Abstract
AIM: To define the histopathological features predictive of post-transplant hepatocellular carcinoma (HCC) recurrence after transarterial chemoembolization, applicable for recipient risk stratification.
METHODS: We retrospectively reviewed the specimens of all suspicious nodules (total 275) from 101 consecutive liver transplant recipients which came to our Pathology Unit over a 6-year period. All nodules were sampled and analyzed, and follow-up data were collected. We finally considered 11 histological variables for each patient: total number of nodules, number of viable nodules, size of the major nodule, size of the major viable nodule, occurrence of microscopic vascular invasion, maximum Edmondson's grade, clear cell/sarcomatous changes, and the residual neoplastic volume. Survival data were computed by means of the Kaplan-Meier procedure and analyzed by means of the Cox proportional hazards model. The multivariate linear regression and a k-means cluster analysis were also used in order to compute the standardized histological score.
RESULTS: The total number of nodules, the residual neoplastic volume (the total volume of all evaluated nodules minus the necrotic portion) and the microvascular invasion entered the Cox multivariate hazard model with HCC recurrence as dependent variable. The histological score was therefore computed and a cluster analysis sorted recipients into 3 risk groups, with 3.3%, 18.5% and 53.8% respectively of tumor recurrence rates and 1.6%, 11.1% and 38.5% of tumor-related mortality respectively at the end of follow-up.
CONCLUSION: The histological score allows a reliable stratification of HCC recurrence risk, especially in those recipients found out to be beyond the Milan criteria after orthotopic liver transplantation (OLT).
Keywords: Chemoembolization, Hepatocellular carcinoma, Histopathology, Orthotopic liver transplantation, Cancer recurrence, Transarterial chemoembolization
Core tip: Transarterial chemoembolization (TACE) of hepatocellular carcinoma (HCC) is used for down-staging before orthotopic liver transplantation (OLT), and as “bridging therapy” to reduce the drop-out rates in the waiting list. A discrepancy exists between the pre-transplant (radiological) and post-transplant (pathological) staging. Few study analyzing the histology of TACE-treated HCC exist. Here we analyzed 11 histological variables in 275 nodules from 101 consecutive OLT recipients and we computed a histological score to stratify recipients into 3 risk groups, with 3.3%, 18.5% and 53.8% respectively of tumor recurrence rates. The histological score allows a better stratification of those recipients beyond the Milan criteria after OLT.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the third cause of cancer death worldwide[1]. While hepatic resection is the choice for single tumors in patients with good liver function, orthotopic liver transplantation (OLT) is considered the best first-line therapeutic approach for multiple HCCs in patients with cirrhosis, not only for the treatment of the neoplastic disease but also for resolving the organ failure. According to the most recent guidelines of the American Association for the Study of Liver Disease, the European Association for the Study of the Liver (EASL) and the European Organization for Research and Treatment of Cancer (EORTC)[2,3], OLT is recommended for patients within the so-called “Milan criteria”[4]. Some studies have shown that post-OLT survival of HCC recipients falling into the Milan criteria seems to be comparable to that of non-HCC OLT recipients[5], and thus Milan criteria have been included both in the Barcelona-Clinic Liver Cancer (BCLC) classification and in the U.S. United Network for Organ Sharing for organ procurement[6,7], although other models have been proposed (and validated), like the “up-to-seven criteria”[8].
The main limitation for OLT is the shortening of the donor pool, with the consequent need to extend the waiting list time for patients with cancer. Although large case-control studies - as well as real guidelines for HCC down-staging prior to OLT - are still lacking in the literature, some authors have emphasized the usefulness of local ablation before OLT in recipient survival[9,10], since it is likely to influence not only the drop-out rate on the waiting list, but also HCC-free survival, at least in selected cases[11-15]. Transarterial chemoembolization (TACE) is the most widely used local treatment[16-18], and it is recommended for patients with > 6 mo of expected time on the waiting list and with BCLC stage B[3,11,19]. In our institution, pre-OLT TACE is used both for down-staging in patients beyond the Milan criteria, and to delay tumor progression of patients within the Milan criteria (“bridging therapy”) in order to grant more time and to reduce the drop-out rate in the waiting list.
