Abstract
Hepatocellular carcinoma (HCC) is the sixth most common cancer and third leading cause of cancer-related death in the world. The Barcelona clinic liver cancer classification is the current standard classification system for the clinical management of patients with HCC and suggests that patients with intermediate-stage HCC benefit from transcatheter arterial chemoembolization (TACE). Interventional treatments such as TACE, balloon-occluded TACE, drug-eluting bead embolization, radioembolization, and combined therapies including TACE and radiofrequency ablation, continue to evolve, resulting in improved patient prognosis. However, patients with advanced-stage HCC typically receive only chemotherapy with sorafenib, a multi-kinase inhibitor, or palliative and conservative therapy. Most patients receive palliative or conservative therapy only, and approximately 50% of patients with HCC are candidates for systemic therapy. However, these patients require therapy that is more effective than sorafenib or conservative treatment. Several researchers try to perform more effective therapies, such as combined therapies (TACE with radiotherapy and sorafenib with TACE), modified TACE for HCC with arterioportal or arteriohepatic vein shunts, TACE based on hepatic hemodynamics, and isolated hepatic perfusion. This review summarizes the published data and data on important ongoing studies concerning interventional treatments for unresectable HCC and discusses the technical improvements in these interventions, particularly for advanced-stage HCC.
Keywords: Unresectable, Hepatocellular carcinoma, Intermediate-stage, Advanced-stage, Interventional
Core tip: Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide. Interventional treatments for intermediate-stage HCC patients such as transcatheter arterial chemoembolization (TACE), drug-eluting bead embolization, and radioembolization, continue to evolve, improving prognosis. However, advanced-stage HCC is typically treated only with sorafenib, with only a modest improvement in overall survival. More effective therapies such as combined TACE and radiotherapy, TACE with special techniques, and isolated hepatic perfusion have been studied extensively. This review summarizes data on published and important ongoing studies concerning interventional treatments for unresectable HCC and discusses technical improvements in these interventions, particularly for advanced-stage HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related deaths in the world[1]. The Barcelona clinic liver cancer (BCLC) classification is the current standard classification system for clinical management of patients with HCC (Table 1)[2] and suggests that patients presenting with very early-stage and early-stage disease (20%-30% of HCC patients) are suitable for curative treatments, such as resection, liver transplantation, local ablation with percutaneous ethanol injection, or radiofrequency ablation (RFA)[3,4]. Typically, patients with intermediate-stage disease show a survival benefit with transarterial chemoembolization (TACE). Patients with advanced-stage HCC with macroscopic vascular invasion (portal vein invasion), extrahepatic spread (lymph nodes and metastases), or cancer-related symptoms (performance status 1-2) may have a modest improvement in prognosis from first-line treatment with sorafenib, a molecularly targeted drug[5], while patients with terminal-stage disease (10%-20% of HCC patients) receive only symptomatic treatment.
Table 1.
Barcelona-clinic liver cancer classification in patients diagnosed with hepatocellular carcinoma
| Stage | Description |
| Very early | PS 0, Child-Pugh A, single HCC < 2 cm |
| Early | PS 0, Child-Pugh A-B, single HCC or 3 nodules < 3 cm |
| Intermediate | PS 0, Child-Pugh A-B, multinodular HCC |
| Advanced | PS 1-2, Child-Pugh A-B, portal neoplastic invasion, nodal metastases, distant metastases |
| End-stage | PS > 2, Child-Pugh C |
This classification is in accordance with that outlined in reference [2]. PS: Performance status; HCC: Hepatocellular carcinoma.
TACE is the current standard of care for patients with intermediate-stage disease and has been reported to prolong survival and allow control of HCC symptoms[6-8]. Patients with advanced-stage HCC receive first-line therapy with sorafenib, according to the BCLC treatment algorithm, and an increase in overall survival and time to progression has been shown in 2 major trials[5,9]. However, the median overall survivals in patients treated with sorafenib for advanced-stage disease were 10.7 and 6.5 mo in the SHARP and Asia-Pacific trials, respectively, and a better treatment modality is needed.
The aims of this review are to summarize the published data and important ongoing studies concerning interventional managements for HCC and to discuss the technical improvements in TACE, particularly for advanced-stage HCC.
UNRESECTABLE HCC
Intermediate-stage HCC
Patients presenting with Child-Pugh class A and B liver function and large or multinodular HCC without cancer-related symptoms, macrovascular invasion, or extrahepatic metastasis are diagnosed with intermediate-stage HCC. Transarterial intervention, particularly TACE, is the recommended BCLC classification treatment and is endorsed by the European Association for the Study of the Liver[10] and the American Association for the Study of Liver Diseases[11] guidelines.
TACE: The liver is a unique organ with a dual blood supply provided by the portal vein and hepatic artery. The portal vein delivers 80% of the blood supply to healthy liver tissue, while, the hepatic artery delivers 99% of the blood supply to hepatic tumors. Based on this observation, TACE for HCC is appropriate for patients for whom surgical or percutaneous ablative treatment is contraindicated. TACE involves the injection of anticancer drugs (doxorubicin, epirubicin, cisplatin or miriplatin) and iodized oil (Lipiodol Ultra Fluide, Laboratoires Guerber, Aulnay-sous-Bois, France) into the hepatic artery, followed by the administration of embolic agents[12,13]. The antitumor effect of TACE is greater than that of either anticancer drugs[14] or iodized oil[15,16] alone. Prognostic factors found to be associated with that a reduction in serum alpha fetoprotein levels after the intervention[17], the number of TACE procedures[18,19], a low model for end-stage liver disease score[20], the absence of diffuse disease[21,22], and small tumor size[23] have been shown to correlate with better survival.
Iodized oil is used as an embolic agent and carrier of anticancer drugs in TACE. Mixtures of anticancer drugs and iodized oil are classified as either emulsions (oil with saline and drugs) or suspensions (drugs in oil)[24,25]. Comparative studies of suspensions versus emulsions in TACE, with cisplatin powder[26] or epirubicin[27] as anticancer agents for the treatment of rabbit VX2 liver tumors, demonstrated that suspensions were superior to emulsions with regard to their antitumor effects. Several factors are thought to explain these findings. In an emulsion, most of the powdered drug is contained in the unstable aqueous phase[13,28], which undergoes rapid dilution in the blood, elimination from the hepatic tissue, and excretion by the kidneys. In a suspension, the powder is directly mixed in the oil phase and distributed in a similar fashion to iodized oil alone through the portal venules and sinusoids over a 24-h period[29,30]. As a result, suspensions show a longer anticancer drug release time at the tumor border and higher continuous drug concentrations. This longer tissue/drug activity period associated with the use of suspensions produces superior antitumor effects, as evaluated by changes to the growth ratio and results of histopathological investigations[24,25,31,32]. However, since most anticancer drugs are hydrophilic, it is hard to make stable drug suspensions in oil. Additionally, tumor uptake of suspensions is poor, most likely due to their high viscosity[33]. As the viscosity of iodized oil has a negative correlation with temperature exceeding 50 mPa.s at 20° but decreasing to 22 mPa.s and 12 mPa.s, at 40° and 60°, respectively[34], we designed a syringe warmer to maintain suspensions at a high temperature. We have obtained significant antitumor effects without major adverse events when treating HCC patients with warmed drug suspensions since January of 2011 (article submitted for publication), and a prospective, comparative study of TACE with warmed suspension at 53° is currently under way.
Drug-eluting beads (DEB), which can be loaded with doxorubicin, represent a novel drug delivery system for chemoembolization. A slow and sustained release of drug is predicted after intra-arterial delivery of DEB, and several studies have reported this method to be safe and efficacious in the treatment of HCC[35,36]. DEB-TACE is proposed to have a better pharmacokinetic profile than conventional Lipiodol-TACE and result in decreased systemic adverse effects[37]. However, a recent study reported that liver/biliary injury was strongly associated with DEB-TACE irrespectively of the type of tumor and underlying liver disease. At least 1 liver/biliary injury was observed after 30.4%-35.7% of DEB-TACE sessions, whereas this event occurred after only 4.2%-7.2% of conventional Lipiodol-TACE (P < 0.001)[38]. Despite encouraging pharmacological and antitumor advantages, a recent large, phase II randomized trial of HCC failed to demonstrate a better tumor response rate to DEB-TACE compared with conventional Lipiodol-TACE[39]. Further randomized, controlled, multicenter trials are necessary to explore the differences in adverse events and to assess the effects of DEB-TACE on short- and long-term outcomes.
