Abstract
AIM: To determine the frequency of occult hepatitis B infection (OHBI) in a group of human immunodeficiency virus (HIV)-1+/ hepatitis B surface antigen negative (HBsAg)- patients from Mexico.
METHODS: We investigated the presence of OHBI in 49 HIV-1+/HBsAg- patients. Hepatitis B virus (HBV) DNA was analyzed using nested PCR to amplify the Core (C) region and by real-time PCR to amplify a region of the S and X genes. The possible associations between the variables and OHBI were investigated using Pearson’s χ2 and/or Fisher’s exact test.
RESULTS: We found that the frequency of OHBI was 49% among the group of 49 HIV-1+/HBsAg- patients studied. The presence of OHBI was significantly associated with the HIV-1 RNA viral load [odds ratio (OR) = 8.75; P = 0.001; 95%CI: 2.26-33.79] and with HIV-antiretroviral treatment with drugs that interfere with HBV replication (lamivudine, tenofovir or emtricitabine) (OR = 0.25; P = 0.05; 95%CI: 0.08-1.05).
CONCLUSION: The OHBI frequency is high among 49 Mexican HIV-1+/HBsAg- patients and it was more frequent in patients with detectable HIV RNA, and less frequent in patients who are undergoing HIV-ARV treatment with drugs active against HBV.
Keywords: Hepatitis B virus, Occult hepatitis B virus infection, Human immunodeficiency virus, Hepatitis B surface antigen negative, Risk factors, Molecular diagnostics
Core tip: In this study we assessed the frequency of occult hepatitis B infection (OHBI) in a group of 49 human immunodeficiency virus (HIV-1)+/ hepatitis B surface antigen negative (HBsAg)- Mexican patients using a highly sensitive in-house core-nested PCR and real-time PCR assays for hepatitis B virus (HBV) DNA detection. In this study we showed that the frequency of OHBI is high among HIV+/HBsAg- patients and suggests the need for testing HBV DNA in bigger populations utilizing more sentitive assays. Then, we propose to utilize the core-nested PCR assay in the initial screening for OHBI. Prospective studies would be needed to assess the clinical value of this diagnostic approach.
INTRODUCTION
Occult hepatitis B infection (OHBI) is characterized by the long-lasting persistence of hepatitis B virus (HBV) DNA in the liver, with detectable or undetectable HBV DNA in the serum, in the absence of HBV surface antigen (HBsAg)[1-3]. The development of more sensitive detection systems for HBV DNA has improved the ability to detect this complex entity[3]. Although OHBI has been associated with the presence of anti-HBV antibodies directed against the viral core antigen (HBc) and/or against HBsAg, a significant proportion of individuals are negative for all HBV serum markers[4,5]. Thus, patients with OHBI can be classified as seropositive (anti-HBc and/or anti-HBs+) or seronegative (anti-HBc and anti-HBs negative)[3]. Prevalence rates for OHBI ranging from < 1% to as high as 87% have been reported from different areas around the world[6-8]. It has been reported that OHBI seems to be more prevalent among subjects at high risk for HBV infection and with concomitant liver disease[9]. Human immunodeficiency virus (HIV)-infected patients are also at risk of acquiring viral hepatitis due to common routes of transmission. Several studies have investigated the prevalence and clinical impact of OHBI in HIV-1-infected individuals, and these studies have shown that co-infection is increasing worldwide[10]. However, the clinical significance of OHBI infection in HIV-infected patients remains unclear. In HIV+/HBsAg-/anti-HBc+ patients, the prevalence of serum HBV DNA is highly variable. For example, a group of injection drug users (IDUs)[11] have no detectable levels of HBV DNA, whereas in other patients, the prevalence of HBV DNA can be as high as 89.5%[12]. The discrepancies in the reported prevalence of OHBI in HIV-infected patients vary depending on the definition used for OHBI, the sensitivity of the assay and the cutoff value for the HBV viral load[13-15]. According to the Taormina statements, the gold standard for OHBI testing is the analysis of HBV DNA in extracts from liver tissues using highly sensitive and specific techniques (nested PCR or real-time PCR) with specific primers for different HBV genomic regions and with highly conserved nucleotide sequences[16]. The aim of this study was to assess the prevalence of OHBI in group of HIV-1+/HBsAg- Mexican patients, Possible associations between the presence of HBV DNA in plasma and antibodies against HBV antigens, the HIV-1 viral load (VL), and anti-retroviral treatment (ARV) that includes drugs active against HBV replication [lamivudine (3TC), tenofovir (TDF) or a combination of TDF/emtricitabine (FTC)] were investigated.
MATERIALS AND METHODS
Patients
Plasma samples were collected from 42 HIV-1-infected patients who were being treated at the La Raza Infectious Diseases Hospital, which is a tertiary referral center of the Instituto Mexicano del Seguro Social (IMSS), and from 13 patients attending Hospital Regional-72. A total samples of 55 were collected during the period of 2009-2011. The study protocol was approved by the Ethical Committee of the IMSS, and written informed consent was obtained from each participant.