An important issue is the objective discrepancy existing between the pre-transplant (radiological) staging and the explant staging on the OLT specimen. This discrepancy depends on the sensitivity of imaging techniques that can fail in the recognition of small-sized atypical/hypovascular HCCs[20,21]. According to Mazzaferro et al[8], the pre-transplant staging fails to predict the actual number of HCC in 25%-35% of patients.
Several clinical and radiological studies are present in the literature, very few analyzing the histological aspect of TACE-treated HCCs and tumor recurrence. Moreover, histological studies assessing the post-TACE tumor regression (and its meaning for HCC recurrence) are still lacking. The aims of this study were: (1) to define the histopathological features predictive of HCC recurrence in patients treated with TACE before OLT; and (2) to establish if these features can be used for defining a histological “score” predictive of HCC recurrence after OLT, that can be applied by pathologists for recipient risk stratification.
MATERIALS AND METHODS
Patient selection and clinical data
We retrospectively reviewed the specimens from consecutive OLT recipients which came to our Pathology Unit over a 6-year period (from January 2005 to December 2010). Eligibility criteria were the TACE ablation of at least one HCC before OLT and the availability of adequate follow-up data. All patients were treated according to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008); informed consent was obtained from each patient at the time of surgery. Data about HCC number and size as seen at computerised tomography (CT) and/or magnetic resonance imaging (MRI) and US analysis and the number of locally ablated nodules were collected, together with the resulting number of patients included in the Milan criteria before TACE.
One-hundred and one patients were finally selected, 85 (84.2%) males and 16 (15.8%) females, mean age at OLT 56.61 ± 7.82 years (range 37-68 years). At pre-transplant evaluation a mean number of 2.42 ± 1.19 HCC/patient was found (range 1-6), with a mean size of the largest nodule of 2.86 ± 0.98 cm (range 1.00-5.00 cm). Forty-four (43.6%) patients were classified beyond the Milan Criteria: the goal of TACE in these patients was the successful downstaging. Conversely, 57 (56.4%) patients were within the Milan criteria, and all their HCCs were treated as ‘‘bridging therapy’’, to prevent drop-out from the waiting list. A total of 195 nodules were treated with TACE, for a mean of 1.93 ± 1.16 nodules/patient (range 1-6).
Follow-up data included HCC recurrence rates after OLT (both graft and systemic localizations) and recipient death rates for HCC recurrence and other causes.
Histopathological analysis
At gross examination of the explanted livers, we performed 1-cm cut sections of the whole parenchyma, and all suspicious nodules, also with a necrotic component, were described and totally sampled. After routine processing, 3-μm-thick sections were cut from all specimen blocks for routine stainings with Hematoxylin-Eosin, Reticulin, Sirius Red, and diastase-periodic acid Schiff.
For each single nodule, the following histopathological features were collected: percentage of necrosis, tumor margins (expansive/infiltrative), growth pattern (solid, acinar and/or trabecular), tumor grade according to Edmondson, peritumoral microvascular invasion, presence of clear cell or sarcomatoid areas. Nodule size was also measured on the histological section and the volume was calculated assuming a hypothetical spherical shape, with the formula V = 4/3(π r3) (where V is the volume expressed in cm3 and r the radius of the HCC)[22-24]. Furthermore, by subtracting the necrotic percentage from the nodule volume, we obtained the viable volume of each nodule (expressed in cm3), as previously described[25].
For each patient, the following parameters were evaluated: the total number of nodules (NNOD, considering both the necrotic and the viable nodules), the effective number of viable neoplastic nodules at OLT, the size of the major nodule, the size of the major viable nodule, the occurrence of microscopic vascular invasion (MVI), the maximum Edmondson’s grade observed, the occurrence of clear cell/sarcomatous changes. We also calculated the Residual Neoplastic Volume (RNV) of each patient, defined as the sum of all viable volumes of all nodules (in cm3), i.e., the total volume of all evaluated nodules minus the necrotic portion. RNV simply represents the total volume of viable neoplasia that was present in the liver at the time of OLT.