Radioembolization involves the catheter-based delivery of yttrium-90 (90Y)-embedded resin (SIR-Spheres®; Sirtex Medical, Sidney, New South Wales, Australia) or glass (TheraSphere®, MDS Nordion, Toronto, Ontario, Canada) microspheres into the hepatic artery[40] to distribute radioisotopes to the tumor. 90Y is a pure beta-emitter, which generates high-energy radiation with a short half-life (2.67 d) and tissue penetration (mean 2.5 mm, maximum 11 mm)[41]. Once administered, these microspheres selectively emit high-energy, low-penetration radiation to the tumor, resulting in necrosis. Although there is currently a lack of sufficient prospective data comparing treatment efficacy in terms of response or survival between radioembolization and TACE for intermediate-stage HCC, a retrospective study[42] comparing the outcomes between radioembolization (n = 38) and TACE (n = 35) determined no significant difference in survival (median 8.0 mo vs 10.3 mo, P = 0.33). However, postembolization syndrome was significantly more severe in patients who underwent TACE[42]. Further evaluation of radioembolization, including direct comparisons with TACE, is needed.
Radioembolization may cause injury to the hepatic tissue and result in fibrotic changes and tissue atrophy[43,44]. The loss of liver function can be compensated for by non-treated liver segments, which tend to undergo hypertrophy after radioembolization (the atrophy-hypertrophy complex)[45]. A few studies have described the hepatic volume changes of treated and non-treated liver segments after radioembolization and report a 40%-55% loss in volume in treated segments and a 30%-40% increase in volume in non-treated segments[46,47].
Combination TACE and RFA: RFA is a curative treatment for very early- and early-stage HCC but is limited in the control of large tumors. The effectiveness of RFA is dependent on thermal necrosis, and blood flow through the tumor promotes heat loss and prevents proper heating. TACE can be performed strategically before RFA treatment to reduce this heat sink effect and increase the ablation volume of the tumor. A recently published, randomized study[48] showed that, when patients with tumors > 3 cm were randomized to receive either TACE, RFA, or TACE-RFA, the combination of TACE-RFA produced a superior median survival (TACE-RFA: 37 mo, TACE: 24 mo, and RFA: 22 mo) and rate of objective tumor response (TACE-RFA: 54%, TACE: 35%, and RFA: 36%). The results of TACE-RFA support the combining of locoregional modalities to improve the outcomes of patients with unresectable HCCs.
Modified TACE to obtain complete necrosis of the tumor: Histopathological investigations of HCCs resected after TACE have shown that the most viable tissue is located at the periphery of the tumor[49]. The efficacy of TACE is limited by the dual blood supply of liver tumors, which makes it impossible to deliver anticancer agents to the entire tumor area or achieve sufficient tumor ischemia without irreversible damage to surrounding normal liver parenchyma. Some studies[50,51] have reported superselective TACE to be useful for the treatment of small HCCs because it can embolize both the tumor and surrounding normal parenchyma. For large liver tumors, however, the therapeutic options are limited to techniques that result in complete necrosis of the tumor.
To obtain complete necrosis of a HCC, including the periphery, several reports[52-54] have investigated the hemodynamic changes occurring in the liver and tumors during hepatic vein balloon occlusion using computed tomography during hepatic arteriography (CTHA) and arterial portography, demonstrated that the occluded area is supplied with arterial blood alone[53,54], and suggested that sufficient embolization may be obtained during TACE with arterial control alone. Since balloon occlusion of the segmental hepatic vein eliminates the dual blood supply, leaving only the arterial supply, TACE performed using this technique can sufficiently embolize both the tumor and the surrounding liver parenchyma. We have performed TACE under balloon occlusion of the hepatic vein for more than 70 patients with advanced HCC, but only 30% of the patients benefited from the procedure due to the complex veno-venous communications in the liver[34,53]. The hepatic veins assume the role of draining veins despite single hepatic vein occlusion[53,55]; therefore, sufficient embolization of large liver tumors using the hepatic vein balloon occlusion technique is difficult.
When portal venous blood flow is decreased or stopped due to tumor thrombus, thromboembolus, or compression of the portal vein, the affected liver parenchyma appears as a hyperattenuated area with straight borders on contrast CT[56-62]. This appearance is similar to that seen with hepatic vein occlusion. One study[63] investigated the hepatic hemodynamic changes under acute balloon occlusion of the portal vein using single-level dynamic CTHA and reported several phenomena. First, a demarcated, hyperattenuated area of the liver parenchyma was noted in the distribution of the occluded portal vein branch, which was significantly higher than that of the non-occluded area (P < 0.01). Second, the balloon-occluded portal branch enhancement appeared to result from arterioportal communications. These findings suggest that, when portal venous flow stoppage occurs, only the hepatic artery supplies the corresponding area and hepatic arterial blood flow is increased. This phenomenon is known as the hepatic artery buffer response. The observed variations in blood flow are due to the degree of adenosine clearance, to which the hepatic artery is very sensitive, as adenosine is a potent vasodilator of the hepatic artery[64-66]. Finally, there is little anatomical variation in portal veins or porto-portal venous anastomosis. Therefore, sufficient embolization may occur, even in large liver tumors, by TACE under the corresponding portal vein branch occlusion (TACE-PVO). We began using TACE-PVO for large HCCs 6 years ago and have seen promising antitumor effects, which will be reported in the near future.
In 1981, Yamada et al[67] first reported a balloon-occluded arterial infusion therapy for liver tumors. In the 1990s, compulsory, superselective, balloon-occluded arterial embolization using a 3-French microballoon catheter[68] was introduced for hypovascular liver tumors. The selective balloon-occluded TACE (B-TACE) method is currently a useful treatment for HCC because of preventing proximal migration and leakage of embolization materials[69]. This technique leads to dense lipiodol emulsion accumulation in the HCC nodule[69]; however, the effectiveness of this therapy for HCC is controversial.
CT arteriography under balloon occlusion of the sub- or segmental hepatic artery demonstrates a much higher attenuation in the corresponding liver parenchyma than in HCC tumors[70]. This phenomenon suggests that B-TACE leads to dense lipiodol emulsion accumulation in nontumorous liver parenchyma and not in HCC nodules. Indeed, arteriography via the balloon-occluded hepatic artery shows dense opacification in the liver parenchyma and faint opacification in the HCC, while arteriography via the non-occluded hepatic artery shows dense opacification in the HCC (Figure 1). Therefore, the effectiveness of B-TACE is controversial. To remedy this, we developed a two-step B-TACE method for liver tumor (Figure 2). First, tumors are sufficiently embolized by TACE, followed by the inflation of a microballoon catheter to occlude the feeding artery in the second step. B-TACE is then performed for full embolization of the peritumoral liver parenchyma. Following this two-step B-TACE procedure, CT images showed dense lipiodol accumulation in the HCC. The two-step B-TACE method has a great advantage for therapy in patients with liver dysfunction because it prevents proximal migration and leakage of embolization materials, the embolized area can be determined, and injury to the liver parenchyma can be prevented. A comparative study of the two-step B-TACE method with conventional TACE obtained promising results for two-step B-TACE.
Figure 1.
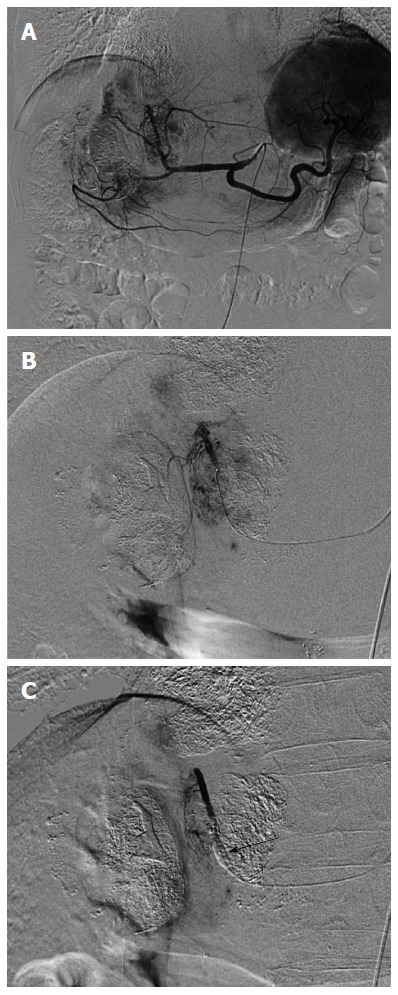
Selective arteriography with and without balloon-occluded feeding artery in a 72 year-old man with multiple hepatocellular carcinomas. A: Celiac arteriography revealed multiple hepatocellular carcinomas (HCCs) in the liver; B: Opacified HCCs were shown by medial segmental arteriography without balloon-occluded feeding artery; C: In contrast, the arteriography with balloon occlusion showed faint opacification of HCCs and nontumoral liver parenchyma was opacified. Arrow indicated an inflated microballoon.