HBV serological markers and determination of HIV RNA-1 viral load
An 8 mL sample of blood was drawn from each of the 55 patients with known diagnoses of HIV-1 infection. All serum samples were screened to detect the following HBV serological markers using commercial ELISA assays (Abbot Diagnostics Laboratories): HBsAg, anti-HBc (IgG), anti-HBs and HBeAg. Six of the 55 patients were co-infected with HBV (HBsAg+) and were eliminated from the study. A total of 49 samples were included for detection of OHBI. The HIV RNA viral load was determined in plasma using the Cobas Amplicor RNA HIV-1 MonitorTM test vs 1.5 (Roche Diagnostics, IN, USA), which has a known sensitivity of 50 copies/mL.
Diagnosis of OHBI infection by amplification of three HBV genome regions
According to Raimondo[2], PCR primers should be designed to span at least three genomic regions of the HBV genome to avoid false-negative or false-positive results. In this study two different approaches, a higly nested PCR assay and a quantitative qPCR with syber green were utilized in order to identify the HBV DNA in the occult HBV coinfected individuals, and confirm the utility of a simple nested assay to screen the presence of very low concentrations of HBV DNA. We tested for HBV DNA using assays specific for three HBV genomic regions, core (C), X and surface (S). To amplify the C region, we used a nested-PCR, whereas for the X and S regions, we designed primers for real-time PCR assays (qPCR).
Definition of OHBI: We considered a patient to be OHBI+ when his or her sample was positive for at least two different viral genomic regions or when positive for the C region and confirmed by sequencing.
RT-PCR for X and S genomic regions
Nucleic acids were extracted from plasma using the High Pure Viral Nucleic Acid Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. The selected X and S genomic regions were amplified using real-time quantitative PCR (qPCR) utilizing SYBR Green technology in a thermal cycler LightCycler-480 (LC480) (Roche Diagnostics, Mannheim, Germany). The following specific primers were designed to specifically amplify a 277 bp fragment of the X-ORF region (nt 1601-1877): forward HBVOX-1 (5′-ACGTCGCATGGAGACCACCG-3′) and reverse HBVOX-2 (5′-GCTTGGAGGCTTGAACAGTGGGA-3′). For the S-ORF, a 145 bp fragment (nt 251-396) was amplified with the following primers: forward HBVV-Lup 5’-GACTCGTGGTGGACTTCTCTCA-3’ and reverse HBV-Ldw (5′-TAAAACGCCGCAGACACATC-3’. The standard curves were calibrated with a sample known to be positive for HBV (HBV-167; VL =4 × 106 IU/mL), with a range of detection from 50 IU/mL to 1 × 105 IU/mL. Amplifications of the S- and X-ORF were carried out in the Thermal Block cycler LC480 in a 10 μL reaction volume, mixing 4 μL DNA and 6 μL of the master mix LightCycler 480 SYBRGreen I (Roche) containing forward and reverse primers. The samples were run in triplicate, and each test was repeated at least two times. Negative controls, lacking nucleic acid, were included in each run. The amplification reactions for ORF-X were performed as follows: 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 10 s and 72 °C for 20 s. Amplification of the S region utilized the same conditions except for the annealing temperature, which was 58 °C. Melting curves were obtained and threshold cycles values (Ct) were calculated with the LC480 Basic software V. 1.2 (Roche Diagnostics).
Nested PCR assay for the HBV-core
A known HBV-positive plasma sample (HBV-84), with a VL of 1330 IU/mL, was used as a positive control for the C-region nested PCR assay as previously described[17]. The limit of detection for the C-nested PCR assay was approximately 5.7 IU/mL (30 copies/mL)[17]. The outer primers that amplify a 560 bp fragment were as follows: sense (5’-TTCAAGCCTCCAAGCTGTGCCTTGG-3’, nt 1863 to 1887) and antisense (5’-TCTGCGACGCGGCGATTGAGA-3’, nt 2402 to 2422). The inner primers that amplify a fragment of 438 bp were as follows: sense (5’-CCTTGGGTGGCTTTGGGGCA-3’, nt 1882-1901) and antisense (5’-AGGATAGGGGCATTTGGTGGTCTATA-3’, nt 2294-2319). The first amplification was performed in a volume of 25 μL, containing Kappa biosystems 1 × PCR buffer, 0.5 μmol/L of each primer, 0.2 mmol/L dNTP mix; 1.25 U Kappa Taq DNA polymerase (Kapa Biosystems) and 5 μL of DNA. The reaction was run in a DNA thermal cycler (Biometra GmbH, Germany) with the following conditions for both PCR rounds: 95 °C for 5 min, followed by 40 cycles at 95 °C for 30 s, 58 °C for 30 s and 72 °C for 1 min with a final extension at 72 °C for 10 min. The second PCR round was done under the same conditions with 5 μL of the first round product. The PCR products were analyzed by electrophoresis on 1% agarose gels. PCR fragments were obtained using the gel purification extraction kit (QIAGEN GMBH, Germany) according to the manufacturer’s instructions.
Sequencing
PCR products were directly sequenced in both directions with corresponding amplification primers. DNA sequencing was performed using the dideoxy method in a BigDye Terminator Cycle Sequencing Ready reaction V.2 using the ABI Prism 3100 (16-capillary) Genetic Analyzers (Applied Biosystems Foster City CA) sequencing kit reaction mix, which contains 40 ng of purified PCR product, according to the manufacturer’s instructions. Sequence data were analyzed using the CLCbio software, and HBV genotypes were determined with the genotyping tool program[18].
