, 3 morbidly obese, and 3 after gastric bypass surgery) had extensive studies of their microbial communities. There were 6 major bacterial divisions identified. Firmicutes were dominant in individuals with normal weight and morbid obesity, but were decreased after gastric bypass. Morbidly obese individuals had increased numbers of hydrogen-producing Prevotellaceae and hydrogen-oxidizing Archaea. The authors hypothesized that interspecies hydrogen transfer is an important mechanism for increasing energy update by the human colon in morbidly obese individuals.
A second study from France examined fecal samples from 13 normal weight individuals and from 30 obese individuals (who had samples obtained before and after RYGB)[127]. The major findings included: (1) Bacteria in the Bacteroides/Prevotella group were lower in obese individals compared to normal weight individuals, but increased 3 mo after RYGB; (2) Escherichia coli had increased 3 mo after RYGB; (3) Lactic acid bacteria decreased by 3 mo after RYBG; and (4) Faecalibacterium prausnitzii was lower in individuals with diabetes mellitus.
The authors hypothesized that components of the gut microbiota adapt to a starvation-like process following RYGB.
A second French study[128] later reported studies of fecal samples and gene expression in white adipose tissue obtained from morbidly obese individuals before and after RYGB. The authors identified enrichment of gut microbiota after RYGB with 37% of increased bacteria being Proteobacteria. It was reported that there were increased associations between gut microbiota composition and gene expression in white adipose tissue after RYGB.
Taken together, it is clear that further work is needed to determine how the composition of the gut microbiota and changes in the gut microbiota after bariatric surgery can be used in decision making for the clinical care of morbidly obese individuals who are seeking interventional therapy to induce weight loss.
HORMONES SECRETED BY ADIPOSE TISSUE
Leptin
The hormone Leptin was originally described as important for suppression of food intake in mice. Human Leptin is a 167 amino acids protein. It is produced by adipocytes of mainly white adipose tissue as well as by brown adipose tissue[129]. Leptin is produced in small amounts by multiple organs. The major Leptin receptor is expressed in the hypothalamus, the site of regulation of body weight and the sense of hunger. It has been reported that blood levels of Leptin are paradoxically increased in obese individuals[129].
The potential influence of Leptin on outcomes after bariatric surgery is unclear. It has been reported[129] that Leptin administration compared to placebo (delivered by subcutaneous injections twice daily for 16 wk) in 27 women enrolled at least 18 mo after RYGB revealed that Leptin treatment had no effect on body weights. This result was disappointing since multiple groups[130-132] have reported marked postoperative decreases in blood Leptin levels after SG and RYGB. Further clinical research is therefore required to determine whether Leptin is involved in the weight loss process after these bariatric surgical procedures.
Adiponectin
The hormone Adiponectin is a 244 amino acids protein. Adiponectin is physiologically involved in the regulation of blood glucose levels and fatty acid breakdown or oxidation[133]. It is secreted by adipose tissue, and, interestingly, by the placenta during pregnancy. In adults, there is an inverse relationship between blood Adiponectin levels and body fat percentage. Adiponectin levels may be paradoxically decreased in obese individuals.
Increases in blood Adiponectin levels after bariatric surgery may be important in postoperative insulin sensitivity[133-135]. It has been reported that Adiponectin serum levels increase after RYGB, even in patients with preoperative body mass index as low as 22 kg/m2[133]. However, investigators in the Swedish Obese Subjects Study have suggested that baseline blood levels of Adiponectin do not predict the treatment benefit of bariatric surgery[136]. Therefore, it is not presently clear how blood levels of Adiponectin could be used to plan future minimally invasive therapy for medically-complicated obesity.
ENDOSCOPY AND THE POTENTIAL FUTURE OF BARIATRIC PROCEDURES
Although bariatric surgery is an effective therapy in the treatment of morbid obesity and has become a cornerstone, it has risks and limitations. Bariatric surgery is the most effective weight loss treatment; however, it is associated with morbidity, mortality, and has a considerable cost. While laparoscopic procedures have reduced the rate of complications, additional advances are actively being sought to further reduce the overall risk and cost. An ideal operation would provide a safe, durable, and reversible procedure with low peri-operative complications and recovery time, without the need for an incision through the abdominal wall. One obvious candidate to help achieve these goals would include endoscopic approaches with interventions performed entirely through the gastrointestinal tract using flexible endoscopes. Not only is this approach becoming more feasible with technological advancements, but it also introduces new categories of therapy.
There are several other types of endoscopic techniques that mimic the anatomic features of bariatric surgery. Two broad categories of endoscopic weight loss modalities include restrictive and malabsorptive therapies. Although these categories are overly simplistic and do not explain the hormonal changes that accompany each type of procedure, they have remained prevalent within the literature. Restrictive procedures attempt to decrease the available volume of the stomach with suturing or stapling devices. Malabsorptive procedures prevent food from contacting the duodenum and jejunum and mixing with biliary secretions until more distal segments of the small bowel. Examples of restrictive procedures include intragastric balloon therapy, endolumenal vertical gastroplasty, transoral gastroplasty, and transoral endoscopic restrictive implant systems. Malabsorptive procedures include the duodeno-jejunal bypass sleeve (DJBS) system, which has certain adaptations that may act as a combination of both restrictive and malabsorptive procedures, and mimics many of the hormonal changes seen in bariatric surgery. These endoscopic methods are summarized in Table 3.
Table 3.
Endoscopic techniques for treatment of obesity
| Technique | Proposed mechanisms of action |
| Intragastric balloon | Increases gastric distension and satiety and delays gastric emptying |
| Endolumenal vertical gastroplasty | Restriction of food intake and Induce early satiety |
| Transoral gastroplasty | Restriction of food intake |
| Trans-oral endoscopic restrictive Implant system | Restriction of food intake |
| Duodeno-jejunal bypass sleeve | Reduced intestinal digestion and absorption And delayed gastric emptying |
| Gastroduodenojejunal bypass sleeve | Prevent absorption of nutrients |
| Aspiration therapy | Reduced presence of available nutrients |
Intragastric balloon
The Garren-Edwards bubble (GEB) was the first intragastric balloon widely used in the United States during the 1980s. It was first introduced in 1982 and approved by the Food and Drug Administration in 1985. The GEB was endoscopically deployed in the stomach and expanded using an air insufflation catheter to fill the polyurethane cylinder, which was freely floating in the stomach. It was designed to decrease caloric intake and induce weight loss by increasing gastric distension and satiety and delay gastric emptying[137]. The GEB was less efficacious than bariatric surgery[138] and did not induce statistically significant weight loss in controlled trials. Due to a lack of efficacy and a relatively high cost of this device, it fell out of favor[139,140]. Furthermore, there were several complications including gastric erosions and ulcers, Mallory-Weis tears, small bowel obstruction, and esophageal laceration. Accordingly, the GEB was withdrawn from the market in 1988.
The BioEnterics intragastric balloon (BIB) was first introduced in 1999. It consists of an adjustable silicone elastomer balloon with a spherical shape. The potential complications are similar to that of GEB, but occur less frequently[137]. The Food and Drug Administration has not given approval for BIB use in the United States, but it has been studied extensively worldwide and is approved in many regions including Europe, several South American countries, Australia, and Canada. The prospects of long-term therapy with BIB remain limited as it is usually left in place for only six months. Retrospective data revealed a low complication rate of approximately 2.8% with EWL of 33.9% and improvement in pre-operative co-morbidities in 44.8% of patients[141]. Similar results have been confirmed by several studies validating BIB as an effective and safe short-term treatment of obesity[142,143]. A small prospective study compared weight loss achieved with BIB and laparoscopic AGB. Since BIB is only approved for six month intervals, the device was removed at six months and replaced with an identical, new BIB during the same procedure. Similar results were noted in the two groups after six months; however, at 12 mo, patients with BIB had greater weight loss than laparoscopic AGB. After 18 mo (six months after the BIB had been removed), overall weight loss results were similar as the BIB group had regained some of the lost weight[144].
The long-term outcomes are not as favorable as short-term outcomes with only one quarter of patients maintaining weight loss 2.5 years after intragastric balloon[145]. Thus, some have proposed intragastric balloons as an effective bridge to bariatric surgery. It helps induce pre-operative weight loss to decrease the technical difficulties associated with bariatric surgeries in morbidly obese individuals, and decreases the risk of general anesthesia[146,147]. Furthermore, while it may not change the total weight loss after surgery, pre-operative treatment with intragastric balloon has been shown to reduce intraoperative complications and risk of conversion to open surgery in morbidly obese patients undergoing laparoscopic AGB[148].
Because of this reported unfavorable long-term outcome, other authors have suggested treatment with serial balloons[149]. In a six year study, 83 individuals with BMI > 40 underwent intragastric balloon placement. After balloon removal, a second balloon was placed in all patients after they had regained ≥ 50% of the weight loss achieved with the initial balloon. A third balloon was placed in 22% of the patients and 1 patient underwent placement of a fourth balloon. At 76 mo of follow up, the mean BMI was 37.6 kg/m2, demonstrating a modest weight loss. Due to the placement of multiple intragastric balloons, this strategy is less likely to be cost effective.
To address some of the limitations experienced with BIB, the spatz adjustable balloon system (SABS) was introduced. The SABS is composed of a spherical silicone balloon mounted on a catheter placed on one surface. The catheter has two perpendicular loops: one is an easily visible white inflation valve, and the other has a metal chain, which prevents collapse of the balloon and maintains its 7 cm diameter within the gastric lumen to prevent migration beyond the stomach. The device may be removed with a standard polypectomy snare by ensnaring a blue clasp at the end of the larger, metal chain containing loop. A pilot study was completed with 18 patients and demonstrated 26.4% EWL at 24 wk and 48.8% EWL at 52 wk. Six successful downward adjustments were made to help alleviate intolerances including abdominal pain, nausea, and vomiting. Upward adjustments were made in ten patients due to weight loss plateau resulting in additional weight loss. Several adverse events were experienced causing premature removal of the device in seven patients. This also prompted several improvements in the design of the device to help prevent complications[150].