Statistical analysis
Data are reported as means ± SD, medians, ranges and frequencies. One-way ANOVA, the Pearson’s correlation, the Fisher’s exact and the linear-by-linear association tests were applied. Survival data (mean survival times and rates together with their standard errors, SE) were computed by means of the Kaplan-Meier procedure and were analyzed by means of the Cox proportional hazards model. The hazard ratios (HRs) were computed together with their 95% confidence intervals (95%CIs). The multivariate linear regression and a k-means cluster analysis were also used in order to compute the standardized histological score (HS). The SPSS (Version 13.0 for Windows; SPSS Inc., Chicago, IL, USA) was used as statistical package. Two-tailed P values less than 0.05 were considered significant.
RESULTS
Histopathological analysis
Two-hundreds and seventy-five neoplastic or necrotic nodules were found and sampled at gross examination in the 101 patients. At histopathological analysis, 32 (31.7%) patients with 1 nodule, 27 (26.7%) with 2, 15 (14.9%) with 3, and 27 (26.7%) patients with 4 or more nodules were found (mean 2.72 ± 1.90 nodules/patient, range 1-8). The histological characteristics of the nodules examined are illustrated in Figure 1 and summarized in Table 1. Considering only the nodules with any amount of viable HCC, 33 (32.7%) patients showed 1 viable nodule, 19 (18.8%) showed 2, 8 (7.9%) showed 3, and 17 (16.8%) showed 4 or more, for a total of 190 viable nodules (mean 1.88 ± 2.00 nodules/patient, range 0-8). Most important, no viable nodules were found in 24 (23.8%) patients, among which one had MVI near necrotic nodules: thus 23 patients were completely free from cancer at the time of OLT, and none of them experienced HCC recurrence in the follow-up.
Figure 1.
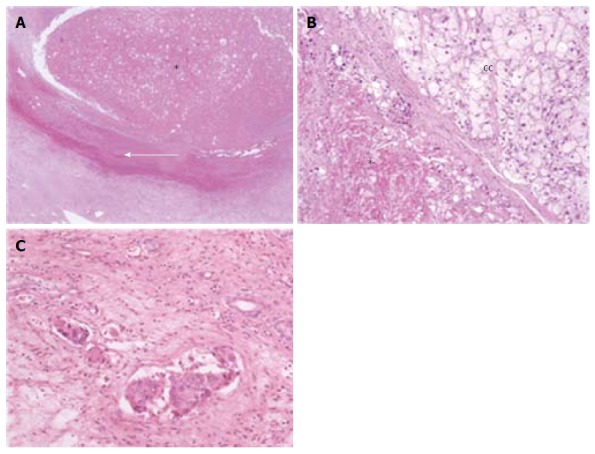
Different histological pictures after transarterial chemoembolization. A: A case of totally necrotic nodule (asterisk), with a well-visible fibrotic capsule (white arrow); B: A case of clear cell (CC) hepatocellular carcinoma with only partial necrosis (asterisk); C: Amicrovascular invasion. Haematoxylin-Eosin stain; Magnification × 2 (A) and × 10 (B, C).
Table 1.
Histological characteristics of all examined nodules (above) and of all viable nodules (any amount of residual tumor, below) n (%)
| Examined nodules (n = 275) | |
| Mean diameter (cm) | 1.89 ± 1.18 (range 0.30-6.00) |
| Mean volume (cm3) | 8.59 ± 16.93 (range 0.01-113.04) |
| Mean viable volume (cm3) | 3.47 ± 9.56 (range 0-78.36) |
| Complete coagulative necrosis | 85 (30.9) |
| Mean necrosis percentage | 48.54% ± 45.58% |
| Microvascular invasion | 81 (29.5) |
| Infiltrative margins | 84 (30.9) |
| Viable nodules (n = 190) | |
| Trabecular/trabeculo-acinar pattern | 129 (67.9) |
| Edmondson’s grade: | |
| 1 | 10 (5.3) |
| 2 | 78 (41.1) |
| 3 | 100 (52.6) |
| 4 | 2 (1.1) |
| Clear cell changes | 14 (7.4) |
The finding of de novo neoplastic nodules at OLT analysis put 34 (33.7%) patients beyond the Milan criteria after TACE, probably due to the small hypovascular nodules currently undetected by dynamic MRI/CT imaging techniques[20,21], and therefore untreatable with TACE.