Statistical analyses
Comparison of the discrete variables age (< 45 or ≥ 45 years old), gender, CD4 cell count (< 200 or ≥ 200 cell/mL), plasma HIV RNA VL (detectable: > 50 copies/mL, or not), antiretroviral (ARV) patient treatment with or without anti HBV-activity (3TC, TDF or FTC) and positivity for HBV DNA (OHBI+ and OHBI- groups) was performed using Pearson’s χ2 test or Fisher’s exact test, as required. Proportions were described together with 95% confidence interval (95%CI). All data with appropriate P values ≤ 0.05 were considered to be statistically significant. All the analyses were performed using SPSS for windows V.12.0 (SPSS, Chicago IL).
RESULTS
Demographic and clinical characteristics of patients
The characteristics of the 49 HIV-1+/HBsAg- patients included in the study are summarized in Table 1. The age ranged from 18 to 73 years with an average age of 45 years. The range of the HIV RNA-1 VL was from non-detectable (ND) to 5.1 log10 HIV-RNA copies/mL. The CD4-T cell counts varied from 70 to 1500 cells/mm. Forty-eight patients were receiving antiviral treatment (ARV), and 33 patients were treated with drugs that exhibited anti-HBV activity: 3TC, TDF, or a combination of TDF/emtricitabine (FTC). According to the HBV serological pattern of OHBI, two groups of HIV-1+/HBsAg- patients were identified: a seropositive (SP) group that included 27 patients anti-HBc+ and a seronegative (SN) group with 22 patients.
Table 1.
Overall demographic, serological, and clinical data of the study population
| HIV-1 infected patients | n = 55 | 95%CI (%) |
| Ratio M:F | 3:1 | |
| Age range (yr) | 18-73 | |
| HIV/HBV coinfection (HBsAg+) | 6 | 10.7 (5.0-21.4) |
| HIV-1 patients included (HbsAg-) | 49 | 89.0 (78.2-94.9) |
| Seropositive OHBI (SP) | 27 | 49.0 (36.4-61.9) |
| Seronegative OHBI (SN) | 22 | 40.0 (28.1-53.2) |
| Range of HIV RNA viral load in plasma (log10 copies/mL) | ND-5.1 | |
| CD4+ cell/mm3 | 70-1500 |
HIV: Human immunodeficiency virus; HBV: hepatitis B virus; SP: One or more HBV serological marker except HbsAg (anti-HBc, anti-HBe, anti-HBs); SN: Negative for all HBV markers; OHBI: Occult hepatitis B infection; ND: No detectable by Cobas Monitor Amplicor Roche V. 1.5.
Variables associated with OHBI
Twenty-four patients (49%) were positive for OHBI, and a detailed description of genome regions amplified, the presence of serologic markers, the HIV RNA VL, and the anti-viral treatment in each of these 24 patients is presented in Table 2. OHBI was identified in 48.1% (13) of the SP group and 50% of the SN group (11). The sequences of the HBV-core amplied fragment were analyzed using the maximum likelihood method based on the Kimura 2-parameter model and compared to HBsAg+/HIV-1 samples and genotype H reference sequence, because the high homology in the sequences to genotype H (Figure 1).
Table 2.
Detection of hepatitis B virus DNA regions, serologic markers, and ART treatment in 24 human immunodeficiency virus-1 patients identified with occult hepatitis B infection
| Patient ID |
HBV serological markers |
qPCR1 |
C-nested PCR | RNA HIV (CV) Log10 c/mm3 | HAART Tx | HBV Tx2 | |||
| αHBc | αHBs | αHBe | S | X | |||||
| SP | |||||||||
| HBVO-04 | + | - | - | + | + | + | 3.1 | + | - |
| HBVO-05 | + | - | - | + | + | + | 3.2 | + | 3TC |
| HBVO-07 | + | + | - | - | - | + | ND | + | - |
| HBVO-17 | + | - | + | + | + | + | 2.4 | + | TDF |
| HBVO-22 | + | + | - | + | + | + | ND | + | 3TC |
| HBVO-27 | + | + | + | + | + | + | ND | + | TDF/FTC |
| HBVO-31 | + | + | + | + | + | + | 4.3 | + | 3TC |
| HBVO-32 | + | + | + | + | + | + | 2.2 | + | 3TC |
| HBVO-36 | + | - | - | + | + | + | 4.3 | + | - |
| HBVO-47 | + | - | + | + | + | + | 5.1 | - | - |
| HBVO-49 | + | + | + | + | + | + | ND | + | - |
| HBVO-54 | + | + | + | + | + | + | 3.6 | + | TDF/FTC |
| HBVO-75 | + | + | + | + | + | + | ND | + | TDF/FTC |
| SN | |||||||||
| HBVO-03 | - | - | - | + | + | + | 5 | O | - |
| HBVO-08 | - | - | - | - | - | + | 1.7 | + | 3TC |
| HBVO-18 | - | - | - | + | + | + | 2.6 | + | - |
| HBVO-20 | - | - | - | + | + | + | 3.3 | + | 3TC |
| HBVO-26 | - | - | - | + | + | + | 3.9 | + | - |
| HBVO-33 | - | - | - | + | - | + | 3 | + | TDF |
| HBVO-34 | - | - | - | + | + | + | 2.7 | + | - |
| HBVO-35 | - | - | - | + | - | + | 2.4 | + | - |
| HBVO-38 | - | - | - | + | - | + | ND | + | - |
| HBVO-39 | - | - | - | + | + | + | ND | + | - |
| HBVO-45 | - | - | - | + | + | + | ND | + | TDF |
| Frequency (%) | 49 | ||||||||
qPCR: Real time PCR utilizing SyberGreen assay;
ARV treatment included tenofovir (TDF). HIV: Human immunodeficiency virus; HBV: hepatitis B virus; SP: Seropositive (one or more HBV serological marker: anti-HBc, anti-HBe, anti-HBs); SN: Negative for all HBV markers; ND: No detectable; S: Surface ORF; X: Protein X ORF; C: Core ORF; Tx: Treatment; TDF/FTC: Tenofovir/emtricitabine; 3TC: Lamivudine; O: Other ARV treatment (IFN).