Despite improvements in the design of the SABS, studies have shown a relatively high rate of adverse events, which remains one of its biggest limitations. Espinet-Coll et al[151] experienced adverse events in 15% of the devices used in a study involving 107 patients. Four devices developed a leak and the other 12 developed intolerances requiring early explantation. The intolerances cited include anchor migration in seven patients with two developing duodenal ulcers (one of which was surgically repaired), one gastric fundus ulcer, and four clinical intolerances. One case report was published with a patient experiencing migration of the SABS to the jejunum causing obstruction and the patient presenting with abdominal pain, nausea, and vomiting 8 mo after implantation of the device. Exploratory laparotomy was performed with removal of the SABS through a small enterotomy[152]. In a direct comparison of SABS and BIB in a case control study, both devices were well tolerated with similar weight loss after 12 mo. However, the SABS group experienced complications in 17.5% compared to only 2.5% of those with BIB. Device migration was noted in 10% of the SABS group and 15% of patients had to have it removed prematurely[153]. The SABS could potentially be used in the same manner as some studies have demonstrated with BIB. The device may be removed one year after the initial procedure with subsequent placement of a new SABS device. This type of approach could allow placement for up to two years[153]. Although preliminary studies are encouraging, the lack of long-term efficacy and concerns about the overall safety necessitate additional research and possibly further improvements in the design of the device prior to its widespread use.
Another potential alternative system has been developed by ReShape Medical. The ReShape Duo has been used in Europe for over 2 years. This devise consists of two connected but separate balloons with a total volume of 900 mL. In one small study, three sites randomized 21 patients to a dual balloon system for 24 wk, while 9 patients treated with diet and exercise alone served as the control group[154]. These 30 patients included 26 women and 4 men with an age range of 26 to 59 years-old. At 24 wk, the mean excess weight lost averaged 32% in the dual balloon group and 18% in the control group. Twenty four weeks later, the dual balloon group had maintained 64% of their weight loss. In the dual balloon group, the treatment-related complications included severe nausea in 4 out of 21 patients (19%), gastritis in 1 patient, and transient hypoxia in 1 patient during removal of the balloon devise. No long term study results are yet available for review.
Endolumenal vertical gastroplasty
Initial studies of endolumenal treatments for gastroesophageal reflux disease (GERD) introduced endoscopic suturing devices developed to create tissue placation using adjacent tissue folds[155]. This system was marketed as the Bard EndoCinch Suturing System and while it has good short-term outcomes in the treatment of GERD, the long-term results show diminishing efficacy over time[156,157]. The possibility of its therapeutic potential has been extended to treatment of obesity. Endolumenal vertical gastroplasty (EVG) was completed by using a continuous suture pattern to create a narrow, tubular shaped stomach to provide restriction of food intake and to cause early satiety. The underlying hormonal effects of this procedure are unknown as they have not yet been studied. The average procedure time for EVG is approximately 45 min with minimal complications and no serious adverse events. EVG provided outcomes comparable to bariatric surgery with 58.1% EWL at one year[158].
A separate device was developed by Bard and named the restore suturing system. This is a single-intubation, multi-stitch device placed at the end of a standard endoscope. An initial pilot study revealed the feasibility and safety of this procedure, but the overall procedure time took an average of 125 min[159]. A one year follow-up revealed only modest decreases in weight, BMI, and waist circumference with a mean of 27.7% EWL. Furthermore, endoscopy at one year found a partial or complete release of plications in 72% of patients[160]. Despite adequate short-term outcomes with EVG, there is a lack of data showing the long-term durability. Given the results noted with the Bard EndoCinch Suturing System in the treatment of gastroesophageal reflux and the limited data about the findings with the restore suturing system, one might expect the efficacy to further decline in longer-term studies.
Transoral gastroplasty
An endolumenal procedure termed transoral gastroplasty (TOGA) was developed by Satiety Inc. and involves endoscopic stapling of the lesser curvature of the stomach to create a restrictive pouch. The pilot study for this device did not have any serious adverse events and had good results with 24.4% EWL at six months post-treatment. The study did note gaps in the original staple line in 62% of patients[161]. The TOGA system was subsequently improved by developing overlapping staple lines to avoid gaps from forming and a small follow-up study involving 11 patients was conducted to test the new advancements. This study confirmed the safety of the procedure and resulted in 46.0% EWL at six months[162].
Although there have been good short-term outcomes, there is limited data about the long-term effects. Outcomes measured at one year in a study with 53 patients revealed a total of 38.7% EWL. Complications were reported with one patient developing respiratory insufficiency requiring mechanical ventilation and another with asymptomatic pneumoperitoneum. Furthermore, 23 of the patients developed gaps in the staple line seen on endoscopy[163]. Another recent study involving a comparison of TOGA to RYGB and BPD revealed good outcomes for a select group of patients with TOGA after two years. Although RYGB and BPD had better overall results in the reduction of BMI, the TOGA patients with a lower initial BMI had higher reductions in their BMI than patients with RYGB and BPD[164]. At this time, the TOGA system is not used within the United States. Clinical trials did not reach set targets and research and development of the product was halted indefinitely due to a lack of approval from the United States Food and Drug Administration.
A variation of TOGA was recently described in a clinical study performed at the Mayo Clinic, Rochester by one of us (Gostout CJ) using the commercially available endoscopic suturing device, Overstitch. This novel procedure accomplishes endoscopic gastric volume reduction in a similar fashion to SG. It introduces a series of closely spaced, full-thickness, interrupted sutures through the gastric wall resulting in volume-reducing plications extending from the gastric antrum to the gastroesophageal junction. A pilot study with four patients demonstrated the technical feasibility of this endoscopic procedure without any intra-operative or serious post-operative adverse events[165].
Two additional intralumenal suturing systems have been recently reported. Espinos and associates have described a per-oral system that is designed to place transmural plications in the gastric fundus and distal gastric body using specialized suture anchors[166]. Their clinical study included 45 patients (76% females). A mean of 8.2 plications were placed in the fundus and a mean of 3.0 plications were placed in the distal gastric body. At 6 mo follow up, patients had lost a mean of 49.4% of their excess body weight with no mortality and no operative morbidity reported. This interesting report will require verification of the authors’ results in additional research centers and long-term follow up of weight loss to confirm the utility of this technique.
In a multicenter report, there has been a recent, partial description of a new transoral suturing device developed by SafeStitch Medical Inc, Miami, Florida, United States[167]. The authors describe four obese patients who underwent proximal gastric placement of gastroplasty sutures after mucosal excisions of gastric tissue. It was reported that at 2 year follow up, the patients had excess body weight lost that ranged from 0% to 68%. This study is quite limited in that it is a small clinical trial with highly variable results. In addition, the physiological basis for the placement of gastroplasty sutures and the mechanisms involved in weight loss are not well defined.
Despite the promising short-term results of TOGA and its variations, long-term results are not yet available. The durability and extent of weight loss remain in question and thus far, these procedures have not demonstrated clear advantages in efficacy over surgery. These procedures may be advantageous in a select subgroup of morbidly obese patients, but further studies are necessary to elucidate the long-term outcomes when compared to other surgical and non-surgical options.
Trans-oral endoscopic restrictive implant system
The trans-oral endoscopic restrictive implant system (TERIS) was developed by BaroSense Inc. and introduced as a new endoscopic therapy for the treatment of obesity[168]. This procedure involves placement of a restrictor with a 10 mm central channel for food passage at the gastric cardia, creating a restrictive pouch. This is considered a permanent implant, but may be removed or modified if necessary. A prospective, observational study was completed to evaluate the short-term safety and efficacy of TERIS. Of a total of 13 patients included in this study, three of them experienced serious adverse events with one developing gastric perforation requiring procedural reversal and laparoscopic treatment and two others developing pneumoperitoneum. The percentage of EWL was 22.2% after three months[169]. While the procedure is feasible and weight loss and improvement in quality of life measures are comparable to restrictive bariatric procedures, the safety profile remains a concern. Technical improvements and long-term data are necessary to make TERIS an effective option in the arsenal of treating obesity. Completion of multi-center feasibility studies would be important in order to evaluate adverse events relating to the procedure and device and the associated overall weight loss. At this time, it does not appear that clinical research studies utilizing TERIS will be proceeding.
DJBS
Developed as the EndoBarrier by GI Dynamics, Inc. (Lexington, Massachusetts, United States), the DJBS is an endoscopically implanted, removable, and impermeable fluoropolymer sleeve with a nitinol anchor. The device is deployed into the duodenal bulb under fluoroscopic guidance during endoscopy and extends 60 cm into the small bowel. It is intended to mimic a bypass procedure without the risks and effects associated with surgery. It does not allow food to contact the mucosa of the duodenum and upper portions of the jejunum. It also prevents food from mixing with biliary and pancreatic secretions until more distal segments of the jejunum. The device was initially studied in porcine models without significant adverse events[170], and was later successfully deployed in the first human subject[171]. An open-label, single-center, prospective study including 12 patients revealed good outcomes with 23.6% EWL after 12 wk. Two patients had explantation of the device after nine days secondary to poor placement and there were no severe device-related events reported[172]. In a larger, multicenter, randomized clinical trial involving 41 patients with 30 undergoing duodeno-jejunal bypass sleeve placement, there were no adverse events. The device could not be implanted successfully in 4 patients, but patients with the device experienced 19% EWL after 3 mo compared to 6.9% EWL in control patients[173].
Another variation of the duodeno-jejunal bypass sleeve has been tested using a restrictor orifice to add a restrictive component. This device, also endoscopically implanted into the duodenal bulb, has a 4 mm orifice to slow gastric emptying. The device was first tested in porcine models and after safety was established, was used in a pilot study including 10 patients. This open-label, single-center trial investigated the modified duodeno-jejunal bypass sleeve with restrictor orifice and found the results of duodenal exclusion and delayed gastric emptying to be additive. Patients had an average of 40% EWL after just 12 wk, which compared favorably to results with the unmodified duodeno-jejunal bypass sleeve after 12 wk. The gastric emptying rate was measured by scintigraphy and noted to be significantly reduced in all patients with the device. There were no clinically significant adverse events, but a majority of patients experienced abdominal pain, while others had nausea and vomiting. These symptoms lead to seven patients requiring balloon dilation of the restrictor orifice[174]. Currently, there are no available data for the long-term outcomes for this device.
The duodeno-jejunal bypass sleeve has also been tested in obese patients with type 2 diabetes mellitus. The study included 22 patients and was an open-label trial lasting 52 wk. Only 13 patients completed the entire duration of the study. Reasons for explantation included device migration in three patients, gastrointestinal bleeding in one patient, abdominal pain in two patients, and unrelated reasons in the remaining three patients. The duodeno-jejunal bypass sleeve improved the overall glycemic status of the 13 patients that completed the entire study and the reported EWL was 39.0%[175]. The duodeno-jejunal bypass sleeve device already has approval and is being used in several countries. A large, randomized, sham-controlled clinical trial is underway in the United States. The long term results of the duodeno-jejunal bypass sleeve remain in question as there is no long-term data. Furthermore, the device is only approved for a short duration of 12 mo and placement for longer intervals has not yet been tested.
