Abstract
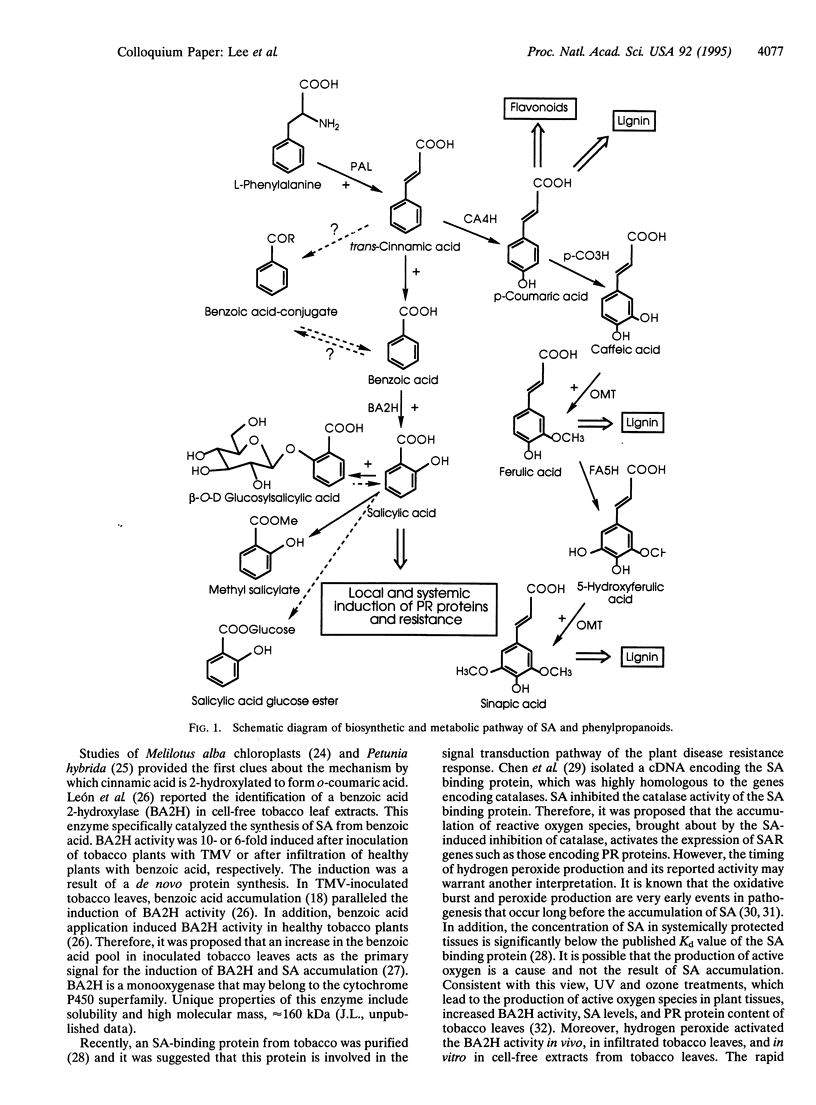
Pathways of salicylic acid (SA) biosynthesis and metabolism in tobacco have been recently identified. SA, an endogenous regulator of disease resistance, is a product of phenylpropanoid metabolism formed via decarboxylation of trans-cinnamic acid to benzoic acid and its subsequent 2-hydroxylation to SA. In tobacco mosaic virus-inoculated tobacco leaves, newly synthesized SA is rapidly metabolized to SA O-beta-D-glucoside and methyl salicylate. Two key enzymes involved in SA biosynthesis and metabolism: benzoic acid 2-hydroxylase, which converts benzoic acid to SA, and UDPglucose:SA glucosyltransferase (EC 2.4.1.35), which catalyzes conversion of SA to SA glucoside have been partially purified and characterized. Progress in enzymology and molecular biology of SA biosynthesis and metabolism will provide a better understanding of signal transduction pathway involved in plant disease resistance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alibert G., Ranjeva R. Recherches sur les enzymes catalysant la biosynthese des acides phénoliques chez Quercus pedunculata (EHRH.): I - Formation des premiers termes des series cinnamique et benzöique. FEBS Lett. 1971 Nov 15;19(1):11–14. doi: 10.1016/0014-5793(71)80593-8. [DOI] [PubMed] [Google Scholar]
- Apostol I., Heinstein P. F., Low P. S. Rapid Stimulation of an Oxidative Burst during Elicitation of Cultured Plant Cells : Role in Defense and Signal Transduction. Plant Physiol. 1989 May;90(1):109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzobohatý B., Moore I., Kristoffersen P., Bako L., Campos N., Schell J., Palme K. Release of active cytokinin by a beta-glucosidase localized to the maize root meristem. Science. 1993 Nov 12;262(5136):1051–1054. doi: 10.1126/science.8235622. [DOI] [PubMed] [Google Scholar]
- COLES R. A., KNIGHT J. J. Effect of jet aircraft noise on hearing. Nature. 1959 Dec 5;184(Suppl 23):1803–1804. doi: 10.1038/1841803b0. [DOI] [PubMed] [Google Scholar]
- Cauthen W. L., Hester W. H. Accidental ingestion of oil of wintergreen. J Fam Pract. 1989 Dec;29(6):680–681. [PubMed] [Google Scholar]
- Chen Z., Ricigliano J. W., Klessig D. F. Purification and characterization of a soluble salicylic acid-binding protein from tobacco. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9533–9537. doi: 10.1073/pnas.90.20.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Silva H., Klessig D. F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993 Dec 17;262(5141):1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- Cleland C. F., Ajami A. Identification of the Flower-inducing Factor Isolated from Aphid Honeydew as being Salicylic Acid. Plant Physiol. 1974 Dec;54(6):904–906. doi: 10.1104/pp.54.6.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi A. J., Raskin I. Induction of UDP-Glucose:Salicylic Acid Glucosyltransferase Activity in Tobacco Mosaic Virus-Inoculated Tobacco (Nicotiana tabacum) Leaves. Plant Physiol. 1993 Apr;101(4):1375–1380. doi: 10.1104/pp.101.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi A. J., Yalpani N., Silverman P., Raskin I. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2480–2484. doi: 10.1073/pnas.89.