Figure 2.
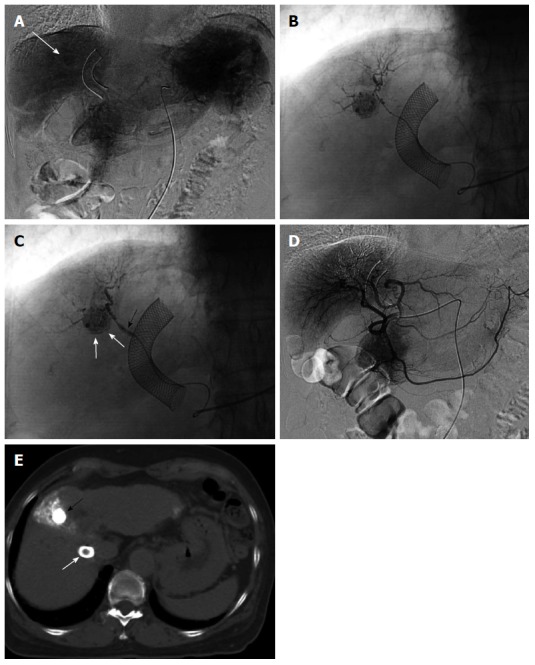
Two-step balloon-occluded transarterial chemoembolization. A 76-year old woman characterized as Child-Pugh class C underwent transjugular intrahepatic portosystemic shunt (TIPS) for massive ascites before several transarterial chemoembolization (TACE) sessions. Celiac arteriography in the venous phase (A) showed a small hepatocellular carcinoma (HCC) (arrow). A microballoon catheter was advanced into the tumor feeding artery. Then, two-step balloon-TACE (B-TACE) was performed. First, tumors were sufficiently embolized by TACE without balloon occlusion of the tumor feeding artery (B); Second, a microballoon catheter was inflated to occlude the feeding artery (C, black arrow), and B-TACE was performed to fully embolize the peritumoral liver parenchyma (C, white arrows). Common hepatic arteriography showed no proximal migration or leakage of embolization materials (D). Following the two-step B-TACE procedure, computed tomography showed dense lipiodol accumulation in the HCC (E, black arrow). There was no evidence of proximal migration or leakage of embolization materials. White arrows indicated a stent for TIPS.
Advanced-stage HCC
HCC patients with Child-Pugh class A and B liver function who present with macroscopic vascular invasion, extrahepatic spread, or cancer-related symptoms (performance status 1-2) are diagnosed with advanced-stage HCC. First-line treatment with sorafenib is recommended for this stage of HCC according to the BCLC treatment algorithm based on the results of sorafenib therapy in randomized trials in Europe and Asia[5,9]. However, overall survival was only modestly improved by 2.8 mo[5]. Patients with advanced-stage HCC require more effective therapy than sorafenib or conservative treatment, and this is currently under consideration.
HCC with vascular invasion: In general, HCCs with portal venous tumor thrombus (PVTT) and significant arterioportal or arteriohepatic vein shunts are contraindicated for TACE. Conventional TACE did not provide a survival benefit for advanced-stage patients with PVTT[71], potentially due to hepatic infarction and acute liver failure. Portal vein invasion is a major prognostic factor for reduced survival in patients with advanced HCC. Advanced HCC with PVTT in the major branches of the portal vein, particularly the main portal vein, has an extremely poor prognosis (3-4 mo without treatment[72]) because portal vein invasion may cause liver failure, massive esophageal variceal bleeding, or extensive tumor spreading throughout the liver[73]. The results of sorafenib therapy in randomized trials in Europe and Asia[5,9] did not provide a substantial survival improvement in patients with PVTT. As the incidence of patients with HCC and PVTT was reported to be between 12.5% and 39.7% at the time of diagnosis using recent imaging techniques[74], effective therapies are needed for advanced HCC patients with vascular invasion.
Combined TACE and radiotherapy (TACE + RT) consists of TACE followed by radiation therapy to the vascular invasions and has shown an overall survival benefit in patients with PVTT[75-77]. Shirai et al[75] reported that HCC patients (n = 19) with PVTT ≥ 8 cm in the first branch or main trunk that received TACE + RT had 1- and 2-year survival rates of 47.4% and 23.7%, respectively. Although prospective data comparing the efficacy of TACE + RT and sorafenib therapy in terms of response or survival in advanced-stage HCC with PVTT are not sufficient, a retrospective study[78] comparing the outcomes of TACE + RT (n = 27) and sorafenib therapy (n = 27) in a propensity score-matched cohort determined that the TACE + RT group had prolonged overall survival compared to the sorafenib group (6.7 mo vs 3.1 mo, P < 0.001). To determine the efficacy of TACE + RT compared to sorafenib in locally advanced HCC, a randomized controlled multicenter trial is necessary.
HCC has a tendency to spread to the portal vein and, to a lesser extent, the hepatic vein[79], which allows the development of arteriovenous shunts. These shunts represent the main impediment to successful TACE because anticancer drugs or mixtures of iodized oil and anticancer drugs easily pass through them[80]. Conventional TACE is therefore not effective for HCC patients with hepatic arteriovenous shunts and may even be harmful, due to the possibility of pulmonary embolism[81-83]. These patients require an effective, low-risk alternative treatment option.
RFA may be a useful treatment for HCCs < 3 cm in diameter; however, most HCCs with intratumoral arteriohepatic vein shunts are large, and RFA cannot be performed. To overcome this limitation, we performed TACE of the HCC feeding arteries with balloon occlusion of the corresponding draining hepatic vein, which was monitored by angiography and CT[34]. If the target HCC is located in the lateral segment of the liver, we recommend TACE with balloon occlusion of the left hepatic vein. If the target HCC is located in the right or middle lobe of the liver, particularly the upper portion, we recommend TACE with balloon occlusion of 2 hepatic veins, typically the right and middle hepatic veins[55]. TACE with balloon occlusion of the corresponding hepatic vein results in both significant tumor growth control and elimination of the intratumoral shunts[34]. After this modified procedure, conventional TACE can be performed for the treatment of residual HCC.
As noted above, HCC is frequently associated with arteriovenous shunts, which typically present as arterioportal shunts. Kojiro[84] analyzed 106 resected HCCs < 2 cm in diameter and found that nodular-type HCC was associated with microscopic portal invasion in up to 25% of cases. Although the presence of small arterioportal shunts does not necessarily preclude TACE therapy for unresectable HCC, larger arterioportal shunts caused by tumor invasion interfere with TACE because anticancer drugs, either alone or mixed with iodized oil, easily pass through the shunts[81,82]. Conventional TACE causes extensive embolization of the portal vein and can induce extensive ischemia of nontumorous liver parenchyma[80]. The presence of significant arterioportal shunts can also lead to liver dysfunction and portal hypertension, resulting in potentially life-threatening conditions such as rupture of the gastroesophageal varices[85-88], refractory ascites, or hepatic encephalopathy.
Several studies have treated significant arterioportal shunts by embolization of the hepatic arteries with materials such as gelatin sponges or coils, and these approaches have yielded good short-term results[89,90]. However, they do not eradicate the HCC and contribute little to patient survival[89,90]. We attempted to overcome this complication by performing TACE of tumor-feeding arteries with occlusion of the corresponding portal vein[91]. The intrahepatic portal branch was punctured under ultrasonographic guidance using an 18-gauge percutaneous transhepatic cholangiography needle. For TACE-PVO, a 5- or 8- French balloon catheter was advanced into the portal vein branch identified by direct portography and hepatic arteriography to contain the arterioportal shunt. The balloon catheter was inflated, and a mixture of lipiodol (up to 15 mL) and anticancer agents, as an emulsion or suspension, was injected via the target feeder artery until reflux into the hepatic artery was confirmed. Particles of gelatin sponge were immediately injected into the feeder artery until the target hepatic artery was occluded. If the target HCC concurrently invades the right and left branches of the portal vein, we recommend TACE with balloon occlusion of the 2 portal vein branches (Figure 3).