Gastroduodeno-jejunal bypass sleeve
A novel device was developed by ValenTx Inc designed to mimic the restrictive and malabsorptive aspects of RYGB without the risks of bariatric surgery. An endolumenal, endoscopic gastroduodeno-jejunal bypass sleeve was proposed to induce short-term weight loss and improvements in co-morbidities. This sleeve is a 120 cm long fluoropolymer deployed through the pylorus with a toposcopic delivery technique with flow and pressure monitoring. Fluoroscopic guidance is used to ensure proper deployment through the duodenum and into the proximal jejunum. The device is then secured at the gastroesophageal junction using endoscopic and laparoscopic techniques. Explantation may be completed with an endoscopic retrieval method. The resulting gastroduodeno-jejunal bypass sleeve consequently prevents absorption of nutrients in the stomach, duodenum, and jejunum and delivers food directly from the esophagus to the small bowel.
An initial prospective, single-center trial included 24 patients. Two patients were excluded pre-procedurally and the device was successfully placed in 22 patients. The 12 wk study was completed by 17 of the patients, who had a reported 39.7% EWL. There were no serious adverse events experienced and the main reason for early explantation in five patients was pain with swallowing[176]. Further development of this device may eliminate laparoscopic visualization and allow the procedure to be performed entirely endoscopically. Although the early results are promising with weight loss results similar to those reported with RYGB and SG, further short-term and long-term data is needed to establish the clinical efficacy of the gastroduodeno-jejunal bypass sleeve in the treatment of morbid obesity.
Aspiration therapy
There is an endoscopic weight loss technique termed “Aspiration Therapy” which is not designed to mimic the physiological effects of bariatric surgery[177]. In this endoscopic technique, a specialized tube called as aspiration tube or A-Tube (Aspire Bariatrics, King of Prussia, Pennsylvania, United States) is placed percutaneously, leaving both an intragastric portion with holes to permit aspiration as well as a skin port. Ten to 14 d after A-Tube placement, individuals in this single center trial were instructed to aspirate their gastric contents 20 min after any breakfast, lunch or dinner that included more than 200 kcal. In this trial involving 11 subjects, ten individuals completed the first year of the study. These ten subjects lost a mean of 49% of their excess body weight at 1 year. Seven of these 10 subjects were able to maintain their weight loss at 2 years after enrollment in this study. This of course is a small, single center clinical study. More extensive validation will be required for assurance that the subjects are not simply being allowed to or encouraged to develop a potentially harmful eating disorder.
OTHER PROCEDURES
Other techniques have been proposed to induce weight loss by attempting to alter normal physiology. These techniques, as shown in Table 4, are summarized in the below section.
Table 4.
Miscellaneous techniques for treatment of obesity
| Technique | Proposed mechanisms of action |
| Electrical stimulation | Reduced gastric accommodation and |
| Delayed gastric emptying | |
| Intragastric botulinum toxin injections | Prolonged gastric emptying time and |
| Reduced maximal gastric capacity | |
| Natural orifice translumenal | Transluminal access to intra-abdominal |
| Endoscopic surgery | Structures |
Electrical stimulation
Several animal and human studies have been completed on the effects of electrical stimulation of the stomach and intestines as a mode of therapy for morbid obesity. Animal studies have shown promising results with electrical stimulation of the intestines, which causes gastric relaxation, delays gastric emptying, and accelerates intestinal transit normally slowed by the ileal brake[178-180]. In humans, gastric electrical stimulation was found to be a safe and feasible therapy for morbid obesity. Several trials showed significant amounts of weight loss due to reduced gastric accommodation, delayed gastric emptying, and increased intestinal transit[181-184]. Direct comparisons of these studies is difficult as several different approaches have been attempted including laparoscopic and endoscopic placement of electrodes. Additionally, the exact placement varies with some studies placing electrodes in the distal stomach and others in the duodenum. Although these results have been encouraging, many questions remain about this modality of therapy and its long-term results.
Another area of active research has been vagus nerve stimulation (VNS). As previously noted, the vagus nerve plays an important role in the hormonal regulation pathways involved with satiety and short-term regulation of food intake. Bodenlos et al[185] demonstrated that depressed patients experience a significant change in ratings of cravings for sweet foods with acute activation of a VNS device. Similarly, providers administering treatments with cervical VNS for adjunctive therapy of severe, treatment-resistant depression noted significant weight loss proportional to the initial BMI, with more severe obesity yielding greater amounts of weight loss[186]. While truncal vagotomy has also successfully been used in the treatment of obesity[187,188], its effects may not be maintained over time, possibly owing to the development of collateral innervations[188].
A new therapy termed VBLOC for Vagal BLOCking therapy was developed for the treatment of obesity using intermittent intra-abdominal vagal blocking with high-frequency electrical currents. During a laparoscopic procedure, electrodes are placed on the anterior and posterior vagal trunks near the gastro-esophageal junction. A neuroregulator is placed subcutaneously and an external controller is used to program the device. This procedure does not require any anatomical modification or tissue compression within the gastrointestinal tract. An open-label, multi-centered trial was conducted to assess the feasibility, safety, and efficacy of VNS. Patients had a mean EWL of 7.5%, 11.6%, and 14.2% at four weeks, 12 wk, and six months, respectively. There were no device-related serious adverse events and patients were noted to have decreased caloric intake, early satiety, reduced hunger, and decreased plasma PP levels[189].
The EMPOWER study was a randomized, prospective, double-blinded trial that included 294 patients from 15 centers. All patients had an implanted VBLOC and were randomized to treated or control groups for 12 mo. Device-related complications were observed in 3% of patients with nearly half due to preexisting conditions. After 12 mo, the EWL for the treated and untreated groups were 17% and 16%, respectively. There was no statistically significant weight loss between the two groups. However, the authors suggest that electrical safety checks of the equipment deliver a low charge for electrical impedance, safety, and diagnostic checks, which may have contributed to the weight loss observed in the control group[190]. Despite promising results seen in the initial studies, the most extensive study to date was unable to demonstrate a clear advantage with the use of VBLOC. Given the favorable safety profile and ease of placement of the device without alteration of the gastrointestinal anatomy, further studies are needed to determine its long-term efficacy and feasibility in the treatment of obesity.
Intragastric botulinum toxin injections
Botulinum toxin (BTX) acts to inhibit acetylcholine release at the neuromuscular junction and causes a local paralysis. This agent has been studied in the treatment of obesity with injections into the wall of the stomach to inhibit motility and delay gastric emptying. Initial experiments in animal models showed that injection of BTX into the gastric antrum caused a reduction in food intake and caused a reduction in body weight[191]. Initial human studies showed the overall safety of the procedure with no clinically significant adverse events. However, the overall outcomes were mixed. Some patients reported early satiety, but BTX injections did not provide a clear benefit for weight loss[192-194]. In double-blinded, randomized, controlled trials with BTX injections in the gastric antrum, the effects of gastric emptying were variable. Although some patients reported a reduced appetite, the amount of weight loss was not statistically significant[195,196].
A slight variation was attempted by Foschi et al[197] with intraparietal endoscopic administration of BTX into the gastric antrum and fundus. This double-blinded controlled study found a prolonged gastric emptying time and reduced maximal gastric capacity for liquids. Patients treated with BTX had significantly greater amounts of weight loss after eight weeks. Given the minimal adverse events noted in studies and the widespread availability, BTX has the potential to have a large role in the treatment of obesity. However, additional investigation into the sites of BTX injection and the optimum dosage are necessary. One major limitation with BTX is its relatively short duration of action, which is approximately three to six months. Furthermore, data regarding long-term outcomes is lacking and additional studies may be warranted.
NOTES
NOTES procedures provide the obvious advantage to patients of a surgical procedure without the externally visible incision and resultant scars. There are several advantages to performing traditional surgical procedures with endoscopic access through natural orifices, including reduced complications and duration of hospitalization and a faster recovery period. Although the endoscope has traditionally been used for treatments within the gastrointestinal lumen, recent ventures have explored translumenal access to intra-abdominal structures. Technological advancements in endoscopy combined with efforts to find less invasive, safer therapies in the field of bariatrics have cultivated endolumenal bariatric surgery.
The basic principles of NOTES began with the description of the first percutaneous endoscopic gastrostomy tube insertions[198]. Multiple novel experimental techniques were described in animal models as this new form of minimally invasive surgery became more feasible[199-205]. With continued development of the technique, one of the main difficulties encountered was achieving adequate spatial orientation. Hybrid versions with the use of standard laparoscopic vision together with endoscopic surgical procedures were first tested for feasibility in animal models[206] and later used in animals to perform more technically advanced procedures including SG[207,208].
Using a hybrid technique with a transvaginal and transgastric approach, the first RYGB was described in a human cadaver to demonstrate the feasibility of the procedure[209]. A similar study by Hagen et al[210] attempted RYGB in cadavers, but they experienced several obstacles. Difficulties included bowel manipulation and measurement, tissue dissection, stapler manipulation, and anvil docking. Furthermore, the total procedure time was approximately 6-9 h. While the authors found the procedure to be feasible, they noted a lack of proper instrumentation as a major barrier. The hybrid form of NOTES was used by Ramos et al[211] in the first description of SG performed in four patients using a transvaginal approach. The total operative time was 90 to 100 min and the procedure was shown to be feasible and safe with no short-term complications reported. In order to enhance the ability of the operators in helping to navigate the instruments, an image registration system has been evaluated in cadavers. Operators were better able to reach their intended targets as this system provided them with enhanced navigation without the need for previous training or knowledge of the system[212]. However, this system has not been widely tested and is not currently used in conjunction with NOTES.
There are several limitations to the growth and expansion of NOTES, which initially generated high expectations. Advanced spatial and endoscopic skills are required by practitioners to apply new techniques and learn to operate new technology. Additionally, the advancement in technology in production of the next generation of endoscopic tools in the United States has been slowed by economic considerations and stringent regulatory standards[213]. With the increasing interest and growing number of studies on NOTES, the Society of American Gastrointestinal and Endoscopic Surgeons and the American Society for Gastrointestinal Endoscopy assembled a Joint Committee to review ongoing issues involving NOTES. As a part of this effort, the Joint Committee formed an organization called the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR). In their most recent publication, NOSCAR notes that a major roadblock to further advancement of NOTES remains within the regulatory and reimbursement arenas. Innovative technology to enable the performance of NOTES is mostly being developed by small startup companies that cannot withstand regulatory and reimbursement difficulties of the industry. Another challenge considered by NOSCAR is the difficulty in defining the optimal human procedures for the first widespread application of NOTES. The best potential human applications are considered to be transanal/vaginal colorectal surgery, endolumenal myotomy for achalasia, and staging peritoneoscopy for GI malignancies. Despite the promising research and growth of NOTES in bariatrics, applications to this field are not currently considered as one of the most promising areas of growth by NOSCAR[214].