Figure 1.
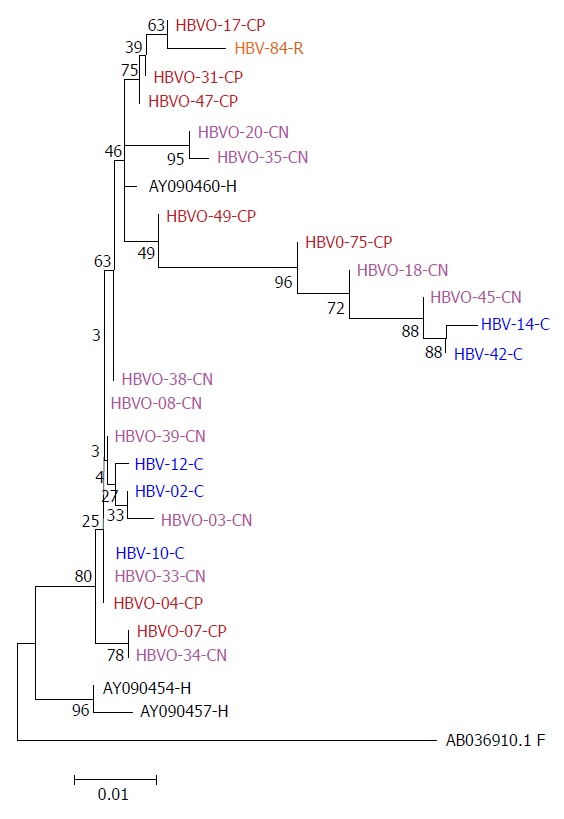
Phylogenetic tree constructed by the Maximum Likelihood method based on the partial nucleotide sequence of the core gen (439 bp) of 18 OHBI isolates (CP and CN); 5 sequences (C) obtained from hepatitis B virus overt co-infection (HbsAg+), using the three genotype H reference sequences from Genbank (AB375159.1, AB375160.1, AB375161.1), as a reference group and one hepatitis B virus sequence from a chronic hepatitis B virus mono-infected patient (HBV-84-R). Genetic distances were estimated using the Kimura two-parameter matrix and Bootstrap values are indicated for the major nodes as a percentage of the data obtained from 1000 replicates. C: Core; -CN: Seronegative OHBI; -CP: Seropositive OHBI; -S: HBsAg+ isolates.
No significant differences in gender, age, CD4+ levels, and the presence or absence of HBV serologic markers were found between OHBI+ patients and OHBI- patients. A positive correlation was found between the presence of OHBI and the HIV RNA-1 VL (OR = 8.75; P = 0.001; 95%CI: 2.26-33.79). ARV treatment with anti-HBV activity was also significantly associated with OHBI (OR = 0.25; P = 0.05; 95%CI: 0.08-1.05) (Table 3).
Table 3.
Analysis of potential risk variables associated with hepatitis B virus infection in human immunodeficiency virus-1 patients
|
HBV DNA |
||||||
| Positive (n = 24) n (%) | Negative (n = 25) n (%) | χ2 | P value | OR | 95%CI | |
| Gender | ||||||
| Male | 17 (71) | 20 (80) | 0.55 | 0.45 | 0.60 | 0.16-2.26 |
| Female | 7 (29) | 5 (20) | ||||
| Age | ||||||
| < 45 yr | 12 (50) | 12 (48) | 0.02 | 0.88 | 0.92 | 0.30-2.83 |
| ≥ 45 yr | 12 (50) | 13 (52) | ||||
| Seropositive for any marker (SP) | ||||||
| Yes | 13 (54) | 14 (56) | 0.01 | 0.89 | 0.92 | 0.30-2.86 |
| No | 11 (46) | 11 (44) | ||||
| HIV RNA VL > 50 copies/mL | ||||||
| Detectable | 15 (63) | 4 (16) | 11.15 | 0.001 | 8.75 | 2.26-33.79 |
| No detectable | 9 (37) | 21 (84) | ||||
| ARV-treatment | ||||||
| Yes | 24 (96) | 23 (96) | - | 1.01 | 1.04 | 0.06-17.68 |
| No | 1 (4) | 1 (4) | ||||
| With TDF/FTC or 3TC | ||||||
| Yes | 13 (54) | 20 (80) | 3.72 | 0.052 | 0.25 | 0.08-1.05 |
| No | 11 (46) | 5 (20) | ||||
| CD4+ | ||||||
| < 200 cell/mm3 | 2 (8) | 3 (12) | - | 1.01 | 0.66 | 0.10-4.38 |
| > 200 cell/mm3 | 22 (92) | 22 (88) | ||||
Fisher’s exact test;
α = 95%, β = 80%, OR = 0.29 with the same percentages.