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C., Brodelius P. E. Phenylpropanoid Metabolism in Suspension Cultures of Vanilla planifolia Andr. : II. Effects of Precursor Feeding and Metabolic Inhibitors. Plant Physiol. 1990 Sep;94(1):95–101. doi: 10.1104/pp.94.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestetner B., Conn E. E. The 2-hydroxylation of trans-cinnamic acid by chloroplasts from Melilotus alba Desr. Arch Biochem Biophys. 1974 Aug;163(2):617–624. doi: 10.1016/0003-9861(74)90522-0. [DOI] [PubMed] [Google Scholar]
- Glass A. D. Influence of phenolic acids on ion uptake: I. Inhibition of phosphate uptake. Plant Physiol. 1973 Jun;51(6):1037–1041. doi: 10.1104/pp.51.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig J., Malamy J., Grynkiewicz G., Indulski J., Klessig D. F. Interconversion of the salicylic acid signal and its glucoside in tobacco. Plant J. 1993 Oct;4(4):593–600. doi: 10.1046/j.1365-313x.1993.04040593.x. [DOI] [PubMed] [Google Scholar]
- Herrmann K. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit Rev Food Sci Nutr. 1989;28(4):315–347. doi: 10.1080/10408398909527504. [DOI] [PubMed] [Google Scholar]
- Leon J., Yalpani N., Raskin I., Lawton M. A. Induction of Benzoic Acid 2-Hydroxylase in Virus-Inoculated Tobacco. Plant Physiol. 1993 Oct;103(2):323–328. doi: 10.1104/pp.103.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J., Carr J. P., Klessig D. F., Raskin I. Salicylic Acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990 Nov 16;250(4983):1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Malamy J., Hennig J., Klessig D. F. Temperature-Dependent Induction of Salicylic Acid and Its Conjugates during the Resistance Response to Tobacco Mosaic Virus Infection. Plant Cell. 1992 Mar;4(3):359–366. doi: 10.1105/tpc.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J. P., Signer H., Ryals J., Ward E., Wyss-Benz M., Gaudin J., Raschdorf K., Schmid E., Blum W., Inverardi B. Increase in salicylic Acid at the onset of systemic acquired resistance in cucumber. Science. 1990 Nov 16;250(4983):1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- Qin X. F., Holuigue L., Horvath D. M., Chua N. H. Immediate early transcription activation by salicylic acid via the cauliflower mosaic virus as-1 element. Plant Cell. 1994 Jun;6(6):863–874. doi: 10.1105/tpc.6.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J., Uknes S., Ward E. Systemic Acquired Resistance. Plant Physiol. 1994 Apr;104(4):1109–1112. doi: 10.1104/pp.104.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N., Balke N. E., Schulz M. Induction of UDP-Glucose:Salicylic Acid Glucosyltransferase in Oat Roots. Plant Physiol. 1992 Nov;100(3):1114–1119. doi: 10.1104/pp.100.3.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N., Leon J., Lawton M. A., Raskin I. Pathway of Salicylic Acid Biosynthesis in Healthy and Virus-Inoculated Tobacco. Plant Physiol. 1993 Oct;103(2):315–321. doi: 10.1104/pp.103.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N., Schulz M., Davis M. P., Balke N. E. Partial purification and properties of an inducible uridine 5'-diphosphate-glucose-salicylic Acid glucosyltransferase from oat roots. Plant Physiol. 1992 Sep;100(1):457–463. doi: 10.1104/pp.100.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]