CONCLUSION
Obesity and its associated co-morbidities are on the rise worldwide and have reached epidemic proportions. To help treat this growing and devastating disorder, the field of bariatrics has been evolving and growing over the past several decades. Surgical procedures have been developed and refined to help manage obesity, and over time, the safety and efficacy of bariatric surgery has improved. Due to the significant benefits and minimal associated risks, bariatric surgery is now the preferred modality of therapy for morbidly obese individuals, especially those with co-morbidities.
Surgical options for medically-complicated obesity currently are supported by the best available data and generally provide the best outcomes. However, attempts to replace invasive techniques, with their related morbidity and mortality, are the driving factors behind studies of newer, minimally invasive procedures. Our therapeutic armamentarium continues to expand for treatment of morbid obesity and its medical complication as new research is completed and novel techniques are assessed. Preliminary results in several of these areas are promising and provide patients and practitioners with a potential future array of options and modes of therapy, yet many questions remain regarding the safety, efficacy, and durability of these new procedures. With continued research and additional technological advancements, new safe and effective minimally invasive treatment options appear to be on the horizon.
Footnotes
P- Reviewer: Hegardt FG, Liu EQ, Sheedfar F S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 4.Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond) 2013;37:889–891. doi: 10.1038/ijo.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 6.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol. 1997;146:214–222. doi: 10.1093/oxfordjournals.aje.a009256. [DOI] [PubMed] [Google Scholar]
- 8.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 9.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S–209S. [PubMed] [Google Scholar]
- 10.Moyer VA. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:373–378. doi: 10.7326/0003-4819-157-5-201209040-00475. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien PE, Dixon JB, Laurie C, Skinner S, Proietto J, McNeil J, Strauss B, Marks S, Schachter L, Chapman L, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med. 2006;144:625–633. doi: 10.7326/0003-4819-144-9-200605020-00005. [DOI] [PubMed] [Google Scholar]
- 12.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 13.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 14.Adams TD, Davidson LE, Litwin SE, Hunt SC. Gastrointestinal surgery: cardiovascular risk reduction and improved long-term survival in patients with obesity and diabetes. Curr Atheroscler Rep. 2012;14:606–615. doi: 10.1007/s11883-012-0286-4. [DOI] [PubMed] [Google Scholar]
- 15.Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen RV, Pinheiro JC, Schiavon CA, Salles JE, Wajchenberg BL, Cummings DE. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35:1420–1428. doi: 10.2337/dc11-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebovitz HE. Type 2 diabetes mellitus--current therapies and the emergence of surgical options. Nat Rev Endocrinol. 2011;7:408–419. doi: 10.1038/nrendo.2011.10. [DOI] [PubMed] [Google Scholar]
- 18.Ryan DH. BMI guidelines for bariatric surgery in diabetes: how low can we go? Diabetes Care. 2012;35:1399–1400. doi: 10.2337/dc12-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker MT. The history and evolution of bariatric surgical procedures. Surg Clin North Am. 2011;91:1181–2001, viii. doi: 10.1016/j.suc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Kremen AJ, Linner JH, Nelson CH. An experimental evaluation of the nutritional importance of proximal and distal small intestine. Ann Surg. 1954;140:439–448. doi: 10.1097/00000658-195409000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scopinaro N. The IFSO and obesity surgery throughout the world. International Federation for the Surgery of Obesity. Obes Surg. 1998;8:3–8. doi: 10.1381/096089298765554971. [DOI] [PubMed] [Google Scholar]
- 22.Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg. 2004;14:1157–1164. doi: 10.1381/0960892042387057. [DOI] [PubMed] [Google Scholar]
- 23.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427–436. doi: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 24.Favretti F, Segato G, Ashton D, Busetto L, De Luca M, Mazza M, Ceoloni A, Banzato O, Calo E, Enzi G. Laparoscopic adjustable gastric banding in 1,791 consecutive obese patients: 12-year results. Obes Surg. 2007;17:168–175. doi: 10.1007/s11695-007-9043-0. [DOI] [PubMed] [Google Scholar]
- 25.Dargent J. Surgical treatment of morbid obesity by adjustable gastric band: the case for a conservative strategy in the case of failure - a 9-year series. Obes Surg. 2004;14:986–990. doi: 10.1381/0960892041719545. [DOI] [PubMed] [Google Scholar]
- 26.Dixon AF, Dixon JB, O’Brien PE. Laparoscopic adjustable gastric banding induces prolonged satiety: a randomized blind crossover study. J Clin Endocrinol Metab. 2005;90:813–819. doi: 10.1210/jc.2004-1546. [DOI] [PubMed] [Google Scholar]
- 27.Burton PR, Brown WA. The mechanism of weight loss with laparoscopic adjustable gastric banding: induction of satiety not restriction. Int J Obes (Lond) 2011;35 Suppl 3:S26–S30. doi: 10.1038/ijo.2011.144. [DOI] [PubMed] [Google Scholar]
- 28.Brethauer SA. Sleeve gastrectomy. Surg Clin North Am. 2011;91:1265–1279, ix. doi: 10.1016/j.suc.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis. 2009;5:469–475. doi: 10.1016/j.soard.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319–324. doi: 10.1097/SLA.0b013e3181e90b31. [DOI] [PubMed] [Google Scholar]
- 31.Papamargaritis D, le Roux CW, Sioka E, Koukoulis G, Tzovaras G, Zacharoulis D. Changes in gut hormone profile and glucose homeostasis after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2013;9:192–201. doi: 10.1016/j.soard.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Dimitriadis E, Daskalakis M, Kampa M, Peppe A, Papadakis JA, Melissas J. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg. 2013;257:647–654. doi: 10.1097/SLA.0b013e31826e1846. [DOI] [PubMed] [Google Scholar]
- 33.Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22:740–748. doi: 10.1007/s11695-012-0622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8:544–556. doi: 10.1038/nrendo.2012.48. [DOI] [PubMed] [Google Scholar]
- 35.Sima LV, Sima AC, Dan RG, Breaza GM, Cretu OM. Complications of Roux-en-Y gastric bypass. Chirurgia (Bucur) 2013;108:180–183. [PubMed] [Google Scholar]
- 36.Vella A. Enteroendocrine secretion after Roux-en-Y gastric bypass: is it important? Neurogastroenterol Motil. 2013;25:1–3. doi: 10.1111/nmo.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodard GA, Encarnacion B, Downey JR, Peraza J, Chong K, Hernandez-Boussard T, Morton JM. Probiotics improve outcomes after Roux-en-Y gastric bypass surgery: a prospective randomized trial. J Gastrointest Surg. 2009;13:1198–1204. doi: 10.1007/s11605-009-0891-x. [DOI] [PubMed] [Google Scholar]
- 38.Scopinaro N, Gianetta E, Civalleri D, Bonalumi U, Bachi V. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg. 1979;66:618–620. doi: 10.1002/bjs.1800660906. [DOI] [PubMed] [Google Scholar]
- 39.Sudan R, Jacobs DO. Biliopancreatic diversion with duodenal switch. Surg Clin North Am. 2011;91:1281–1293, ix. doi: 10.1016/j.suc.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Kenler HA, Brolin RE, Cody RP. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am J Clin Nutr. 1990;52:87–92. doi: 10.1093/ajcn/52.1.87. [DOI] [PubMed] [Google Scholar]
- 41.Halmi KA, Mason E, Falk JR, Stunkard A. Appetitive behavior after gastric bypass for obesity. Int J Obes. 1981;5:457–464. [PubMed] [Google Scholar]
- 42.Olbers T, Björkman S, Lindroos A, Maleckas A, Lönn L, Sjöström L, Lönroth H. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 44.Howard L, Malone M, Michalek A, Carter J, Alger S. Gastric Bypass and Vertical Banded Gastroplasty- a Prospective Randomized Comparison and 5-Year Follow-up. Obes Surg. 1995;5:55–60. doi: 10.1381/096089295765558169. [DOI] [PubMed] [Google Scholar]
- 45.Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg. 1987;205:613–624. doi: 10.1097/00000658-198706000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Näslund I, Wickbom G, Christoffersson E, Agren G. A prospective randomized comparison of gastric bypass and gastroplasty. Complications and early results. Acta Chir Scand. 1986;152:681–689. [PubMed] [Google Scholar]
- 47.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–481. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 48.Tsoli M, Chronaiou A, Kehagias I, Kalfarentzos F, Alexandrides TK. Hormone changes and diabetes resolution after biliopancreatic diversion and laparoscopic sleeve gastrectomy: a comparative prospective study. Surg Obes Relat Dis. 2013;9:667–677. doi: 10.1016/j.soard.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 51.Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46:261–274. doi: 10.1016/j.npep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roddy DR, Koch TR, Reilly WM, Carney JA, Go VL. Identification and distribution of immunoreactive peptide YY in the human, canine, and murine gastrointestinal tracts: species-related antibody recognition differences. Regul Pept. 1987;18:201–212. doi: 10.1016/0167-0115(87)90008-5. [DOI] [PubMed] [Google Scholar]
- 53.Akkary E. Bariatric surgery evolution from the malabsorptive to the hormonal era. Obes Surg. 2012;22:827–831. doi: 10.1007/s11695-012-0623-2. [DOI] [PubMed] [Google Scholar]