DISCUSSION
In Mexico HBV infection endemicity is characterized by a low HBsAg seroprevalence (0.03%), apparently due to a rapid resolution of the infection, low viral loads and a high prevalence of occult Hepatitis B infection[19]. However, high endemic areas of HBV infection have been detected in native population using anti-HBc marker and molecular diagnosis of HBV genomes[20,21]. The present study reports a high prevalence (49%) of occult hepatitis B virus infection in a group 49 HIV-1-infected individuals in Mexico. Previous reports on the prevalence of OHBI in HIV-1 patients have shown controversial results, ranging from 0 to 89.5%. For example, Núñez et al[11] did not detect HBV DNA in HIV-positive injection drug users, whereas a prevalence of 0.8% was reported in French HIV-infected patients[22]. Additional reports from Brazil[23], and the Netherlands[24] found OHBI in 5% of HIV-infected patients. In contrast, prevalences of 14% in the UK[25] as high as 89.5% in Switzerland[12] and 88.4% in South Africa[13] have been reported. The discrepancies in the rate of OHBI may reflect the varying prevalences of HBV and HIV infections in different countries and the sensitivity of the assays used to detect HBV DNA. The prevalence of OHBI generally correlates with the level of HBV endemicity in different geographic areas. Thus, in regions with a low prevalence of HBV infection, like our country, OHBI would be high only in groups with a risk factor such as HIV-1 infection.
The C nested-PCR assay used in this study for HBV DNA detection improved our ability to detect OHBI because we had two cases negative for the X and S region but positive for nested-C PCR assay. In these cases, the identity of the OHBI circulating in the patient was confirmed by sequencing. One limitation of this study was the inability to sequence all the positive samples; we were unable to obtain the sequence in 7 of the 24 positive samples, because the amount of amplicon was very low. However, from the samples that were sequenced it is important to emphasize that they belong to genotype H. In Mexico, HBV genotype H is predominant during chronic infections and it is detected in mixtures with other genotypes and associated with other comorbities such a coinfection with HCV or HIV-1[19]. In particular, OHBI has been identified in 6.4% of blood donors, whereas genotype H was detected in 66.7% of the samples[17]. However, there is only one previous report of 18.4% OHBI in a group of HIV-1 Mexican patients[26].
Several studies have examined the prevalence of OHBI in HIV-1 patients, and few have evaluated risk factors. We analyzed the correlations between diverse variables and OHBI. We found no association between age, sex, ARV treatment duration, or CD4-cell count and OHBI. In our study, the distribution of positive cases for HBV serologic markers was similar in both SP and SN patients. This finding is relevant because previous studies have suggested that OHBI is more prevalent in SP individuals with anti-HBc[4,27]. Some studies have reported this in 13.6% of Iranian HIV-positive patients[28], compared to 24.5% of Indian[29] and 28.7% of Lebanese HIV-infected patients[30] although other studies have reported OHBI in SN HIV-1 patients[29,31]. In contrast, we found a significant inverse association (OR = 0.25; P = 0.05; 95%CI: 0.08-1.05) in patients treated with HBV-active drugs (3TC, TDF, or FTC) and OHBI, which would suggest a protective role for OHBI prevention. This agrees with a previous report in which the use of ARV treatment with anti-HBV activity decreased the risk for OHBI[7]. However, other studies have found no correlation between 3TC treatment and OHBI[32-34]. Although the P value was at the limits of statistical significance (P = 0.05) and the statistical power of the size sample is only of 48%, the data could suggest a protective role for prevention of OHBI. These results suggest the necessity to perform the screening for OHBI in a larger group of HIV-1 patients.
Other studies have also shown that OHBI was more frequently detected in individuals with a low CD4+ cell count[24,35], an association that was not found in our study. We found that a detectable level of HIV RNA was a significant risk factor for OHBI in the group of patients studied (OR = 8.75), suggesting that an immune dysfunction other than a low CD4 cell count might impair the elimination of HBV and allow low levels of replication. In accordance with this, a study reported that occult HBV was associated with an HIV RNA level > 1000 copies/mL but not with CD4 cell counts < 200 cells/mm3[7]. However, other studies have not found a significant association with HIV-RNA levels[22,25,36,37].
Several patients in the SN group (8/18) exhibit the coexistence of anti-HBs with HBV DNA detectable in plasma (Table 2), this prevalence is high and it would be interesting in the future to define by additional sequencing the presence of mutations at “a” major determinant at S gene, as potential mutants to escape neutralizing antibodies.
The results of this study suggest that HIV+/HBsAg- patients should be tested for OHBI, particularly those with detectable levels of HIV RNA, regardless of the presence of HBV serological markers. Our results also suggest that the C-nested PCR amplification could be used for the initial screening for OHBI. Furthermore, this approach would make routine serological testing unnecessary, significantly reducing the cost of HBV diagnosis. Prospective studies would be needed to assess the clinical value of this diagnostic approach.