At histology, the mean size of the major nodule for each patient was 2.72 ± 1.29 cm (range 0.6-6.0 cm), the mean size of the major viable nodule was 2.57 ± 1.30 cm (range 0.4-5.5 cm), MVI was observed in 38 (37.6%) patients, clear cell component was present in 11 (10.9%) cases, the maximum Edmondson’s grade was 1 in one case (1.0%), 2 in 26 (25.7%), 3 in 49 (48.5%) and 4 in one (1.0%) case. The mean RNV was 9.44 ± 19.14 cm3 (range 0-106.35 cm3).
Correlations among nodule characteristics
The histological features of the 275 nodules were correlated with each other: the presence of MVI adjacent to a nodule was significantly related to a high Edmondson’s grade (76 nodules showed MVI, among which 50 had Edmondson’s grade 3 or 4, P = 0.001, linear-by-linear association) and to infiltrative margins (in 50 nodules, P < 0.001, Fisher’s exact test); Edmondson’s grade and tumor infiltrative margins were correlated too (of 84 HCC with infiltrative margins 56, 66.7%, were grade 3 or 4, of 105 HCC with expansive margins 45, 42.8%, were grade 3 and none of grade 4, P < 0.001, linear-by-linear association). These findings should not be surprising, since all these features are typical of more aggressive HCCs. Nodule necrosis was directly correlated with tumor size (P = 0.001, Pearson’s R), in line with what was previously reported in our Institution[12], and inversely related to the perinodular MVI (mean necrosis 32.8% ± 37.4% in the 81 nodules with thrombosis vs 55.1% ± 47.1% in the 194 nodules without thrombosis, P < 0.001, one-way ANOVA). Solid architecture was correlated with a clear cell morphology [10 of 52 (19.2%) clear-cell HCC vs 4 of 136 (2.9%), P = 0.001, Fisher’s exact test].
Follow-up
Mean follow-up from OLT was 3.55 ± 1.73 years (range from 14 d to 6.81 years, median 3.37). At the end of follow-up, 14 (13.9%) recipients experienced HCC recurrence either in the graft or extrahepatic localizations, after a mean of 1.12 ± 0.89 years (range 69 d to 3.01 years, median 1.00). Twelve of these 14 (85.7%) recurrences occurred in patients discovered beyond the Milan criteria at histology. Conversely, 65 (74.7%) out of 87 not-recurrent cases occurred in patients within the Milan criteria after the histological examination.
Eighteen (17.8%) recipients died, 9 for HCC recurrence, i.e., the 64.3% of the 14 recipients with HCC recurrence. The other causes of death included HCV recurrence (4 cases), sepsis/infection (3 cases), and cerebrovascular events (2 cases).
Calculation of a Histological Score (HS) predictive of HCC recurrence
At univariate Cox analysis, the only donor variables significantly related to post-OLT recurrence were: the MVI (P = 0.002, HR = 7.23, 95%CI: 2.01-25.94), the total number of nodules (P < 0.001, HR = 1.54, 95%CI: 1.25-1.89), the number of viable nodules (P < 0.001, HR = 1.47, 95%CI: 1.22-1.77), and the RNV (P = 0.002, HR = 1.03, 95%CI: 1.01-1.04). In particular, 11 out of 14 HCC recurrence cases had MVI (i.e., MVI had a 78.6% sensitivity), while 60 of the 87 patients without recurrence did not show histological MVI (i.e., specificity of the 69.0%). Furthermore, these variables were also confirmed as the only ones significantly predictive of HCC-related deaths after recurrence in the subset of patients who had had recurrence (data not shown). Age (P = 0.872), sex (P = 0.373), inclusion in Milan criteria prior to TACE (P = 0.639) and dimension of the major nodule (P = 0.080) were not significantly related to post-OLT recurrence.