- 54.Morimoto R, Satoh F, Murakami O, Totsune K, Saruta M, Suzuki T, Sasano H, Ito S, Takahashi K. Expression of peptide YY in human brain and pituitary tissues. Nutrition. 2008;24:878–884. doi: 10.1016/j.nut.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez Bartolomé M, Borque M, Martinez-Sarmiento J, Aparicio E, Hernández C, Cabrerizo L, Fernández-Represa JA. Peptide YY secretion in morbidly obese patients before and after vertical banded gastroplasty. Obes Surg. 2002;12:324–327. doi: 10.1381/096089202321088084. [DOI] [PubMed] [Google Scholar]
- 57.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 58.Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A, Nanni G, Castagneto M, Calvani M, Mingrone G. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–2031. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 59.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 60.Madsbad S. The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabetes Obes Metab. 2014;16:9–21. doi: 10.1111/dom.12119. [DOI] [PubMed] [Google Scholar]
- 61.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 62.Marathe CS, Rayner CK, Jones KL, Horowitz M. Glucagon-like peptides 1 and 2 in health and disease: a review. Peptides. 2013;44:75–86. doi: 10.1016/j.peptides.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Deane AM, Nguyen NQ, Stevens JE, Fraser RJ, Holloway RH, Besanko LK, Burgstad C, Jones KL, Chapman MJ, Rayner CK, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab. 2010;95:215–221. doi: 10.1210/jc.2009-1503. [DOI] [PubMed] [Google Scholar]
- 64.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flint A, Raben A, Rehfeld JF, Holst JJ, Astrup A. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. Int J Obes Relat Metab Disord. 2000;24:288–298. doi: 10.1038/sj.ijo.0801126. [DOI] [PubMed] [Google Scholar]
- 66.Wettergren A, Wøjdemann M, Meisner S, Stadil F, Holst JJ. The inhibitory effect of glucagon-like peptide-1 (GLP-1) 7-36 amide on gastric acid secretion in humans depends on an intact vagal innervation. Gut. 1997;40:597–601. doi: 10.1136/gut.40.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wettergren A, Wøjdemann M, Holst JJ. Glucagon-like peptide-1 inhibits gastropancreatic function by inhibiting central parasympathetic outflow. Am J Physiol. 1998;275:G984–G992. doi: 10.1152/ajpgi.1998.275.5.G984. [DOI] [PubMed] [Google Scholar]
- 68.Imeryüz N, Yeğen BC, Bozkurt A, Coşkun T, Villanueva-Peñacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol. 1997;273:G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- 69.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pournaras DJ, Osborne A, Hawkins SC, Mahon D, Ghatei MA, Bloom SR, Welbourn R, le Roux CW. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes Surg. 2010;20:56–60. doi: 10.1007/s11695-009-9989-1. [DOI] [PubMed] [Google Scholar]
- 72.Zarrinpar A, Loomba R. Review article: the emerging interplay among the gastrointestinal tract, bile acids and incretins in the pathogenesis of diabetes and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;36:909–921. doi: 10.1111/apt.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vilsbøll T, Krarup T, Sonne J, Madsbad S, Vølund A, Juul AG, Holst JJ. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–2713. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 74.Gautier JF, Choukem SP, Girard J. Physiology of incretins (GIP and GLP-1) and abnormalities in type 2 diabetes. Diabetes Metab. 2008;34 Suppl 2:S65–S72. doi: 10.1016/S1262-3636(08)73397-4. [DOI] [PubMed] [Google Scholar]
- 75.Jones IR, Owens DR, Luzio S, Williams S, Hayes TM. The glucose dependent insulinotropic polypeptide response to oral glucose and mixed meals is increased in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:668–677. doi: 10.1007/BF00274255. [DOI] [PubMed] [Google Scholar]
- 76.Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 77.Knop FK, Vilsbøll T, Højberg PV, Larsen S, Madsbad S, Vølund A, Holst JJ, Krarup T. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes. 2007;56:1951–1959. doi: 10.2337/db07-0100. [DOI] [PubMed] [Google Scholar]
- 78.Khoo CM, Muehlbauer MJ, Stevens RD, Pamuklar Z, Chen J, Newgard CB, Torquati A. Postprandial metabolite profiles reveal differential nutrient handling after bariatric surgery compared with matched caloric restriction. Ann Surg. 2014;259:687–693. doi: 10.1097/SLA.0b013e318296633f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–1407. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 80.Ionut V, Bergman RN. Mechanisms responsible for excess weight loss after bariatric surgery. J Diabetes Sci Technol. 2011;5:1263–1282. doi: 10.1177/193229681100500536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zac-Varghese S, Tan T, Bloom SR. Hormonal interactions between gut and brain. Discov Med. 2010;10:543–552. [PubMed] [Google Scholar]
- 82.Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab. 2003;88:4696–4701. doi: 10.1210/jc.2003-030421. [DOI] [PubMed] [Google Scholar]
- 83.Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54:2390–2395. doi: 10.2337/diabetes.54.8.2390. [DOI] [PubMed] [Google Scholar]
- 84.Laferrère B, Swerdlow N, Bawa B, Arias S, Bose M, Oliván B, Teixeira J, McGinty J, Rother KI. Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab. 2010;95:4072–4076. doi: 10.1210/jc.2009-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki K, Jayasena CN, Bloom SR. Obesity and appetite control. Exp Diabetes Res. 2012;2012:824305. doi: 10.1155/2012/824305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 88.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 89.Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschöp M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145:669–673. [PubMed] [Google Scholar]
- 90.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 91.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87:2984. doi: 10.1210/jcem.87.6.8738. [DOI] [PubMed] [Google Scholar]
- 92.le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab. 2005;90:1068–1071. doi: 10.1210/jc.2004-1216. [DOI] [PubMed] [Google Scholar]
- 93.Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 94.Christou NV, Look D, McLean AP. Pre- and post-prandial plasma ghrelin levels do not correlate with satiety or failure to achieve a successful outcome after Roux-en-Y gastric bypass. Obes Surg. 2005;15:1017–1023. doi: 10.1381/0960892054621071. [DOI] [PubMed] [Google Scholar]
- 95.Leonetti F, Silecchia G, Iacobellis G, Ribaudo MC, Zappaterreno A, Tiberti C, Iannucci CV, Perrotta N, Bacci V, Basso MS, et al. Different plasma ghrelin levels after laparoscopic gastric bypass and adjustable gastric banding in morbid obese subjects. J Clin Endocrinol Metab. 2003;88:4227–4231. doi: 10.1210/jc.2003-030133. [DOI] [PubMed] [Google Scholar]
- 96.Frühbeck G, Diez Caballero A, Gil MJ. Fundus functionality and ghrelin concentrations after bariatric surgery. N Engl J Med. 2004;350:308–309. doi: 10.1056/NEJM200401153500323. [DOI] [PubMed] [Google Scholar]
- 97.Roth CL, Reinehr T, Schernthaner GH, Kopp HP, Kriwanek S, Schernthaner G. Ghrelin and obestatin levels in severely obese women before and after weight loss after Roux-en-Y gastric bypass surgery. Obes Surg. 2009;19:29–35. doi: 10.1007/s11695-008-9568-x. [DOI] [PubMed] [Google Scholar]
- 98.Langer FB, Reza Hoda MA, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, Schindler K, Luger A, Ludvik B, Prager G. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024–1029. doi: 10.1381/0960892054621125. [DOI] [PubMed] [Google Scholar]
- 99.Carroll JF, Franks SF, Smith AB, Phelps DR. Visceral adipose tissue loss and insulin resistance 6 months after laparoscopic gastric banding surgery: a preliminary study. Obes Surg. 2009;19:47–55. doi: 10.1007/s11695-008-9642-4. [DOI] [PubMed] [Google Scholar]
- 100.Mingrone G, Granato L, Valera-Mora E, Iaconelli A, Calvani MF, Bracaglia R, Manco M, Nanni G, Castagneto M. Ultradian ghrelin pulsatility is disrupted in morbidly obese subjects after weight loss induced by malabsorptive bariatric surgery. Am J Clin Nutr. 2006;83:1017–1024. doi: 10.1093/ajcn/83.5.1017. [DOI] [PubMed] [Google Scholar]
- 101.Kotidis EV, Koliakos GG, Baltzopoulos VG, Ioannidis KN, Yovos JG, Papavramidis ST. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment--a prospective study. Obes Surg. 2006;16:1425–1432. doi: 10.1381/096089206778870058. [DOI] [PubMed] [Google Scholar]
- 102.Frühbeck G, Diez-Caballero A, Gil MJ, Montero I, Gómez-Ambrosi J, Salvador J, Cienfuegos JA. The decrease in plasma ghrelin concentrations following bariatric surgery depends on the functional integrity of the fundus. Obes Surg. 2004;14:606–612. doi: 10.1381/096089204323093363. [DOI] [PubMed] [Google Scholar]
- 103.Frühbeck G, Rotellar F, Hernández-Lizoain JL, Gil MJ, Gómez-Ambrosi J, Salvador J, Cienfuegos JA. Fasting plasma ghrelin concentrations 6 months after gastric bypass are not determined by weight loss or changes in insulinemia. Obes Surg. 2004;14:1208–1215. doi: 10.1381/0960892042386904. [DOI] [PubMed] [Google Scholar]
- 104.Chronaiou A, Tsoli M, Kehagias I, Leotsinidis M, Kalfarentzos F, Alexandrides TK. Lower ghrelin levels and exaggerated postprandial peptide-YY, glucagon-like peptide-1, and insulin responses, after gastric fundus resection, in patients undergoing Roux-en-Y gastric bypass: a randomized clinical trial. Obes Surg. 2012;22:1761–1770. doi: 10.1007/s11695-012-0738-5. [DOI] [PubMed] [Google Scholar]
- 105.Maier C, Schaller G, Buranyi B, Nowotny P, Geyer G, Wolzt M, Luger A. The cholinergic system controls ghrelin release and ghrelin-induced growth hormone release in humans. J Clin Endocrinol Metab. 2004;89:4729–4733. doi: 10.1210/jc.2004-0656. [DOI] [PubMed] [Google Scholar]