Figure 3.
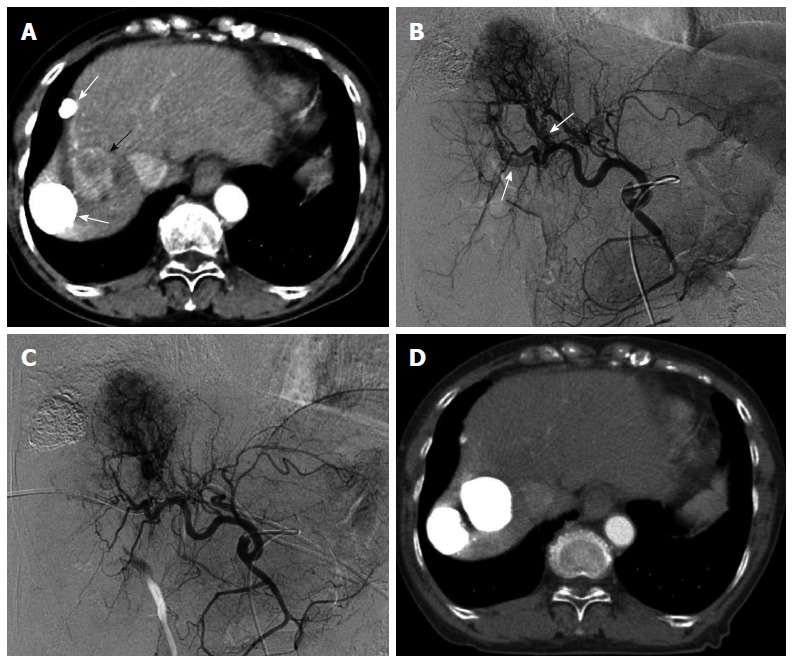
Multiple hepatocellular carcinomas with significant arterioportal shunts in a 71-year-old woman. Enhanced computed tomography (CT) imaging revealed viable hepatocellular carcinomas (HCCs) (A, black arrow) with lipiodol deposit HCCs (A, white arrows). Common hepatic arteriography in the arterial phase (B) demonstrated HCC with significant arterioportal shunts (B, arrows), consisting of P8 and P4. Common hepatic arteriography under balloon occlusion of the portal vein branches of P8 and P4 showed an opacified tumor with non-visualized arterioportal shunts (C). CT images 2 mo after transcatheter arterial chemoembolization under occlusion of the 2 portal vein branches (D) demonstrated a dense lipiodol deposit in the HCC and a complete response, according to modified Response Evaluation Criteria in Solid Tumors.
The effectiveness of TACE-PVO for arterioportal shunts was ascribed to balloon occlusion of the corresponding portal veins, resulting in adequate embolization of the entire tumor, including the portions involving arterioportal shunts. Consequently, TACE-PVO may prevent the development of collateral anastomoses to arterioportal shunts.
We performed a comparative, prospective, but not randomized study of standard transarterial embolization (TAE) or TACE versus TACE-PVO for HCC with significant arterioportal shunts[91]. Subjects differed fundamentally by patients’ choice of treatment. We found that TACE-PVO was significantly better (P = 0.009) than standard TAE for arterioportal shunt treatment, and subsequent angiographic findings suggested the superiority of TACE-PVO (P = 0.028). Antitumor response (P = 0.002) and patient outcome (P = 0.032) were significantly better in the TACE-PVO group than in the standard treatment group. Furuse et al[90] reported that HCC patients with significant arterioportal shunts due to PVTT who underwent embolization had 1- and 2-year survival rates of 12% and 0%, respectively. In our study, 1- and 2-year survival rates in the standard treatment group were 28.6% and 0%, respectively, similar to that observed by Furuse et al[90]. We obtained both a good target tumor response and dramatic improvement in arterioportal shunts with TACE-PVO therapy, with favorable 1-, 2-, and 3-year survival rates of 85.7%, 64.3%, and 42.9%, respectively[91]. This survival was markedly better than that in the previous series.
To our knowledge, no effective treatment for both HCC and significant arterioportal shunts has been previously reported. We have performed TACE-PVO on 37 patients with significant arterioportal shunts at our institution, resulting in good tumor responses and prolonged survival. Randomized controlled multicenter trials are necessary to further explore differences in quality of life and to assess the effects of TACE-PVO on short- and long-term outcomes.
Finally, we introduce a specialized embolization technique for HCC with arterioportal shunt. A patient presented to our institution with multiple HCCs and underwent several sessions of TACE. Following therapy, 1 HCC, fed by a cystic artery with an arterioportal shunt, remained. We attempted superselective TACE-PVO via the branch of the cystic artery, thus avoiding embolization; however, the procedure was not successful (Figure 4). We then performed chemoembolization via the portal vein branch with an arterioportal shunt during balloon occlusion of the portal vein (TPCE) and observed a dense accumulation of lipiodol in the tumor. Angiography 3 mo after the TPCE procedure revealed no arterioportal shunt, and conventional TACE was performed for the residual HCC. To our knowledge, this is the first report of successful TPCE, and this technique may be a useful and safe method for selected patients with HCC and arterioportal shunts.
Figure 4.
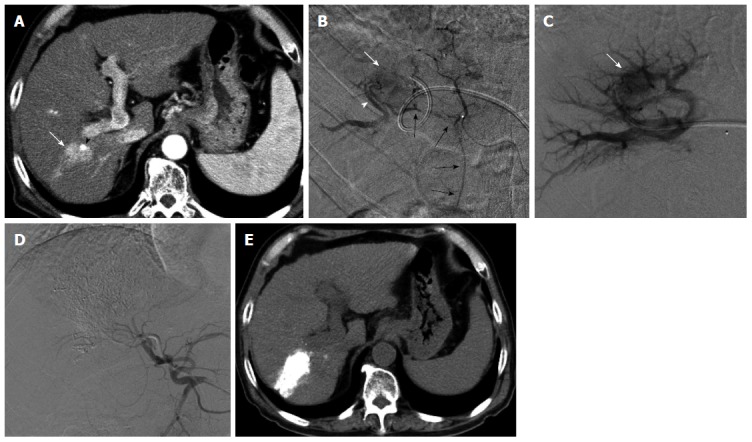
Transportal chemoembolization for hepatocellular carcinoma with an arterioportal shunt in a 70-year-old man. Enhanced abdominal computed tomography (CT) examination (A) demonstrated a recurrent hepatocellular carcinoma (HCC) (A, arrow) in the posterior segment with a lipiodol deposit (arrowhead). Hepatic arteriography via the anterior branch (B) revealed a HCC (B, white arrow) fed by the cystic artery (B, black arrows) and an intratumoral arterioportal shunt (B, arrowhead). A 5-French sheath was inserted into the portal vein via the lateral superior branch of the left portal vein. Direct portography via the portal vein branch contributed an arterioportal shunt showed a tumor stain (C, arrow). Following transcatheter portal chemoembolization, proper hepatic arteriography showed non-visualized tumor stain and arterioportal shunt (D). Non-enhanced CT 7 d after therapy (E) showed a dense accumulation of lipiodol in the tumor and peritumoral liver parenchyma.
Isolated hepatic perfusion (IHP) is an attractive option for achieving higher tumor exposure to any given agent while avoiding systemic toxicity by decreasing systemic drug exposure. Surgical IHP has shown promising results[92-94]; however, this technique is therapeutically limited because it requires an aggressive surgical intervention that is associated with considerable morbidity and mortality. Further, this technique is not amenable to repeated interventions, mainly due to the formation of adhesions[92,94,95]. Therefore, several studies have attempted to develop safe and repeatable percutaneous IHP techniques without using laparotomy with balloon occlusion catheters. Ku et al[96] reported on advanced-stage HCC patients (n = 25) that underwent percutaneous IHP after reductive surgery and determined their 5-year overall survival rate to be 42%. However, percutaneous IHP techniques result in higher rates of leakage from the perfusion circuit into the systemic circulation[97-99], mainly because the distance between the right atrium and hepatic vein origins is often too short to allow balloon occlusion of the suprahepatic inferior vena cava (IVC) above the hepatic veins without occluding the hepatic veins themselves[97,99]. IHP involves an orthograde approach, employing inflow via the hepatic artery and outflow via the IVC through the hepatic veins. All non-surgical orthograde IHP techniques may be associated with this problem, including combinations of orthograde percutaneous and surgical IHP techniques that employ a double-balloon catheter for IVC occlusion.