The histological cancer variables (Edmondson’s grade, clear cell component, dimension of the major viable nodule) were available only in the 77 patients with residual HCC and resulted not significantly related to post-OLT HCC recurrence (P = 0.336, P = 0.193, and P = 0.229, respectively); therefore, they have been excluded from the multivariate model. The model finally considered 101 cases and 8 variables: recipient age and sex, inclusion in Milan criteria prior to TACE, RNV, number of total nodules, number of viable nodules, MVI, dimension of the major nodule.
The number of total nodules (P = 0.073), the residual neoplastic volume (RNV, P = 0.035) and the neoplastic microvascular invasion (MVI, P = 0.062) entered the Cox multivariate hazard model with HCC recurrence as dependent variable.
For the purposes of our study, we computed a score based on predictive variables only and unrelated to time-dependent components, the histological score (HS) was computed by means of a multivariate linear regression having the rescaled hazard level evaluated by the Cox analysis in each patient as dependent variable and these three parameters as the independent ones. In order to find a solid classification of the patients into 3 groups at different risk levels, a cluster analysis was made. Finally, the coefficients of the model were standardized in order to obtain the values of 1 and 2 as cut-off separating the 3 groups at different risk levels (i.e., low risk class: HS ranging from 0 to 0.99; moderate risk class: HS ranging from 1 to 1.99; high risk class: HS equal to, or greater than, 2).
The final score resulted:
HS = (0.164 × NNOD) + (0.012 × RNV) + (1.015 × MVI)
where NNOD is the total number of total nodules, RNV is the residual neoplastic volume and the notation “1.015 × MVI” means that the 1.015 value should be added to the sum of the previous two terms in patients with presence of microvascular invasion only.
In our population of 101 patients the HS ranged between 0.164 and 3.393 (mean 0.942 ± 0.816) showing an accuracy of 84.2% in discriminating between HCC recurrent and non-recurrent cases (AUC ± SE: 0.842 ± 0.047). By using the best cut-off (HS ranging between 0.664 and 0.667) a sensitivity of 100% (14/14) and a specificity of 60.9% (53/87) were reached. The cluster analysis sorted the population into the following three classes: 61 patients (60.4%) with low risk; 27 patients (26.7%) with moderate risk; and 13 patients (12.9%) with high risk.
The cumulative recurrence-free survival of the three classes is shown in Figure 2, while survival data, together with the hazard ratios vs the low risk class, are shown in Table 2. Of note, the 5-year recurrence-free survival rate decreases from the 96.7% of the low-risk group to the 77.9% of moderate-risk group and the 40.0% of high-risk group, and the HR for the moderate- and high-risk groups were 6.37 and 23.18 respectively when compared to the low-risk group.
Figure 2.
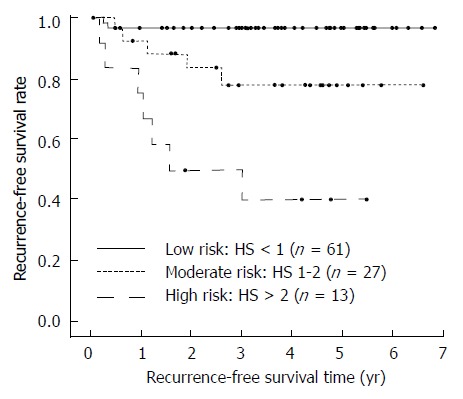
Recurrence-free survival according to the three histological score classes. The actuarial recurrence-free survival rate is 96.7% in the low-risk group, 77.9% in the moderate-risk group and 40.0% in group the high-risk group.
Table 2.
Recurrence-free survival data and hazard ratios of hepatocellular carcinoma recurrence according to the histological score classes n (%)
| HS risk classes | Mean recurrence-free survival time (± SE; years) | 5-yr recurrence-free survival rate (± SE) | Hazard ratio (95%CI)1 | HCC recurrence cases | HCC-related deaths |
| Low (HS: 0-1; n = 61) | 6.60 ± 0.15 | 96.7% ± 2.3% | 1.00 | 2 (3.3) | 1 (1.6) |
| Moderate (HS: 1-2; n = 27) | 5.43 ± 0.45 | 77.9% ± 8.9% | 6.37 (1.23-32.86) | 5 (18.5) | 3 (11.1) |
| High (HS: >2; n = 13) | 2.92 ± 0.21 | 40.0% ± 14.6% | 23.18 (4.80-111.96) | 7 (53.8) | 5 (38.5) |
Univariate Cox proportional hazards model. SE: Standard error; CI: Confidence interval; HCC: Hepatocellular carcinoma; HS: Histological score.