- 106.Broglio F, Gottero C, Van Koetsveld P, Prodam F, Destefanis S, Benso A, Gauna C, Hofland L, Arvat E, van der Lely AJ, et al. Acetylcholine regulates ghrelin secretion in humans. J Clin Endocrinol Metab. 2004;89:2429–2433. doi: 10.1210/jc.2003-031517. [DOI] [PubMed] [Google Scholar]
- 107.le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, Ghatei MA, Theodorou NA, Bloom SR. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab. 2005;90:4521–4524. doi: 10.1210/jc.2004-2537. [DOI] [PubMed] [Google Scholar]
- 108.Katsuura G, Asakawa A, Inui A. Roles of pancreatic polypeptide in regulation of food intake. Peptides. 2002;23:323–329. doi: 10.1016/s0196-9781(01)00604-0. [DOI] [PubMed] [Google Scholar]
- 109.Schwartz TW, Holst JJ, Fahrenkrug J, Jensen SL, Nielsen OV, Rehfeld JF, de Muckadell OB, Stadil F. Vagal, cholinergic regulation of pancreatic polypeptide secretion. J Clin Invest. 1978;61:781–789. doi: 10.1172/JCI108992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schwartz TW. Pancreatic polypeptide: a hormone under vagal control. Gastroenterology. 1983;85:1411–1425. [PubMed] [Google Scholar]
- 111.Sundbom M, Holdstock C, Engström BE, Karlsson FA. Early changes in ghrelin following Roux-en-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17:304–310. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 112.Perathoner A, Weiss H, Santner W, Brandacher G, Laimer E, Höller E, Aigner F, Klaus A. Vagal nerve dissection during pouch formation in laparoscopic Roux-Y-gastric bypass for technical simplification: does it matter? Obes Surg. 2009;19:412–417. doi: 10.1007/s11695-008-9657-x. [DOI] [PubMed] [Google Scholar]
- 113.Ramón JM, Salvans S, Crous X, Puig S, Goday A, Benaiges D, Trillo L, Pera M, Grande L. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012;16:1116–1122. doi: 10.1007/s11605-012-1855-0. [DOI] [PubMed] [Google Scholar]
- 114.D’Alessio D. Intestinal hormones and regulation of satiety: the case for CCK, GLP-1, PYY, and Apo A-IV. JPEN J Parenter Enteral Nutr. 2008;32:567–568. doi: 10.1177/0148607108322401. [DOI] [PubMed] [Google Scholar]
- 115.Liddle RA, Rushakoff RJ, Morita ET, Beccaria L, Carter JD, Goldfine ID. Physiological role for cholecystokinin in reducing postprandial hyperglycemia in humans. J Clin Invest. 1988;81:1675–1681. doi: 10.1172/JCI113505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suzuki K, Jayasena CN, Bloom SR. The gut hormones in appetite regulation. J Obes. 2011;2011:528401. doi: 10.1155/2011/528401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lieverse RJ, Jansen JB, Masclee AA, Rovati LC, Lamers CB. Effect of a low dose of intraduodenal fat on satiety in humans: studies using the type A cholecystokinin receptor antagonist loxiglumide. Gut. 1994;35:501–505. doi: 10.1136/gut.35.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gutzwiller JP, Drewe J, Ketterer S, Hildebrand P, Krautheim A, Beglinger C. Interaction between CCK and a preload on reduction of food intake is mediated by CCK-A receptors in humans. Am J Physiol Regul Integr Comp Physiol. 2000;279:R189–R195. doi: 10.1152/ajpregu.2000.279.1.R189. [DOI] [PubMed] [Google Scholar]
- 119.Lieverse RJ, Jansen JB, Masclee AM, Lamers CB. Satiety effects of cholecystokinin in humans. Gastroenterology. 1994;106:1451–1454. doi: 10.1016/0016-5085(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 120.Lieverse RJ, Jansen JB, Masclee AA, Lamers CB. Satiety effects of a physiological dose of cholecystokinin in humans. Gut. 1995;36:176–179. doi: 10.1136/gut.36.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hill P, Garbaczewski L, Koppeschaar H, Thijssen JH, de Waard F, Wynder EL. Peptide and steroid hormones in subjects at different risk for diet-related diseases. Am J Clin Nutr. 1988;48:782–786. doi: 10.1093/ajcn/48.3.782. [DOI] [PubMed] [Google Scholar]
- 122.Tomita T, Greeley G, Watt L, Doull V, Chance R. Protein meal-stimulated pancreatic polypeptide secretion in Prader-Willi syndrome of adults. Pancreas. 1989;4:395–400. doi: 10.1097/00006676-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 123.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kellum JM, Kuemmerle JF, O’Dorisio TM, Rayford P, Martin D, Engle K, Wolf L, Sugerman HJ. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg. 1990;211:763–770; discussion 770-771. doi: 10.1097/00000658-199006000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Foschi D, Corsi F, Pisoni L, Vago T, Bevilacqua M, Asti E, Righi I, Trabucchi E. Plasma cholecystokinin levels after vertical banded gastroplasty: effects of an acidified meal. Obes Surg. 2004;14:644–647. doi: 10.1381/096089204323093426. [DOI] [PubMed] [Google Scholar]
- 126.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, Zucker JD, Doré J, Clément K. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 129.Korner J, Conroy R, Febres G, McMahon DJ, Conwell I, Karmally W, Aronne LJ. Randomized double-blind placebo-controlled study of leptin administration after gastric bypass. Obesity (Silver Spring) 2013;21:951–956. doi: 10.1002/oby.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Terra X, Auguet T, Guiu-Jurado E, Berlanga A, Orellana-Gavaldà JM, Hernández M, Sabench F, Porras JA, Llutart J, Martinez S, et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23:1790–1798. doi: 10.1007/s11695-013-1033-9. [DOI] [PubMed] [Google Scholar]
- 131.Perathoner A, Weißenbacher A, Sucher R, Laimer E, Pratschke J, Mittermair R. Significant weight loss and rapid resolution of diabetes and dyslipidemia during short-term follow-up after laparoscopic sleeve gastrectomy. Obes Surg. 2013;23:1966–1972. doi: 10.1007/s11695-013-1038-4. [DOI] [PubMed] [Google Scholar]
- 132.Gumbau V, Bruna M, Canelles E, Guaita M, Mulas C, Basés C, Celma I, Puche J, Marcaida G, Oviedo M, et al. A prospective study on inflammatory parameters in obese patients after sleeve gastrectomy. Obes Surg. 2014;24:903–908. doi: 10.1007/s11695-014-1186-1. [DOI] [PubMed] [Google Scholar]
- 133.Shrestha C, He H, Liu Y, Zhu S, Xiong J, Mo Z. Changes in Adipokines following Laparoscopic Roux-en-Y Gastric Bypass Surgery in Chinese Individuals with Type 2 Diabetes Mellitus and BMI of 22-30 kg·m(-2.) Int J Endocrinol. 2013;2013:240971. doi: 10.1155/2013/240971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bachmayer C, Lammert A, Hasenberg T, Hammes HP. Healthy obese and post bariatric patients - metabolic and vascular patterns. Exp Clin Endocrinol Diabetes. 2013;121:483–487. doi: 10.1055/s-0033-1347248. [DOI] [PubMed] [Google Scholar]
- 135.Lee YJ, Heo YS, Park HS, Lee SH, Lee SK, Jang YJ. Serum SPARC and matrix metalloproteinase-2 and metalloproteinase-9 concentrations after bariatric surgery in obese adults. Obes Surg. 2014;24:604–610. doi: 10.1007/s11695-013-1111-z. [DOI] [PubMed] [Google Scholar]
- 136.Herder C, Peltonen M, Svensson PA, Carstensen M, Jacobson P, Roden M, Sjöström L, Carlsson L. Adiponectin and bariatric surgery: associations with diabetes and cardiovascular disease in the Swedish Obese Subjects Study. Diabetes Care. 2014;37:1401–1409. doi: 10.2337/dc13-1362. [DOI] [PubMed] [Google Scholar]
- 137.Lee SM, Pryor AD. Future directions in bariatric surgery. Surg Clin North Am. 2011;91:1373–1395, x. doi: 10.1016/j.suc.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 138.Kirby DF, Wade JB, Mills PR, Sugerman HJ, Kellum JM, Zfass AM, Starkey JV, Birkenhauer R, Hamer RM. A prospective assessment of the Garren-Edwards Gastric Bubble and bariatric surgery in the treatment of morbid obesity. Am Surg. 1990;56:575–580. [PubMed] [Google Scholar]
- 139.Hogan RB, Johnston JH, Long BW, Sones JQ, Hinton LA, Bunge J, Corrigan SA. A double-blind, randomized, sham-controlled trial of the gastric bubble for obesity. Gastrointest Endosc. 1989;35:381–385. doi: 10.1016/s0016-5107(89)72839-x. [DOI] [PubMed] [Google Scholar]
- 140.Benjamin SB, Maher KA, Cattau EL, Collen MJ, Fleischer DE, Lewis JH, Ciarleglio CA, Earll JM, Schaffer S, Mirkin K. Double-blind controlled trial of the Garren-Edwards gastric bubble: an adjunctive treatment for exogenous obesity. Gastroenterology. 1988;95:581–588. doi: 10.1016/s0016-5085(88)80001-5. [DOI] [PubMed] [Google Scholar]
- 141.Genco A, Bruni T, Doldi SB, Forestieri P, Marino M, Busetto L, Giardiello C, Angrisani L, Pecchioli L, Stornelli P, et al. BioEnterics Intragastric Balloon: The Italian Experience with 2,515 Patients. Obes Surg. 2005;15:1161–1164. doi: 10.1381/0960892055002202. [DOI] [PubMed] [Google Scholar]
- 142.Lopez-Nava G, Rubio MA, Prados S, Pastor G, Cruz MR, Companioni E, Lopez A. BioEnterics® intragastric balloon (BIB®). Single ambulatory center Spanish experience with 714 consecutive patients treated with one or two consecutive balloons. Obes Surg. 2011;21:5–9. doi: 10.1007/s11695-010-0093-3. [DOI] [PubMed] [Google Scholar]
- 143.Stimac D, Majanović SK, Turk T, Kezele B, Licul V, Orlić ZC. Intragastric balloon treatment for obesity: results of a large single center prospective study. Obes Surg. 2011;21:551–555. doi: 10.1007/s11695-010-0310-0. [DOI] [PubMed] [Google Scholar]
- 144.Peker Y, Coskun H, Bozkurt S, Cin N, Atak T, Genc H. Comparison of results of laparoscopic gastric banding and consecutive intragastric balloon application at 18 months: a clinical prospective study. J Laparoendosc Adv Surg Tech A. 2011;21:471–475. doi: 10.1089/lap.2010.0439. [DOI] [PubMed] [Google Scholar]
- 145.Dastis NS, François E, Deviere J, Hittelet A, Ilah Mehdi A, Barea M, Dumonceau JM. Intragastric balloon for weight loss: results in 100 individuals followed for at least 2.5 years. Endoscopy. 2009;41:575–580. doi: 10.1055/s-0029-1214826. [DOI] [PubMed] [Google Scholar]