Our study had some limitations. The sample size was relatively small and it was a single time point testing without any follow-up. Secondly, since it was a cross-sectional study, liver and clinical hepatic characteristics other than CD4 and ARV treatment were not determined in our study.
In conclusion, this study shows that the prevalence of OHBI is high among 49 HIV+/HBsAg- patients in Mexico and suggests the need for a sensitive test for HBV DNA detection. We suggest that C-nested PCR assay might be useful in the initial screening for OHBI especially in absence of HBsAg marker.
ACKNOWLEDGMENTS
We thank Mario Valle and Remedios Sandoval for the serological data and Ana Ma Cevallos for a critical review of the manuscript. We would like to acknowledge Bernal Leon for S primer design and Ivan Romero (Roche advisor) for technical advice on the real-time PCR assays. JT is a recipient of an Exclusivity scholarship from Fundacion-IMSS, Mexico. Preliminary results were presented at XXII Congreso de ALEH in Lima, Perú, September 5-7, 2012, and the 3rd Conference of Virology Baltimore, MD, November 20-22, 2013. Ethical Approval IMSS: 2008-86-717.
COMMENTS
Background
The presence of hepatitis B virus (HBV) DNA in individuals hepatitis B surface antigen negative (HBsAg)-negative is defined as occult HBV infection (OHBI). Human immunodeficiency virus (HIV)-infected patients are at risk of acquiring viral hepatitis, due to common routes of transmission. Current evidences have shown that is relatively frequent in HIV-patients, however there are conflicting reports on the impact of OHBI on the natural history of HIV disease. In Mexico, few studies have been perfomed on OHBI prevalence. More studies are needed to determine the frequency of infection in this group HIV patients should be screened for evidence of occult hepatitis B infection.
Research frontiers
The clinical significance of OHBI is not defined yet. However, this infection has been involved in different clinical context, including acute reactivation when an immunosuppressive status occurs, and there are also evidence suggesting that it may contribute to the development of cirrhosis and may have an important role in hepatocarcinogenesis.
Innovations and breakthroughs
The frequency of OHBI in HIV-1 patients is now well recognized due to advances in molecular. This is the first study in Mexico, that demonstrates the frequency of OHBI in HIV-patients utilizing more than one genetic marker in the analysis. The data presented here is a valuable contribution in the area of clinical virology because HIV-1 infection in our country is a very important health problem. Other problem is the low amount of HBV DNA that can be detected in the serum, of coinfected patients, for that more sensitive assay must be developed. The data clearly shows that the determination of the HBsAg solely may underestimate the actual prevalence of HBVO in this group of patients.
Applications
The results of this study suggest that HIV/OHBI coinfected patients remain undiagnosed, if only conventional serological markers for HBV are used. The authors propose that C-nested PCR assay might be useful in the initial screening for HBV DNA detection in bigger population groups, this approach will allow us to screen a bigger population.
Peer review
The author investigated the presence of OHBI in 49 HIV-1/HBsAg- patients. They would like to determine the frequency of OHBI in HIV-1+/HBsAg- patients in Mexico. They used nested PCR or real time PCR to amplify a region of the C, S and X genes. They found that the OHBI frequency is high among Mexican HIV-1+/HBsAg- patients and is more frequency in patients with detectable HIV RNA. The findings in this report are very important for managing HIV-1+/HBsAg patients. The study population is small, but the results are sufficient to perhaps merit an exploration in a bigger population.
Footnotes
Supported by Consejo Nacional de Ciencia y Tecnologia, Mexico CONACYT 2008-C01-86717, (to Alvarez-Muñoz MT and Lira R)
P- Reviewer: Fanning LJ, Lonardo A, Kondo Y S- Editor: Ding Y L- Editor: A E- Editor: Wang CH
References
- 1.Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol. 2007;46:160–170. doi: 10.1016/j.jhep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Raimondo G, Pollicino T, Romanò L, Zanetti AR. A 2010 update on occult hepatitis B infection. Pathol Biol (Paris) 2010;58:254–257. doi: 10.1016/j.patbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Raimondo G, Caccamo G, Filomia R, Pollicino T. Occult HBV infection. Semin Immunopathol. 2013;35:39–52. doi: 10.1007/s00281-012-0327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2:479–486. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 5.Shire NJ, Rouster SD, Stanford SD, Blackard JT, Martin CM, Fichtenbaum CJ, Sherman KE. The prevalence and significance of occult hepatitis B virus in a prospective cohort of HIV-infected patients. J Acquir Immune Defic Syndr. 2007;44:309–314. doi: 10.1097/QAI.0b013e31802e29a9. [DOI] [PubMed] [Google Scholar]