DISCUSSION
The application of TACE in the pre-transplant setting is still controversial[25,26]. In our setting, TACE is performed according to the most recent guidelines for downstaging in patients beyond the Milan criteria, or for patients within the Milan criteria but with > 6 mo of expected time on the waiting list (“bridging therapy”)[19,27]. Despite the abundance of clinical and radiological studies on the pre-transplant staging of HCC patients, studies analyzing the histological aspect of tumor regression after TACE and its impact on HCC recurrence are still rare in the literature, since in the past few years only the percentage of TACE-induced necrosis has been histologically studied, mainly in comparison with MRI[12,28]. The principal aims of our study were to define the histopathological features predictive of HCC recurrence in patients treated with TACE, and to combine them into a histological “score” predictive of HCC recurrence after OLT.
The histological examination of the single nodules (grade, architecture, growth, etc.) did not provide contributions about prognosis, while the multivariate analysis of recipient variables allowed us to identify three histological parameters predictive both of HCC recurrence and HCC-related mortality: MVI, the number of nodules, and RNV. The Histological Score (HS) could be considered a variation of the well-established Milan criteria: in fact, it includes the number of nodules and the tumor volume (instead of dimensions)[2,4,6]. At any rate, the availability of the whole liver specimens allows the pathologist to sample and characterize all the suspicious nodules in each case, as well as to assess the real amount of viable HCC in each nodule, and MVI too. The first result of this approach was that the number of nodules proved to be predictive independently on their being viable or necrotic. A possible explanation is that a higher number of HCC nodules implies a higher risk of vascular or intrahepatic dissemination that can manifest itself also after the necrosis of the main nodules (and after OLT). This does not contradict what previously reported by our group, i.e. that partial tumor necrosis has a higher risk for HCC recurrence[29]: indeed, since the Residual Neoplastic Volume (RNV) is part of the HS too, it is evident that incomplete necrosis has a worse impact on disease-free survival than complete necrosis. At any chance, no patients with complete tumor necrosis (absence of viable tumor) experienced HCC recurrence in our series.
RNV was calculated assuming a hypothetical spherical shape of the HCCs[22-24] minus the amount of necrosis, and it is likely to be a fairly realistic model for tumor quantification. At a glance, the calculation of RNV might seem complicated, but only the nodule diameter and the percentage of necrosis, which are commonly included in every histopathological report, are needed. The presence of MVI in the HS is not surprising, since MVI is a well-known prognostic parameter of HCC recurrence both in non-transplanted patients and in OLT recipients with or without local therapy[2]. At any chance, since MVI resulted as a part of the HS, the diagnostic performance (computed as the balance between sensitivity and specificity) of MVI alone was obviously lower than HS diagnostic performance (73.8% vs 80.5%). In particular, the finding that no patients in the low risk HS class had MVI, suggests that the absence of MVI well predicts low risk of recurrence. Conversely, since MVI occurred similarly in the moderate risk (25/27, 92.6%) and high risk (13/13, 100%) recipients, the other two histological features (the number of nodules and the RNV) are likely to be needed to discriminate between moderate risk and high risk of HCC recurrence.
It is noteworthy that in our proposed model the inclusion in the Milan criteria prior to TACE had not an independent role in HCC recurrence prediction both at univariate and multivariate analyses: it seems that the indication to TACE (i.e., down-staging vs “bridging therapy”) did not influence tumor recurrence. This confirms previous findings from our group that the recurrence rate after downstaging to Milan criteria is similar to the recurrence rate of patients within Milan criteria[10].