To overcome this disadvantage, we developed a percutaneous IHP circulation system - retrograde-outflow percutaneous IHP (R-PIHP) - by redirecting hepatic outflow through the portal vein[100,101] based on hepatic circulation under hepatic vein occlusion[53,54]. This technique inhibits systemic leakage and maintains a high concentration of drug delivery to the liver. A phase I/II trial of R-PIHP for unresectable liver malignancies, including HCC, was begun 3 years ago, and we have obtained good tumor responses and survival benefits although R-PIHP recommends expert interventional techniques (Figure 5).
Figure 5.
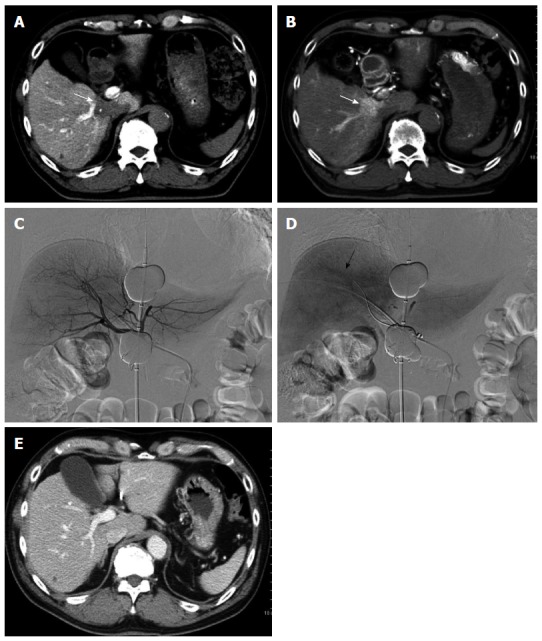
Retrograde-outflow percutaneous isolated hepatic perfusion for multiple hepatocellular carcinomas in a 64-year-old man. A patients received 2 sessions of transcatheter arterial chemoembolization for multiple hepatocellular carcinomas (HCCs) at other hospital. Computed tomography (CT) during arterial portography (A) and hepatic arteriography (B) demonstrated multiple HCCs with portal vein invasion (arrows). Retrograde-outflow percutaneous isolated hepatic perfusion (C, arterial phase; D, venous phase) was performed twice (arrow of D indicated a portal branch), and enhanced CT imaging 6 mo after therapy (E) revealed that the HCC was diminished.
Unresectable HCC usually presents as a hypervascular tumor. TACE results in both tumor hypoxia and longer activity periods for anticancer drugs remaining in the tumor tissues. However, TACE also induces a post-treatment surge of angiogenic factors, such as vascular endothelial growth factor (VEGF), which can occur as early as a few hours post-TACE[102]. This process may contribute to the revascularization of the tumor, thus reducing the efficacy of TACE[103,104]. Sorafenib is an antiangiogenic drug that blocks tumor cell proliferation and angiogenesis by inhibiting the activity of VEGF receptors. Combining sorafenib with TACE may potentially improve the treatment outcome[105]. Several studies have evaluated the efficacy and safety of this combination treatment[106,107], and clinical trials investigating this therapeutic approach are currently ongoing. The dosing schedule of antiangiogenic drugs in relation to TACE is a key factor for this method of therapy. A randomized phase III study comparing the advent of sorafenib treatment with placebo 1-3 mo after TACE failed to show a survival benefit[106]. In contrast, a single-arm phase II study with sorafenib beginning 1 wk after TACE reported a disease control rate of 95%[107], according to the response evaluation criteria for solid tumors. Optimal scheduling of antiangiogenic agents with TACE is essential to improve the patient prognosis. Moreover, if locoregional treatments are superior to conventional TACE, the combination of sorafenib with these other interventional treatments, such as DEB-TACE or radioembolization, is also promising.
CONCLUSION
In this review, we have summarized the efforts of many studies to improve the treatment outcomes of patients with intermediate- or advanced-stage HCC. For intermediate-stage HCC patients, interventional treatments, such as TACE, drug-eluting beads embolization, radioembolization, and combination therapies, for prognostic improvement are continually evolving. For advanced-stage HCC patients, sorafenib chemotherapy, TACE-based therapies combined with sorafenib treatment, TACE with radiotherapy, TACE based on hepatic hemodynamics, and IHP are all effective therapies. In conclusion, the evolution of therapy continues to improve the prognosis of patients with unresectable HCC.
Footnotes
P- Reviewer: Liaskou E S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera R, Nelson DR. Review article: the management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;31:461–476. doi: 10.1111/j.1365-2036.2009.04200.x. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 7.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 8.Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 9.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 10.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura H, Hashimoto T, Oi H, Sawada S, Furui S, Mizumoto S, Monden M. Treatment of hepatocellular carcinoma by segmental hepatic artery injection of adriamycin-in-oil emulsion with overflow to segmental portal veins. Acta Radiol. 1990;31:347–349. [PubMed] [Google Scholar]
- 13.Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1989;170:783–786. doi: 10.1148/radiology.170.3.2536946. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa M, Saisho H, Ebara M, Iijima T, Iwama S, Endo F, Kimura M, Shimamura Y, Suzuki Y, Nakano T. A randomized trial of intrahepatic arterial infusion of 4’-epidoxorubicin with Lipiodol versus 4’-epidoxorubicin alone in the treatment of hepatocellular carcinoma. Cancer Chemother Pharmacol. 1994;33 Suppl:S149–S152. doi: 10.1007/BF00686689. [DOI] [PubMed] [Google Scholar]
- 15.Yoon CJ, Chung JW, Park JH, Yoon YH, Lee JW, Jeong SY, Chung H. Transcatheter arterial chemoembolization with paclitaxel-lipiodol solution in rabbit VX2 liver tumor. Radiology. 2003;229:126–131. doi: 10.1148/radiol.2291021029. [DOI] [PubMed] [Google Scholar]
- 16.Takayasu K, Shima Y, Muramatsu Y, Moriyama N, Yamada T, Makuuchi M, Hasegawa H, Hirohashi S. Hepatocellular carcinoma: treatment with intraarterial iodized oil with and without chemotherapeutic agents. Radiology. 1987;163:345–351. doi: 10.1148/radiology.163.2.3031724. [DOI] [PubMed] [Google Scholar]
- 17.Berger DH, Carrasco CH, Hohn DC, Curley SA. Hepatic artery chemoembolization or embolization for primary and metastatic liver tumors: post-treatment management and complications. J Surg Oncol. 1995;60:116–121. doi: 10.1002/jso.2930600210. [DOI] [PubMed] [Google Scholar]
- 18.Farinati F, De Maria N, Marafin C, Herszènyi L, Del Prato S, Rinaldi M, Perini L, Cardin R, Naccarato R. Unresectable hepatocellular carcinoma in cirrhosis: survival, prognostic factors, and unexpected side effects after transcatheter arterial chemoembolization. Dig Dis Sci. 1996;41:2332–2339. doi: 10.1007/BF02100123. [DOI] [PubMed] [Google Scholar]
- 19.Okuda K, Obata H, Nakajima Y, Ohtsuki T, Okazaki N, Ohnishi K. Prognosis of primary hepatocellular carcinoma. Hepatology. 1984;4:3S–6S. doi: 10.1002/hep.1840040703. [DOI] [PubMed] [Google Scholar]
- 20.Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Prognostic factors for survival in patients with unresectable hepatocellular carcinoma undergoing chemoembolization with doxorubicin drug-eluting beads: a preliminary study. HPB (Oxford) 2010;12:174–180. doi: 10.1111/j.1477-2574.2009.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji SK, Cho YK, Ahn YS, Kim MY, Park YO, Kim JK, Kim WT. Multivariate analysis of the predictors of survival for patients with hepatocellular carcinoma undergoing transarterial chemoembolization: focusing on superselective chemoembolization. Korean J Radiol. 2008;9:534–540. doi: 10.3348/kjr.2008.9.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez RR, Pan SH, Hoffman AL, Ramirez C, Rojter SE, Ramos H, McMonigle M, Lois J. Comparison of transarterial chemoembolization in patients with unresectable, diffuse vs focal hepatocellular carcinoma. Arch Surg. 2002;137:653–657; discussion 657-658. doi: 10.1001/archsurg.137.6.653. [DOI] [PubMed] [Google Scholar]