- 6.Kazemi-Shirazi L, Petermann D, Müller C. Hepatitis B virus DNA in sera and liver tissue of HBsAg negative patients with chronic hepatitis C. J Hepatol. 2000;33:785–790. doi: 10.1016/s0168-8278(00)80311-6. [DOI] [PubMed] [Google Scholar]
- 7.Lo Re V, Frank I, Gross R, Dockter J, Linnen JM, Giachetti C, Tebas P, Stern J, Synnestvedt M, Localio AR, et al. Prevalence, risk factors, and outcomes for occult hepatitis B virus infection among HIV-infected patients. J Acquir Immune Defic Syndr. 2007;44:315–320. doi: 10.1097/QAI.0b013e31802ea499. [DOI] [PubMed] [Google Scholar]
- 8.Yuan Q, Ou SH, Chen CR, Ge SX, Pei B, Chen QR, Yan Q, Lin YC, Ni HY, Huang CH, et al. Molecular characteristics of occult hepatitis B virus from blood donors in southeast China. J Clin Microbiol. 2010;48:357–362. doi: 10.1128/JCM.01781-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuoka S, Nirei K, Tamura A, Nakamura H, Matsumura H, Oshiro S, Arakawa Y, Yamagami H, Tanaka N, Moriyama M. Influence of occult hepatitis B virus coinfection on the incidence of fibrosis and hepatocellular carcinoma in chronic hepatitis C. Intervirology. 2008;51:352–361. doi: 10.1159/000187720. [DOI] [PubMed] [Google Scholar]
- 10.Gilson RJ, Hawkins AE, Beecham MR, Ross E, Waite J, Briggs M, McNally T, Kelly GE, Tedder RS, Weller IV. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS. 1997;11:597–606. doi: 10.1097/00002030-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Núñez M, Ríos P, Pérez-Olmeda M, Soriano V. Lack of ‘occult’ hepatitis B virus infection in HIV-infected patients. AIDS. 2002;16:2099–2101. doi: 10.1097/00002030-200210180-00024. [DOI] [PubMed] [Google Scholar]
- 12.Hofer M, Joller-Jemelka HI, Grob PJ, Lüthy R, Opravil M. Frequent chronic hepatitis B virus infection in HIV-infected patients positive for antibody to hepatitis B core antigen only. Swiss HIV Cohort Study. Eur J Clin Microbiol Infect Dis. 1998;17:6–13. doi: 10.1007/BF01584356. [DOI] [PubMed] [Google Scholar]
- 13.Firnhaber C, Viana R, Reyneke A, Schultze D, Malope B, Maskew M, Di Bisceglie A, MacPhail P, Sanne I, Kew M. Occult hepatitis B virus infection in patients with isolated core antibody and HIV co-infection in an urban clinic in Johannesburg, South Africa. Int J Infect Dis. 2009;13:488–492. doi: 10.1016/j.ijid.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukhwareni A, Burnett RJ, Selabe SG, Mzileni MO, Mphahlele MJ. Increased detection of HBV DNA in HBsAg-positive and HBsAg-negative South African HIV/AIDS patients enrolling for highly active antiretroviral therapy at a Tertiary Hospital. J Med Virol. 2009;81:406–412. doi: 10.1002/jmv.21418. [DOI] [PubMed] [Google Scholar]
- 15.Barth RE, Huijgen Q, Tempelman HA, Mudrikova T, Wensing AM, Hoepelman AI. Presence of occult HBV, but near absence of active HBV and HCV infections in people infected with HIV in rural South Africa. J Med Virol. 2011;83:929–934. doi: 10.1002/jmv.22026. [DOI] [PubMed] [Google Scholar]
- 16.Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, Colombo M, Craxì A, Donato F, Ferrari C, Gaeta GB, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49:652–657. doi: 10.1016/j.jhep.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 17.García-Montalvo BM, Ventura-Zapata LP. Molecular and serological characterization of occult hepatitis B infection in blood donors from Mexico. Ann Hepatol. 2011;10:133–141. [PubMed] [Google Scholar]
- 18.Rozanov M, Plikat U, Chappey C, Kochergin A, Tatusova T. A web-based genotyping resource for viral sequences. Nucleic Acids Res. 2004;32:W654–W659. doi: 10.1093/nar/gkh419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roman S, Panduro A. HBV endemicity in Mexico is associated with HBV genotypes H and G. World J Gastroenterol. 2013;19:5446–5453. doi: 10.3748/wjg.v19.i33.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez-Muñoz T, Bustamante-Calvillo E, Martínez-García C, Moreno-Altamirando L, Guiscafre-Gallardo H, Guiscafre JP, Muñoz O. Seroepidemiology of the hepatitis B and delta in the southeast of Chiapas, Mexico. Arch Invest Med (Mex) 1989;20:189–195. [PubMed] [Google Scholar]
- 21.Roman S, Panduro A, Aguilar-Gutierrez Y, Maldonado M, Vazquez-Vandyck M, Martinez-Lopez E, Ruiz-Madrigal B, Hernandez-Nazara Z. A low steady HBsAg seroprevalence is associated with a low incidence of HBV-related liver cirrhosis and hepatocellular carcinoma in Mexico: a systematic review. Hepatol Int. 2009;3:343–355. doi: 10.1007/s12072-008-9115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloquel B, Jeulin H, Burty C, Letranchant L, Rabaud C, Venard V. Occult hepatitis B infection in patients infected with HIV: report of two cases of hepatitis B reactivation and prevalence in a hospital cohort. J Med Virol. 2010;82:206–212. doi: 10.1002/jmv.21685. [DOI] [PubMed] [Google Scholar]