The application of the Milan criteria on OLT specimens showed a diagnostic performance comparable to the HS (80.2% and 80.5%, respectively), but it should be pointed out that these values were determined by two measures of discrimination quite different between the two binary classification rules: i.e., HS had higher sensitivity (100% vs 85.7%) while the Milan criteria had higher specificity (74.7% vs 60.9%). Finally, among the 34 patients who were beyond the Milan criteria, 9 (26.5%) were assigned to the low risk class (with only 1 case of HCC recurrence), 13 (38.2%) to the moderate risk class (with 4 cases of recurrence), and 12 (35.3%) to the high risk class (with 7 cases of recurrence). The recipients in our HS low risk class actually showed a low frequency of HCC recurrence comparable to the recipients within the Milan criteria (3.3% and 3.0%, respectively), while the histopathological analysis and the HS allowed once more a better stratification of the recipients with a higher risk: in fact the moderate-risk class reached a recurrence frequency assessment of 18.5% (5/27), and the high-risk class reached a recurrence frequency assessment of 53.8% (7/13), vs 35.3% of the Milan criteria alone. Briefly, our HS better stratifies the “moderate risk” from the “high risk” in those recipients that turn out to be beyond the Milan criteria after OLT, as for 33.7% of patients in our series. It is true that this percentage of patients with de novo nodules is likely to decrease in the future, with a more widespread use of hepatospecific contrast media on MRI, which have demonstrated an increased sensitivity for the detection of early atypical HCC, although we surmise that the histopathological analysis will be necessary also in the future.
In conclusion, our results strengthen the role of the pathologist in the management of HCC recipients after OLT. Despite the improvement of imaging techniques[21,25], only a thorough gross examination and histological analysis of all suspicious liver nodules can provide data about MVI and the finding of de novo early HCCs, which can put the patients beyond the Milan criteria. In these cases HS might prove to be useful in order to identify those patients with a higher risk of HCC recurrence. Our “score” is not meant to be a universal score, applicable to all HCC patients, but it might be useful for the pathologists in a liver transplantation setting to predict the recipient’s real risk of HCC recurrence after TACE and after OLT, and to help the transplant surgeon in the post-transplant management of this group of patients.
COMMENTS
Background
Transarterial chemoembolization (TACE) of hepatocellular carcinoma (HCC) is used for down-staging before orthotopic liver transplantation (OLT) in patients beyond the Milan criteria, and to delay tumor progression in patients within the Milan criteria (“bridging therapy”) in order to grant more time and to reduce the drop-out rate in the waiting list.
Research frontiers
Imaging techniques can fail in the recognition of small-sized atypical and/or hypovascular HCCs, so that a discrepancy between the radiological pre-transplant staging and the pathological post-transplant staging exists. According to the literature, the pre-transplant staging fails to predict the actual number of HCC in 25%-35% of patients. Apart from the number of neoplastic nodules, single pathological features such as microvascular invasion, tumor grade, etc. have already been described as important in the post-transplant HCC recurrence, but comprehensive histopathological studies analyzing their global prognostic impact have not been performed yet.
Innovations and breakthroughs
We retrospectively reviewed 275 suspicious nodules from 101 consecutive OLT recipients, collecting 11 histological variables. Survival data were analyzed: the total number of nodules, the residual neoplastic volume and the microvascular invasion entered the Cox proportional hazards model, with HCC recurrence as dependent variable. A standardized Histological Score (HS) was therefore computed, and a cluster analysis sorted the recipients into 3 risk groups according to the HS, with 3.3%, 18.5% and 53.8% respectively of tumor recurrence rates and 1.6%, 11.1% and 38.5% of tumor-related mortality respectively. This is the first time that histological tumor features are quantified and applied in a post-treatment HCC tumoral regression score, as it is already done in other tumors (e.g., breast, colon-rectum).
Applications
A thorough gross examination and histological analysis of all suspicious liver nodules is mandatory to obtain reliable data about the real amount of tumor necrosis after TACE, the microvascular invasion, as well as to find de novo early HCCs. The HS might prove to be useful in order to identify those patients with a higher risk of HCC recurrence after OLT. In a liver transplantation setting, the application of a standardized tumoral regression score after TACE and after OLT, can help the transplant surgeon in the recipients management.