- 23.Savastano S, Miotto D, Casarrubea G, Teso S, Chiesura-Corona M, Feltrin GP. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with Child’s grade A or B cirrhosis: a multivariate analysis of prognostic factors. J Clin Gastroenterol. 1999;28:334–340. doi: 10.1097/00004836-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto K, Shimizu T, Narabayashi I. Intraarterial infusion chemotherapy with lipiodol-CDDP suspension for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2000;23:26–39. doi: 10.1007/s002709910005. [DOI] [PubMed] [Google Scholar]
- 25.Demachi H, Matsui O, Abo H, Tatsu H. Simulation model based on non-newtonian fluid mechanics applied to the evaluation of the embolic effect of emulsions of iodized oil and anticancer drug. Cardiovasc Intervent Radiol. 2000;23:285–290. doi: 10.1007/s002700010070. [DOI] [PubMed] [Google Scholar]
- 26.Mine T, Murata S, Ueda T, Onozawa S, Onda M, Naito Z, Kumita S. Comparative study of cisplatin-iodized oil suspension and emulsion for transcatheter arterial chemoembolization of rabbit VX2 liver tumors. Hepatol Res. 2012;42:473–481. doi: 10.1111/j.1872-034X.2011.00942.x. [DOI] [PubMed] [Google Scholar]
- 27.Ueda T, Murata S, Mine T, Onozawa S, Onda M, Naito Z, Amano Y, Kumita S. Comparison of epirubicin-iodized oil suspension and emulsion for transcatheter arterial chemoembolization in VX2 tumor. ScientificWorldJournal. 2012;2012:961986. doi: 10.1100/2012/961986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Baere T, Dufaux J, Roche A, Counnord JL, Berthault MF, Denys A, Pappas P. Circulatory alterations induced by intra-arterial injection of iodized oil and emulsions of iodized oil and doxorubicin: experimental study. Radiology. 1995;194:165–170. doi: 10.1148/radiology.194.1.7997545. [DOI] [PubMed] [Google Scholar]
- 29.Kan Z, Sato M, Ivancev K, Uchida B, Hedgpeth P, Lunderquist A, Rosch J, Yamada R. Distribution and effect of iodized poppyseed oil in the liver after hepatic artery embolization: experimental study in several animal species. Radiology. 1993;186:861–866. doi: 10.1148/radiology.186.3.8381552. [DOI] [PubMed] [Google Scholar]
- 30.Miller DL, O’Leary TJ, Girton M. Distribution of iodized oil within the liver after hepatic arterial injection. Radiology. 1987;162:849–852. doi: 10.1148/radiology.162.3.3027747. [DOI] [PubMed] [Google Scholar]
- 31.Kasai K, Ushio A, Kasai Y, Sawara K, Miyamoto Y, Oikawa K, Takikawa Y, Suzuki K. Therapeutic efficacy of transarterial chemo-embolization with a fine-powder formulation of cisplatin for hepatocellular carcinoma. World J Gastroenterol. 2013;19:2242–2248. doi: 10.3748/wjg.v19.i14.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yodono H, Matsuo K, Shinohara A. A retrospective comparative study of epirubicin-lipiodol emulsion and cisplatin-lipiodol suspension for use with transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma. Anticancer Drugs. 2011;22:277–282. doi: 10.1097/cad.0b013e328342231d. [DOI] [PubMed] [Google Scholar]
- 33.Iwazawa J, Ohue S, Hashimoto N, Mitani T. Local tumor progression following lipiodol-based targeted chemoembolization of hepatocellular carcinoma: a retrospective comparison of miriplatin and epirubicin. Cancer Manag Res. 2012;4:113–119. doi: 10.2147/CMAR.S30431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murata S, Mine T, Ueda T, Nakazawa K, Onozawa S, Yasui D, Kumita S. Transcatheter arterial chemoembolization based on hepatic hemodynamics for hepatocellular carcinoma. ScientificWorldJournal. 2013;2013:479805. doi: 10.1155/2013/479805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, Dourakis S, Delis S, Gouliamos A, Kelekis D. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol. 2008;31:269–280. doi: 10.1007/s00270-007-9226-z. [DOI] [PubMed] [Google Scholar]
- 37.Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12:2563–2567. doi: 10.1158/1078-0432.CCR-05-2225. [DOI] [PubMed] [Google Scholar]
- 38.Guiu B, Deschamps F, Aho S, Munck F, Dromain C, Boige V, Malka D, Leboulleux S, Ducreux M, Schlumberger M, et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol. 2012;56:609–617. doi: 10.1016/j.jhep.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ibrahim SM, Lewandowski RJ, Sato KT, Gates VL, Kulik L, Mulcahy MF, Ryu RK, Omary RA, Salem R. Radioembolization for the treatment of unresectable hepatocellular carcinoma: a clinical review. World J Gastroenterol. 2008;14:1664–1669. doi: 10.3748/wjg.14.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464–473. doi: 10.1016/j.jhep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Lance C, McLennan G, Obuchowski N, Cheah G, Levitin A, Sands M, Spain J, Srinivas S, Shrikanthan S, Aucejo FN, et al. Comparative analysis of the safety and efficacy of transcatheter arterial chemoembolization and yttrium-90 radioembolization in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1697–1705. doi: 10.1016/j.jvir.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Nalesnik MA, Federle M, Buck D, Fontes P, Carr BI. Hepatobiliary effects of 90yttrium microsphere therapy for unresectable hepatocellular carcinoma. Hum Pathol. 2009;40:125–134. doi: 10.1016/j.humpath.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 44.Jakobs TF, Saleem S, Atassi B, Reda E, Lewandowski RJ, Yaghmai V, Miller F, Ryu RK, Ibrahim S, Sato KT, et al. Fibrosis, portal hypertension, and hepatic volume changes induced by intra-arterial radiotherapy with 90yttrium microspheres. Dig Dis Sci. 2008;53:2556–2563. doi: 10.1007/s10620-007-0148-z. [DOI] [PubMed] [Google Scholar]
- 45.Kim RD, Kim JS, Watanabe G, Mohuczy D, Behrns KE. Liver regeneration and the atrophy-hypertrophy complex. Semin Intervent Radiol. 2008;25:92–103. doi: 10.1055/s-2008-1076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaba RC, Lewandowski RJ, Kulik LM, Riaz A, Ibrahim SM, Mulcahy MF, Ryu RK, Sato KT, Gates V, Abecassis MM, et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587–1596. doi: 10.1245/s10434-009-0454-0. [DOI] [PubMed] [Google Scholar]
- 47.Theysohn JM, Ertle J, Müller S, Schlaak JF, Nensa F, Sipilae S, Bockisch A, Lauenstein TC. Hepatic volume changes after lobar selective internal radiation therapy (SIRT) of hepatocellular carcinoma. Clin Radiol. 2014;69:172–178. doi: 10.1016/j.crad.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Cheng BQ, Jia CQ, Liu CT, Fan W, Wang QL, Zhang ZL, Yi CH. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299:1669–1677. doi: 10.1001/jama.299.14.1669. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura H, Tanaka T, Hori S, Yoshioka H, Kuroda C, Okamura J, Sakurai M. Transcatheter embolization of hepatocellular carcinoma: assessment of efficacy in cases of resection following embolization. Radiology. 1983;147:401–405. doi: 10.1148/radiology.147.2.6300959. [DOI] [PubMed] [Google Scholar]
- 50.Uchida H, Matsuo N, Sakaguchi H, Nagano N, Nishimine K, Ohishi H. Segmental embolotherapy for hepatic cancer: keys to success. Cardiovasc Intervent Radiol. 1993;16:67–71. doi: 10.1007/BF02602980. [DOI] [PubMed] [Google Scholar]
- 51.Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, Miyayama S, Takashima T, Unoura M, Kogayashi K. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79–83. doi: 10.1148/radiology.188.1.8390073. [DOI] [PubMed] [Google Scholar]