- 23.Jardim RN, Gonçales NS, Pereira JS, Fais VC, Gonçales Junior FL. Occult hepatitis B virus infection in immunocompromised patients. Braz J Infect Dis. 2008;12:300–305. doi: 10.1590/s1413-86702008000400008. [DOI] [PubMed] [Google Scholar]
- 24.Cohen Stuart JW, Velema M, Schuurman R, Boucher CA, Hoepelman AI. Occult hepatitis B in persons infected with HIV is associated with low CD4 counts and resolves during antiretroviral therapy. J Med Virol. 2009;81:441–445. doi: 10.1002/jmv.21422. [DOI] [PubMed] [Google Scholar]
- 25.Nebbia G, Garcia-Diaz A, Ayliffe U, Smith C, Dervisevic S, Johnson M, Gilson R, Tedder R, Geretti AM. Predictors and kinetics of occult hepatitis B virus infection in HIV-infected persons. J Med Virol. 2007;79:1464–1471. doi: 10.1002/jmv.20954. [DOI] [PubMed] [Google Scholar]
- 26.Torres-Baranda R, Bastidas-Ramírez BE, Maldonado-González M, Sánchez-Orozco LV, Vázquez-Vals E, Rodríguez-Noriega E, Panduro A. Occult hepatitis B in Mexican patients with HIV, an analysis using nested polymerase chain reaction. Ann Hepatol. 2006;5:34–40. [PubMed] [Google Scholar]
- 27.Aghakhani A, Banifazl M, Kalantar E, Eslamifar A, Ahmadi F, Razeghi E, Atabak S, Amini M, Khadem-Sadegh A, Ramezani A. Occult hepatitis B virus infection in hemodialysis patients with isolated hepatitis B core antibody: a multicenter study. Ther Apher Dial. 2010;14:349–353. doi: 10.1111/j.1744-9987.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 28.Azadmanesh K, Mohraz M, Aghakhani A, Edalat R, Jam S, Eslamifar A, Banifazl M, Moradmand-Badie B, Ramezani A. Occult hepatitis B virus infection in HIV-infected patients with isolated hepatitis B core antibody. Intervirology. 2008;51:270–274. doi: 10.1159/000160217. [DOI] [PubMed] [Google Scholar]
- 29.Rundell S. Care about care plans! Nurs Times. 2010;87:32. [PubMed] [Google Scholar]
- 30.Ramia S, Mokhbat J, Ramlawi F, El-Zaatari M. Occult hepatitis B virus infection in HIV-infected Lebanese patients with isolated antibodies to hepatitis B core antigen. Int J STD AIDS. 2008;19:197–199. doi: 10.1258/ijsa.2007.007200. [DOI] [PubMed] [Google Scholar]
- 31.Minuk GY, Sun DF, Uhanova J, Zhang M, Caouette S, Nicolle LE, Gutkin A, Doucette K, Martin B, Giulivi A. Occult hepatitis B virus infection in a North American community-based population. J Hepatol. 2005;42:480–485. doi: 10.1016/j.jhep.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 32.Chen SJ, Zhao YX, Fang Y, Xu WZ, Ma YX, Song ZW, Teng X, Gu HX. Viral deletions among healthy young Chinese adults with occult hepatitis B virus infection. Virus Res. 2012;163:197–201. doi: 10.1016/j.virusres.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 33.Morsica G, Ancarani F, Bagaglio S, Maracci M, Cicconi P, Cozzi Lepri A, Antonucci G, Bruno R, Santantonio T, Tacconi L, et al. Occult hepatitis B virus infection in a cohort of HIV-positive patients: correlation with hepatitis C virus coinfection, virological and immunological features. Infection. 2009;37:445–449. doi: 10.1007/s15010-008-8194-9. [DOI] [PubMed] [Google Scholar]
- 34.Martin CM, Welge JA, Shire NJ, Rouster SD, Shata MT, Sherman KE, Blackard JT. Genomic variability associated with the presence of occult hepatitis B virus in HIV co-infected individuals. J Viral Hepat. 2010;17:588–597. doi: 10.1111/j.1365-2893.2009.01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsui JI, French AL, Seaberg EC, Augenbraun M, Nowicki M, Peters M, Tien PC. Prevalence and long-term effects of occult hepatitis B virus infection in HIV-infected women. Clin Infect Dis. 2007;45:736–740. doi: 10.1086/520989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagaglio S, Bianchi G, Danise A, Porrino L, Uberti-Foppa C, Lazzarin A, Castagna A, Morsica G. Longitudinal evaluation of occult hepatitis B infection in HIV-1 infected individuals during highly active antiretroviral treatment interruption and after HAART resumption. Infection. 2011;39:121–126. doi: 10.1007/s15010-011-0093-9. [DOI] [PubMed] [Google Scholar]
- 37.Khamduang W, Ngo-Giang-Huong N, Gaudy-Graffin C, Jourdain G, Suwankornsakul W, Jarupanich T, Chalermpolprapa V, Nanta S, Puarattana-Aroonkorn N, Tonmat S, et al. Prevalence, risk factors, and impact of isolated antibody to hepatitis B core antigen and occult hepatitis B virus infection in HIV-1-infected pregnant women. Clin Infect Dis. 2013;56:1704–1712. doi: 10.1093/cid/cit166. [DOI] [PMC free article] [PubMed] [Google Scholar]