- 146.Melissas J, Mouzas J, Filis D, Daskalakis M, Matrella E, Papadakis JA, Sevrisarianos N, Charalambides D. The intragastric balloon - smoothing the path to bariatric surgery. Obes Surg. 2006;16:897–902. doi: 10.1381/096089206777822188. [DOI] [PubMed] [Google Scholar]
- 147.Spyropoulos C, Katsakoulis E, Mead N, Vagenas K, Kalfarentzos F. Intragastric balloon for high-risk super-obese patients: a prospective analysis of efficacy. Surg Obes Relat Dis. 2007;3:78–83. doi: 10.1016/j.soard.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 148.Busetto L, Segato G, De Luca M, Bortolozzi E, MacCari T, Magon A, Inelmen EM, Favretti F, Enzi G. Preoperative weight loss by intragastric balloon in super-obese patients treated with laparoscopic gastric banding: a case-control study. Obes Surg. 2004;14:671–676. doi: 10.1381/096089204323093471. [DOI] [PubMed] [Google Scholar]
- 149.Alfredo G, Roberta M, Massimiliano C, Michele L, Nicola B, Adriano R. Long-term multiple intragastric balloon treatment--a new strategy to treat morbid obese patients refusing surgery: prospective 6-year follow-up study. Surg Obes Relat Dis. 2014;10:307–311. doi: 10.1016/j.soard.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 150.Machytka E, Klvana P, Kornbluth A, Peikin S, Mathus-Vliegen LE, Gostout C, Lopez-Nava G, Shikora S, Brooks J. Adjustable intragastric balloons: a 12-month pilot trial in endoscopic weight loss management. Obes Surg. 2011;21:1499–1507. doi: 10.1007/s11695-011-0424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Espinet-Coll E, Nebreda-Durán J, Gómez-Valero JA, Muñoz-Navas M, Pujol-Gebelli J, Vila-Lolo C, Martínez-Gómez A, Juan-Creix-Comamala A. Current endoscopic techniques in the treatment of obesity. Rev Esp Enferm Dig. 2012;104:72–87. doi: 10.4321/s1130-01082012000200006. [DOI] [PubMed] [Google Scholar]
- 152.Vilallonga R, Valverde S, Caubet E. Intestinal occlusion as unusual complication of new intragastric balloon Spatz Adjustable Balloon system for treatment of morbid obesity. Surg Obes Relat Dis. 2013;9:e16–e17. doi: 10.1016/j.soard.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 153.Genco A, Dellepiane D, Baglio G, Cappelletti F, Frangella F, Maselli R, Dante MC, Camoirano R, Lorenzo M, Basso N. Adjustable intragastric balloon vs non-adjustable intragastric balloon: case-control study on complications, tolerance, and efficacy. Obes Surg. 2013;23:953–958. doi: 10.1007/s11695-013-0891-5. [DOI] [PubMed] [Google Scholar]
- 154.Ponce J, Quebbemann BB, Patterson EJ. Prospective, randomized, multicenter study evaluating safety and efficacy of intragastric dual-balloon in obesity. Surg Obes Relat Dis. 2013;9:290–295. doi: 10.1016/j.soard.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 155.Kadirkamanathan SS, Evans DF, Gong F, Yazaki E, Scott M, Swain CP. Antireflux operations at flexible endoscopy using endoluminal stitching techniques: an experimental study. Gastrointest Endosc. 1996;44:133–143. doi: 10.1016/s0016-5107(96)70130-x. [DOI] [PubMed] [Google Scholar]
- 156.Rothstein RI, Filipi CJ. Endoscopic suturing for gastroesophageal reflux disease: clinical outcome with the Bard EndoCinch. Gastrointest Endosc Clin N Am. 2003;13:89–101. doi: 10.1016/s1052-5157(02)00107-1. [DOI] [PubMed] [Google Scholar]
- 157.Montgomery M, Håkanson B, Ljungqvist O, Ahlman B, Thorell A. Twelve months’ follow-up after treatment with the EndoCinch endoscopic technique for gastro-oesophageal reflux disease: a randomized, placebo-controlled study. Scand J Gastroenterol. 2006;41:1382–1389. doi: 10.1080/00365520600735738. [DOI] [PubMed] [Google Scholar]
- 158.Fogel R, De Fogel J, Bonilla Y, De La Fuente R. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc. 2008;68:51–58. doi: 10.1016/j.gie.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 159.Brethauer SA, Chand B, Schauer PR, Thompson CC. Transoral gastric volume reduction for weight management: technique and feasibility in 18 patients. Surg Obes Relat Dis. 2010;6:689–694. doi: 10.1016/j.soard.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 160.Brethauer SA, Chand B, Schauer PR, Thompson CC. Transoral gastric volume reduction as intervention for weight management: 12-month follow-up of TRIM trial. Surg Obes Relat Dis. 2012;8:296–303. doi: 10.1016/j.soard.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 161.Devière J, Ojeda Valdes G, Cuevas Herrera L, Closset J, Le Moine O, Eisendrath P, Moreno C, Dugardeyn S, Barea M, de la Torre R, et al. Safety, feasibility and weight loss after transoral gastroplasty: First human multicenter study. Surg Endosc. 2008;22:589–598. doi: 10.1007/s00464-007-9662-5. [DOI] [PubMed] [Google Scholar]
- 162.Moreno C, Closset J, Dugardeyn S, Baréa M, Mehdi A, Collignon L, Zalcman M, Baurain M, Le Moine O, Devière J. Transoral gastroplasty is safe, feasible, and induces significant weight loss in morbidly obese patients: results of the second human pilot study. Endoscopy. 2008;40:406–413. doi: 10.1055/s-2007-995748. [DOI] [PubMed] [Google Scholar]
- 163.Familiari P, Costamagna G, Bléro D, Le Moine O, Perri V, Boskoski I, Coppens E, Barea M, Iaconelli A, Mingrone G, et al. Transoral gastroplasty for morbid obesity: a multicenter trial with a 1-year outcome. Gastrointest Endosc. 2011;74:1248–1258. doi: 10.1016/j.gie.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 164.Nanni G, Familiari P, Mor A, Iaconelli A, Perri V, Rubino F, Boldrini G, Salerno MP, Leccesi L, Iesari S, et al. Effectiveness of the Transoral Endoscopic Vertical Gastroplasty (TOGa®): a good balance between weight loss and complications, if compared with gastric bypass and biliopancreatic diversion. Obes Surg. 2012;22:1897–1902. doi: 10.1007/s11695-012-0770-5. [DOI] [PubMed] [Google Scholar]
- 165.Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc. 2013;78:530–535. doi: 10.1016/j.gie.2013.04.197. [DOI] [PubMed] [Google Scholar]
- 166.Espinós JC, Turró R, Mata A, Cruz M, da Costa M, Villa V, Buchwald JN, Turró J. Early experience with the Incisionless Operating Platform™ (IOP) for the treatment of obesity: the Primary Obesity Surgery Endolumenal (POSE) procedure. Obes Surg. 2013;23:1375–1383. doi: 10.1007/s11695-013-0937-8. [DOI] [PubMed] [Google Scholar]
- 167.Legner A, Altorjay A, Juhasz A, Stadlhuber R, Reich V, Hunt B, Rothstein R, Filipi C. Transoral Mucosal Excision Sutured Gastroplasty: A Pilot Study for GERD and Obesity With Two-Year Follow-Up. Surg Innov. 2014;21:456–463. doi: 10.1177/1553350613508228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Biertho L, Hould FS, Lebel S, Biron S. Transoral endoscopic restrictive implant system: a new endoscopic technique for the treatment of obesity. Surg Obes Relat Dis. 2010;6:203–205. doi: 10.1016/j.soard.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 169.de Jong K, Mathus-Vliegen EM, Veldhuyzen EA, Eshuis JH, Fockens P. Short-term safety and efficacy of the Trans-oral Endoscopic Restrictive Implant System for the treatment of obesity. Gastrointest Endosc. 2010;72:497–504. doi: 10.1016/j.gie.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 170.Tarnoff M, Shikora S, Lembo A, Gersin K. Chronic in-vivo experience with an endoscopically delivered and retrieved duodenal-jejunal bypass sleeve in a porcine model. Surg Endosc. 2008;22:1023–1028. doi: 10.1007/s00464-007-9652-7. [DOI] [PubMed] [Google Scholar]
- 171.Gersin KS, Keller JE, Stefanidis D, Simms CS, Abraham DD, Deal SE, Kuwada TS, Heniford BT. Duodenal- jejunal bypass sleeve: a totally endoscopic device for the treatment of morbid obesity. Surg Innov. 2007;14:275–278. doi: 10.1177/1553350607312901. [DOI] [PubMed] [Google Scholar]
- 172.Rodriguez-Grunert L, Galvao Neto MP, Alamo M, Ramos AC, Baez PB, Tarnoff M. First human experience with endoscopically delivered and retrieved duodenal-jejunal bypass sleeve. Surg Obes Relat Dis. 2008;4:55–59. doi: 10.1016/j.soard.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 173.Schouten R, Rijs CS, Bouvy ND, Hameeteman W, Koek GH, Janssen IM, Greve JW. A multicenter, randomized efficacy study of the EndoBarrier Gastrointestinal Liner for presurgical weight loss prior to bariatric surgery. Ann Surg. 2010;251:236–243. doi: 10.1097/SLA.0b013e3181bdfbff. [DOI] [PubMed] [Google Scholar]
- 174.Escalona A, Yáñez R, Pimentel F, Galvao M, Ramos AC, Turiel D, Boza C, Awruch D, Gersin K, Ibáñez L. Initial human experience with restrictive duodenal-jejunal bypass liner for treatment of morbid obesity. Surg Obes Relat Dis. 2010;6:126–131. doi: 10.1016/j.soard.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 175.de Moura EG, Martins BC, Lopes GS, Orso IR, de Oliveira SL, Galvão Neto MP, Santo MA, Sakai P, Ramos AC, Garrido Júnior AB, et al. Metabolic improvements in obese type 2 diabetes subjects implanted for 1 year with an endoscopically deployed duodenal-jejunal bypass liner. Diabetes Technol Ther. 2012;14:183–189. doi: 10.1089/dia.2011.0152. [DOI] [PubMed] [Google Scholar]
- 176.Sandler BJ, Rumbaut R, Swain CP, Torres G, Morales L, Gonzales L, Schultz S, Talamini M, Horgan S. Human experience with an endoluminal, endoscopic, gastrojejunal bypass sleeve. Surg Endosc. 2011;25:3028–3033. doi: 10.1007/s00464-011-1665-6. [DOI] [PubMed] [Google Scholar]
- 177.Sullivan S, Stein R, Jonnalagadda S, Mullady D, Edmundowicz S. Aspiration therapy leads to weight loss in obese subjects: a pilot study. Gastroenterology. 2013;145:1245–1252.e1-5. doi: 10.1053/j.gastro.2013.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen JD, Lin HC. Electrical pacing accelerates intestinal transit slowed by fat-induced ileal brake. Dig Dis Sci. 2003;48:251–256. doi: 10.1023/a:1021911023155. [DOI] [PubMed] [Google Scholar]