- 52.Kanazawa S, Wright KC, Kasi LP, Charnsangavej C, Wallace S. Preliminary experimental evaluation of temporary segmental hepatic venous occlusion: angiographic, pathologic, and scintigraphic findings. J Vasc Interv Radiol. 1993;4:759–766. doi: 10.1016/s1051-0443(93)71969-7. [DOI] [PubMed] [Google Scholar]
- 53.Murata S, Itai Y, Asato M, Kobayashi H, Nakajima K, Eguchi N, Saida Y, Kuramoto K, Tohno E. Effect of temporary occlusion of the hepatic vein on dual blood in the liver: evaluation with spiral CT. Radiology. 1995;197:351–356. doi: 10.1148/radiology.197.2.7480676. [DOI] [PubMed] [Google Scholar]
- 54.Murata S, Itai Y, Satake M, Asato M, Kobayashi H, Eguchi N, Moriyama N. Changes in contrast enhancement of hepatocellular carcinoma and liver: effect of temporary occlusion of a hepatic vein evaluated with spiral CT. Radiology. 1997;202:715–720. doi: 10.1148/radiology.202.3.9051023. [DOI] [PubMed] [Google Scholar]
- 55.Murata S, Tajima H, Abe Y, Fukunaga T, Nakazawa K, Mohamad RA, Kumazaki T. Temporary occlusion of two hepatic veins for chemoembolization of hepatocellular carcinoma with arteriohepatic vein shunts. AJR Am J Roentgenol. 2005;184:415–417. doi: 10.2214/ajr.184.2.01840415. [DOI] [PubMed] [Google Scholar]
- 56.Itai Y, Moss AA, Goldberg HI. Transient hepatic attenuation difference of lobar or segmental distribution detected by dynamic computed tomography. Radiology. 1982;144:835–839. doi: 10.1148/radiology.144.4.6287520. [DOI] [PubMed] [Google Scholar]
- 57.Mathieu D, Vasile N, Grenier P. Portal thrombosis: dynamic CT features and course. Radiology. 1985;154:737–741. doi: 10.1148/radiology.154.3.3881793. [DOI] [PubMed] [Google Scholar]
- 58.Irie T, Terahata S, Hatsuse K, Takeshita K, Yamauchi T, Aoki H, Kusano S. Postsurgical intrahepatic portal thromboembolism: a possible cause of perfusion defects on CT during arterial portography. J Comput Assist Tomogr. 1995;19:204–210. [PubMed] [Google Scholar]
- 59.Matsui O, Takashima T, Kadoya M, Kitagawa K, Kamimura R, Itoh H, Suzuki M, Ida M. Segmental staining on hepatic arteriography as a sign of intrahepatic portal vein obstruction. Radiology. 1984;152:601–606. doi: 10.1148/radiology.152.3.6087404. [DOI] [PubMed] [Google Scholar]
- 60.Itai Y, Ohtomo K, Kokubo T, Okada Y, Yamauchi T, Yoshida H. Segmental intensity differences in the liver on MR images: a sign of intrahepatic portal flow stoppage. Radiology. 1988;167:17–19. doi: 10.1148/radiology.167.1.2831561. [DOI] [PubMed] [Google Scholar]
- 61.Itai Y, Murata S, Kurosaki Y. Straight border sign of the liver: spectrum of CT appearances and causes. Radiographics. 1995;15:1089–1102. doi: 10.1148/radiographics.15.5.7501852. [DOI] [PubMed] [Google Scholar]
- 62.Itai Y, Matsui O. Blood flow and liver imaging. Radiology. 1997;202:306–314. doi: 10.1148/radiology.202.2.9015047. [DOI] [PubMed] [Google Scholar]
- 63.Komada Y, Murata S, Tajima H, Kumita S, Kanazawa H, Tajiri T. Haemodynamic changes in the liver under balloon occlusion of a portal vein branch: evaluation with single-level dynamic computed tomography during hepatic arteriography. Clin Radiol. 2007;62:579–586. doi: 10.1016/j.crad.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 64.Lautt WW, Legare DJ, Ezzat WR. Quantitation of the hepatic arterial buffer response to graded changes in portal blood flow. Gastroenterology. 1990;98:1024–1028. doi: 10.1016/0016-5085(90)90029-z. [DOI] [PubMed] [Google Scholar]
- 65.Rocheleau B, Ethier C, Houle R, Huet PM, Bilodeau M. Hepatic artery buffer response following left portal vein ligation: its role in liver tissue homeostasis. Am J Physiol. 1999;277:G1000–G1007. doi: 10.1152/ajpgi.1999.277.5.G1000. [DOI] [PubMed] [Google Scholar]
- 66.Mücke I, Richter S, Menger MD, Vollmar B. Significance of hepatic arterial responsiveness for adequate tissue oxygenation upon portal vein occlusion in cirrhotic livers. Int J Colorectal Dis. 2000;15:335–341. doi: 10.1007/s003840000247. [DOI] [PubMed] [Google Scholar]
- 67.Yamada R, Yamaguchi S, Nakatsuka H, Nakamura K, Sato M, Kobayashi N, Takashima S, Sangen H. [Balloon-occluded arterial infusion--a new method for administration of anticancer drugs (author’s transl)] Nihon Igaku Hoshasen Gakkai Zasshi. 1981;41:894–896. [PubMed] [Google Scholar]
- 68.Ishizaka H, Ishijima H, Katsuya T, Horikoshi H, Koyama Y. Compulsory superselective arterial embolization in hypovascular local hepatic tumor ablation. Microballoon coaxial catheterization. Acta Radiol. 1997;38:836–839. doi: 10.1080/02841859709172420. [DOI] [PubMed] [Google Scholar]
- 69.Irie T, Kuramochi M, Takahashi N. Dense accumulation of lipiodol emulsion in hepatocellular carcinoma nodule during selective balloon-occluded transarterial chemoembolization: measurement of balloon-occluded arterial stump pressure. Cardiovasc Intervent Radiol. 2013;36:706–713. doi: 10.1007/s00270-012-0476-z. [DOI] [PubMed] [Google Scholar]
- 70.Koizumi J, Kurata T, Yamashita T, Kominami M, Fujiwara H, Narimatsu Y, Hiramatsu K. Computed tomography during arterial portography under temporary balloon occlusion of the hepatic artery: evaluation of pseudolesions caused by arterio-portal venous shunts. Abdom Imaging. 2000;25:583–586. [PubMed] [Google Scholar]
- 71.Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, Vilana R, Rodés J. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578–1583. doi: 10.1002/hep.510270617. [DOI] [PubMed] [Google Scholar]
- 72.Villa E, Moles A, Ferretti I, Buttafoco P, Grottola A, Del Buono M, De Santis M, Manenti F. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32:233–238. doi: 10.1053/jhep.2000.9603. [DOI] [PubMed] [Google Scholar]
- 73.Lladó L, Virgili J, Figueras J, Valls C, Dominguez J, Rafecas A, Torras J, Fabregat J, Guardiola J, Jaurrieta E. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50–57. doi: 10.1002/(sici)1097-0142(20000101)88:1<50::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 74.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7567. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shirai S, Sato M, Suwa K, Kishi K, Shimono C, Sonomura T, Kawai N, Tanihata H, Minamiguchi H, Nakai M. Feasibility and efficacy of single photon emission computed tomography-based three-dimensional conformal radiotherapy for hepatocellular carcinoma 8 cm or more with portal vein tumor thrombus in combination with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2010;76:1037–1044. doi: 10.1016/j.ijrobp.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 76.Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005;25:1189–1196. doi: 10.1111/j.1478-3231.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 77.Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 78.Cho JY, Paik YH, Park HC, Yu JI, Sohn W, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, et al. The feasibility of combined transcatheter arterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma. Liver Int. 2014;34:795–801. doi: 10.1111/liv.12445. [DOI] [PubMed] [Google Scholar]
- 79.Okuda K, Musha H, Yamasaki T, Jinnouchi S, Nagasaki Y, Kubo Y, Shimokawa Y, Nakayama T, Kojiro M, Sakamoto K, et al. Angiographic demonstration of intrahepatic arterio-portal anastomoses in hepatocellular carcinoma. Radiology. 1977;122:53–58. doi: 10.1148/122.1.53. [DOI] [PubMed] [Google Scholar]