- 179.Zhao X, Yin J, Chen J, Song G, Wang L, Zhu H, Brining D, Chen JD. Inhibitory effects and mechanisms of intestinal electrical stimulation on gastric tone, antral contractions, pyloric tone, and gastric emptying in dogs. Am J Physiol Regul Integr Comp Physiol. 2009;296:R36–R42. doi: 10.1152/ajpregu.90627.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu X, Lei Y, Chen JD. Duodenum electrical stimulation delays gastric emptying, reduces food intake and accelerates small bowel transit in pigs. Obesity (Silver Spring) 2011;19:442–448. doi: 10.1038/oby.2010.247. [DOI] [PubMed] [Google Scholar]
- 181.D’Argent J. Gastric electrical stimulation as therapy of morbid obesity: preliminary results from the French study. Obes Surg. 2002;12 Suppl 1:21S–25S. doi: 10.1381/096089202762552638. [DOI] [PubMed] [Google Scholar]
- 182.Yao S, Ke M, Wang Z, Xu D, Zhang Y, Chen JD. Visceral sensitivity to gastric stimulation and its correlation with alterations in gastric emptying and accommodation in humans. Obes Surg. 2005;15:247–253. doi: 10.1381/0960892053268363. [DOI] [PubMed] [Google Scholar]
- 183.Liu S, Hou X, Chen JD. Therapeutic potential of duodenal electrical stimulation for obesity: acute effects on gastric emptying and water intake. Am J Gastroenterol. 2005;100:792–796. doi: 10.1111/j.1572-0241.2005.40511.x. [DOI] [PubMed] [Google Scholar]
- 184.Liu J, Xiang Y, Qiao X, Dai Y, Chen JD. Hypoglycemic effects of intraluminal intestinal electrical stimulation in healthy volunteers. Obes Surg. 2011;21:224–230. doi: 10.1007/s11695-010-0326-5. [DOI] [PubMed] [Google Scholar]
- 185.Bodenlos JS, Kose S, Borckardt JJ, Nahas Z, Shaw D, O’Neil PM, George MS. Vagus nerve stimulation acutely alters food craving in adults with depression. Appetite. 2007;48:145–153. doi: 10.1016/j.appet.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 186.Pardo JV, Sheikh SA, Kuskowski MA, Surerus-Johnson C, Hagen MC, Lee JT, Rittberg BR, Adson DE. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes (Lond) 2007;31:1756–1759. doi: 10.1038/sj.ijo.0803666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kral JG. Vagotomy for treatment of severe obesity. Lancet. 1978;1:307–308. doi: 10.1016/s0140-6736(78)90074-0. [DOI] [PubMed] [Google Scholar]
- 188.Smith DK, Sarfeh J, Howard L. Truncal vagotomy in hypothalamic obesity. Lancet. 1983;1:1330–1331. doi: 10.1016/s0140-6736(83)92437-6. [DOI] [PubMed] [Google Scholar]
- 189.Camilleri M, Toouli J, Herrera MF, Kulseng B, Kow L, Pantoja JP, Marvik R, Johnsen G, Billington CJ, Moody FG, et al. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery. 2008;143:723–731. doi: 10.1016/j.surg.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 190.Sarr MG, Billington CJ, Brancatisano R, Brancatisano A, Toouli J, Kow L, Nguyen NT, Blackstone R, Maher JW, Shikora S, et al. The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg. 2012;22:1771–1782. doi: 10.1007/s11695-012-0751-8. [DOI] [PubMed] [Google Scholar]
- 191.Gui D, De Gaetano A, Spada PL, Viggiano A, Cassetta E, Albanese A. Botulinum toxin injected in the gastric wall reduces body weight and food intake in rats. Aliment Pharmacol Ther. 2000;14:829–834. doi: 10.1046/j.1365-2036.2000.00765.x. [DOI] [PubMed] [Google Scholar]
- 192.Albani G, Petroni ML, Mauro A, Liuzzi A, Lezzi G, Verti B, Marzullo P, Cattani L. Safety and efficacy of therapy with botulinum toxin in obesity: a pilot study. J Gastroenterol. 2005;40:833–835. doi: 10.1007/s00535-005-1669-x. [DOI] [PubMed] [Google Scholar]
- 193.García-Compean D, Mendoza-Fuerte E, Martínez JA, Villarreal I, Maldonado H. Endoscopic injection of botulinum toxin in the gastric antrum for the treatment of obesity. Results of a pilot study. Gastroenterol Clin Biol. 2005;29:789–791. doi: 10.1016/s0399-8320(05)86349-3. [DOI] [PubMed] [Google Scholar]
- 194.Júnior AC, Savassi-Rocha PR, Coelho LG, Spósito MM, Albuquerque W, Diniz MT, Paixão Ade M, Garcia FD, Lasmar LF. Botulinum A toxin injected into the gastric wall for the treatment of class III obesity: a pilot study. Obes Surg. 2006;16:335–343. doi: 10.1381/096089206776116408. [DOI] [PubMed] [Google Scholar]
- 195.Gui D, Mingrone G, Valenza V, Spada PL, Mutignani M, Runfola M, Scarfone A, Di Mugno M, Panunzi S. Effect of botulinum toxin antral injection on gastric emptying and weight reduction in obese patients: a pilot study. Aliment Pharmacol Ther. 2006;23:675–680. doi: 10.1111/j.1365-2036.2006.02773.x. [DOI] [PubMed] [Google Scholar]
- 196.Mittermair R, Keller C, Geibel J. Intragastric injection of botulinum toxin A for the treatment of obesity. Obes Surg. 2007;17:732–736. doi: 10.1007/s11695-007-9135-x. [DOI] [PubMed] [Google Scholar]
- 197.Foschi D, Corsi F, Lazzaroni M, Sangaletti O, Riva P, La Tartara G, Bevilacqua M, Osio M, Alciati A, Bianchi Porro G, et al. Treatment of morbid obesity by intraparietogastric administration of botulinum toxin: a randomized, double-blind, controlled study. Int J Obes (Lond) 2007;31:707–712. doi: 10.1038/sj.ijo.0803451. [DOI] [PubMed] [Google Scholar]
- 198.Gauderer MW, Ponsky JL, Izant RJ. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872–875. doi: 10.1016/s0022-3468(80)80296-x. [DOI] [PubMed] [Google Scholar]
- 199.Fritscher-Ravens A, Mosse CA, Mukherjee D, Mills T, Park PO, Swain CP. Transluminal endosurgery: single lumen access anastomotic device for flexible endoscopy. Gastrointest Endosc. 2003;58:585–591. doi: 10.1016/s0016-5107(03)02006-6. [DOI] [PubMed] [Google Scholar]
- 200.Park AE, Adrales GL, McKinlay R, Knapp C. A novel intestinal anastomotic device in a porcine model. Am Surg. 2004;70:767–773; discussion 773-774. [PubMed] [Google Scholar]
- 201.Swanstrom LL, Kozarek R, Pasricha PJ, Gross S, Birkett D, Park PO, Saadat V, Ewers R, Swain P. Development of a new access device for transgastric surgery. J Gastrointest Surg. 2005;9:1129–1136; discussion 1136-1137. doi: 10.1016/j.gassur.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 202.Kantsevoy SV, Jagannath SB, Niiyama H, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Magee CA, Vaughn CA, et al. Endoscopic gastrojejunostomy with survival in a porcine model. Gastrointest Endosc. 2005;62:287–292. doi: 10.1016/s0016-5107(05)01565-8. [DOI] [PubMed] [Google Scholar]
- 203.Kantsevoy SV, Niiyama H, Jagannath SB, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Magee CA, Vaughn CA, et al. The endoscopic transilluminator: an endoscopic device for identification of the proximal jejunum for transgastric endoscopic gastrojejunostomy. Gastrointest Endosc. 2006;63:1055–1058. doi: 10.1016/j.gie.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 204.Park PO, Bergström M, Ikeda K, Fritscher-Ravens A, Swain P. Experimental studies of transgastric gallbladder surgery: cholecystectomy and cholecystogastric anastomosis (videos) Gastrointest Endosc. 2005;61:601–606. doi: 10.1016/s0016-5107(04)02774-9. [DOI] [PubMed] [Google Scholar]
- 205.Bergström M, Ikeda K, Swain P, Park PO. Transgastric anastomosis by using flexible endoscopy in a porcine model (with video) Gastrointest Endosc. 2006;63:307–312. doi: 10.1016/j.gie.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 206.Mintz Y, Horgan S, Cullen J, Ramamoorthy S, Chock A, Savu MK, Easter DW, Talamini MA. NOTES: the hybrid technique. J Laparoendosc Adv Surg Tech A. 2007;17:402–406. doi: 10.1089/lap.2006.0225. [DOI] [PubMed] [Google Scholar]
- 207.Mintz Y, Horgan S, Savu MK, Cullen J, Chock A, Ramamoorthy S, Easter DW, Talamini MA. Hybrid natural orifice translumenal surgery (NOTES) sleeve gastrectomy: a feasibility study using an animal model. Surg Endosc. 2008;22:1798–1802. doi: 10.1007/s00464-008-9915-y. [DOI] [PubMed] [Google Scholar]
- 208.Marchesini JC, Cardoso AR, Nora M, Galvão Neto M, Mottin CC, Baretta G, Padoin AV, Moretto M, Maggioni L, Alves LB, et al. Laparoscopic sleeve gastrectomy with NOTES visualization--a step toward NOTES procedures. Surg Obes Relat Dis. 2008;4:773–776. doi: 10.1016/j.soard.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 209.Madan AK, Tichansky DS, Khan KA. Natural orifice transluminal endoscopic gastric bypass performed in a cadaver. Obes Surg. 2008;18:1192–1199. doi: 10.1007/s11695-008-9553-4. [DOI] [PubMed] [Google Scholar]
- 210.Hagen ME, Wagner OJ, Swain P, Pugin F, Buchs N, Caddedu M, Jamidar P, Fasel J, Morel P. Hybrid natural orifice transluminal endoscopic surgery (NOTES) for Roux-en-Y gastric bypass: an experimental surgical study in human cadavers. Endoscopy. 2008;40:918–924. doi: 10.1055/s-2008-1077720. [DOI] [PubMed] [Google Scholar]
- 211.Ramos AC, Zundel N, Neto MG, Maalouf M. Human hybrid NOTES transvaginal sleeve gastrectomy: initial experience. Surg Obes Relat Dis. 2008;4:660–663. doi: 10.1016/j.soard.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 212.Azagury DE, Ryou M, Shaikh SN, San José Estépar R, Lengyel BI, Jagadeesan J, Vosburgh KG, Thompson CC. Real-time computed tomography-based augmented reality for natural orifice transluminal endoscopic surgery navigation. Br J Surg. 2012;99:1246–1253. doi: 10.1002/bjs.8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bingener J, Gostout CJ. Update on natural orifice translumenal endoscopic surgery. Gastroenterol Hepatol (N Y) 2012;8:384–389. [PMC free article] [PubMed] [Google Scholar]
- 214.Rattner DW, Hawes R, Schwaitzberg S, Kochman M, Swanstrom L. The Second SAGES/ASGE White Paper on natural orifice transluminal endoscopic surgery: 5 years of progress. Surg Endosc. 2011;25:2441–2448. doi: 10.1007/s00464-011-1605-5. [DOI] [PubMed] [Google Scholar]