- 80.Sugano S, Miyoshi K, Suzuki T, Kawafune T, Kubota M. Intrahepatic arteriovenous shunting due to hepatocellular carcinoma and cirrhosis, and its change by transcatheter arterial embolization. Am J Gastroenterol. 1994;89:184–188. [PubMed] [Google Scholar]
- 81.Bledin AG, Kantarjian HM, Kim EE, Wallace S, Chuang VP, Patt YZ, Haynie TP. 99mTc-labeled macroaggregated albumin in intrahepatic arterial chemotherapy. AJR Am J Roentgenol. 1982;139:711–715. doi: 10.2214/ajr.139.4.711. [DOI] [PubMed] [Google Scholar]
- 82.Ziessman HA, Thrall JH, Yang PJ, Walker SC, Cozzi EA, Niederhuber JE, Gyves JW, Ensminger WD, Tuscan MC. Hepatic arterial perfusion scintigraphy with Tc-99m-MAA. Use of a totally implanted drug delivery system. Radiology. 1984;152:167–172. doi: 10.1148/radiology.152.1.6233632. [DOI] [PubMed] [Google Scholar]
- 83.Lai CL, Wu PC, Chan GC, Lok AS, Lin HJ. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer. 1988;62:479–483. doi: 10.1002/1097-0142(19880801)62:3<479::aid-cncr2820620306>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 84.Kojiro M. Focus on dysplastic nodules and early hepatocellular carcinoma: an Eastern point of view. Liver Transpl. 2004;10:S3–S8. doi: 10.1002/lt.20042. [DOI] [PubMed] [Google Scholar]
- 85.Lazaridis KN, Kamath PS. Images in hepatology. Arterio-portal fistula causing recurrent variceal bleeding. J Hepatol. 1998;29:142. doi: 10.1016/s0168-8278(98)80189-x. [DOI] [PubMed] [Google Scholar]
- 86.Okuyama M, Fujiwara Y, Hayakawa T, Shiba M, Watanabe T, Tominaga K, Tamori A, Oshitani N, Higuchi K, Matsumoto T, et al. Esophagogastric varices due to arterioportal shunt in a serous cystadenoma of the pancreas in von Hippel-Lindau disease. Dig Dis Sci. 2003;48:1948–1954. doi: 10.1023/a:1026262019552. [DOI] [PubMed] [Google Scholar]
- 87.Morse SS, Sniderman KW, Galloway S, Rapoport S, Ross GR, Glickman MG. Hepatoma, arterioportal shunting, and hyperkinetic portal hypertension: therapeutic embolization. Radiology. 1985;155:77–82. doi: 10.1148/radiology.155.1.2983375. [DOI] [PubMed] [Google Scholar]
- 88.Velázquez RF, Rodríguez M, Navascués CA, Linares A, Pérez R, Sotorríos NG, Martínez I, Rodrigo L. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520–527. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]
- 89.Tarazov PG. Intrahepatic arterioportal fistulae: role of transcatheter embolization. Cardiovasc Intervent Radiol. 1993;16:368–373. doi: 10.1007/BF02603142. [DOI] [PubMed] [Google Scholar]
- 90.Furuse J, Iwasaki M, Yoshino M, Konishi M, Kawano N, Kinoshita T, Ryu M, Satake M, Moriyama N. Hepatocellular carcinoma with portal vein tumor thrombus: embolization of arterioportal shunts. Radiology. 1997;204:787–790. doi: 10.1148/radiology.204.3.9280260. [DOI] [PubMed] [Google Scholar]
- 91.Murata S, Tajima H, Nakazawa K, Onozawa S, Kumita S, Nomura K. Initial experience of transcatheter arterial chemoembolization during portal vein occlusion for unresectable hepatocellular carcinoma with marked arterioportal shunts. Eur Radiol. 2009;19:2016–2023. doi: 10.1007/s00330-009-1349-y. [DOI] [PubMed] [Google Scholar]
- 92.Vahrmeijer AL, van Dierendonck JH, Keizer HJ, Beijnen JH, Tollenaar RA, Pijl ME, Marinelli A, Kuppen PJ, van Bockel JH, Mulder GJ, et al. Increased local cytostatic drug exposure by isolated hepatic perfusion: a phase I clinical and pharmacologic evaluation of treatment with high dose melphalan in patients with colorectal cancer confined to the liver. Br J Cancer. 2000;82:1539–1546. doi: 10.1054/bjoc.2000.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bartlett DL, Libutti SK, Figg WD, Fraker DL, Alexander HR. Isolated hepatic perfusion for unresectable hepatic metastases from colorectal cancer. Surgery. 2001;129:176–187. doi: 10.1067/msy.2001.110365. [DOI] [PubMed] [Google Scholar]
- 94.Alexander HR, Bartlett DL, Libutti SK, Pingpank JF, Fraker DL, Royal R, Steinberg SM, Helsabeck CB, Beresneva TH. Analysis of factors associated with outcome in patients undergoing isolated hepatic perfusion for unresectable liver metastases from colorectal center. Ann Surg Oncol. 2009;16:1852–1859. doi: 10.1245/s10434-009-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Iersel LB, Gelderblom H, Vahrmeijer AL, van Persijn van Meerten EL, Tijl FG, Putter H, Hartgrink HH, Kuppen PJ, Nortier JW, Tollenaar RA, et al. Isolated hepatic melphalan perfusion of colorectal liver metastases: outcome and prognostic factors in 154 patients. Ann Oncol. 2008;19:1127–1134. doi: 10.1093/annonc/mdn032. [DOI] [PubMed] [Google Scholar]
- 96.Ku Y, Iwasaki T, Tominaga M, Fukumoto T, Takahashi T, Kido M, Ogata S, Takahashi M, Kuroda Y, Matsumoto S, et al. Reductive surgery plus percutaneous isolated hepatic perfusion for multiple advanced hepatocellular carcinoma. Ann Surg. 2004;239:53–60. doi: 10.1097/01.sla.0000103133.03688.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Savier E, Azoulay D, Huguet E, Lokiec F, Gil-Delgado M, Bismuth H. Percutaneous isolated hepatic perfusion for chemotherapy: a phase 1 study. Arch Surg. 2003;138:325–332. doi: 10.1001/archsurg.138.3.325. [DOI] [PubMed] [Google Scholar]
- 98.Pingpank JF, Libutti SK, Chang R, Wood BJ, Neeman Z, Kam AW, Figg WD, Zhai S, Beresneva T, Seidel GD, et al. Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J Clin Oncol. 2005;23:3465–3474. doi: 10.1200/JCO.2005.00.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rothbarth J, Tollenaar RA, Schellens JH, Nortier JW, Kool LJ, Kuppen PJ, Mulder GJ, van de Velde CJ. Isolated hepatic perfusion for the treatment of colorectal metastases confined to the liver: recent trends and perspectives. Eur J Cancer. 2004;40:1812–1824. doi: 10.1016/j.ejca.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 100.Murata S, Ivancev K, Jeppsson B, Lunderquist A. Hemodynamic changes in the liver during complete hepatic venous occlusion: applicability in treatment of hepatic malignancy. Cardiovasc Intervent Radiol. 1998:99 (Suppl): 116. [Google Scholar]
- 101.Murata S, Jeppsson B, Lunderquist A, Ivancev K. Hemodynamics in rat liver tumor model during retrograde-outflow isolated hepatic perfusion with aspiration from the portal vein: angiography and in vivo microscopy. Acta Radiol. 2013;55:737–744. doi: 10.1177/0284185113505258. [DOI] [PubMed] [Google Scholar]
- 102.Chan SL, Mok T, Ma BB. Management of hepatocellular carcinoma: beyond sorafenib. Curr Oncol Rep. 2012;14:257–266. doi: 10.1007/s11912-012-0233-0. [DOI] [PubMed] [Google Scholar]
- 103.Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878–2882. doi: 10.3748/wjg.v10.i19.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523–529. doi: 10.1080/02841850801958890. [DOI] [PubMed] [Google Scholar]
- 105.Jiang H, Meng Q, Tan H, Pan S, Sun B, Xu R, Sun X. Antiangiogenic therapy enhances the efficacy of transcatheter arterial embolization for hepatocellular carcinomas. Int J Cancer. 2007;121:416–424. doi: 10.1002/ijc.22655. [DOI] [PubMed] [Google Scholar]
- 106.Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–2127. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29:3960–3967. doi: 10.1200/JCO.2011.37.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]