Peer review
In this paper the authors evaluate the prognostic impact of histopathology in post-transplant HCC recurrence after TACE treatment. The authors used appropriate methods of analysis and results are of interest.
Footnotes
P- Reviewer: Fassan M S- Editor: Nan J L- Editor: A E- Editor: Du P
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 7.Freeman RB, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, Merion R, Wolfe R, Turcotte J, Teperman L. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 8.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 9.Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43–58. doi: 10.1148/radiol.11110144. [DOI] [PubMed] [Google Scholar]
- 10.Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, Vivarelli M, Golfieri R, D’Errico Grigioni A, Panzini I, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547–2557. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 12.Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E, Ravaioli M, D’Errico-Grigioni A, Pinna AD, Bolondi L. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (< 5 cm) hepatocellular carcinomas. Hepatology. 2011;53:1580–1589. doi: 10.1002/hep.24246. [DOI] [PubMed] [Google Scholar]
- 13.Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557–563. doi: 10.1053/jlts.2003.50106. [DOI] [PubMed] [Google Scholar]
- 14.Harnois DM, Steers J, Andrews JC, Rubin JC, Pitot HC, Burgart L, Wiesner RH, Gores GJ. Preoperative hepatic artery chemoembolization followed by orthotopic liver transplantation for hepatocellular carcinoma. Liver Transpl Surg. 1999;5:192–199. doi: 10.1002/lt.500050307. [DOI] [PubMed] [Google Scholar]
- 15.Spreafico C, Marchianò A, Regalia E, Frigerio LF, Garbagnati F, Andreola S, Milella M, Lanocita R, Mazzaferro V. Chemoembolization of hepatocellular carcinoma in patients who undergo liver transplantation. Radiology. 1994;192:687–690. doi: 10.1148/radiology.192.3.8058934. [DOI] [PubMed] [Google Scholar]
- 16.Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224–1229. doi: 10.1053/jhep.2000.20456. [DOI] [PubMed] [Google Scholar]
- 17.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796–802. doi: 10.1002/cncr.20426. [DOI] [PubMed] [Google Scholar]
- 19.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rimola J, Forner A, Tremosini S, Reig M, Vilana R, Bianchi L, Rodríguez-Lope C, Solé M, Ayuso C, Bruix J. Non-invasive diagnosis of hepatocellular carcinoma ≤ 2 cm in cirrhosis. Diagnostic accuracy assessing fat, capsule and signal intensity at dynamic MRI. J Hepatol. 2012;56:1317–1323. doi: 10.1016/j.jhep.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Lencioni R. Evolving strategies in the diagnosis of hepatocellular carcinoma. J Hepatol. 2011;54:184–186. doi: 10.1016/j.jhep.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 22.Zhang JW, Feng XY, Liu HQ, Yao ZW, Yang YM, Liu B, Yu YQ. CT volume measurement for prognostic evaluation of unresectable hepatocellular carcinoma after TACE. World J Gastroenterol. 2010;16:2038–2045. doi: 10.3748/wjg.v16.i16.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macaron C, Hanouneh IA, Lopez R, Aucejo F, Zein NN. Total tumor volume predicts recurrence of hepatocellular carcinoma after liver transplantation in patients beyond Milan or UCSF criteria. Transplant Proc. 2010;42:4585–4592. doi: 10.1016/j.transproceed.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Hanson JA, Ason R, Weinreb J, Van Dyke A, Mitchell KA. Radiology estimates of viable tumor percentage in hepatocellular carcinoma ablation cavities correlate poorly with pathology assessment. Arch Pathol Lab Med. 2013;137:392–399. doi: 10.5858/arpa.2012-0126-OA. [DOI] [PubMed] [Google Scholar]
- 25.Oldhafer KJ, Chavan A, Frühauf NR, Flemming P, Schlitt HJ, Kubicka S, Nashan B, Weimann A, Raab R, Manns MP, et al. Arterial chemoembolization before liver transplantation in patients with hepatocellular carcinoma: marked tumor necrosis, but no survival benefit? J Hepatol. 1998;29:953–959. doi: 10.1016/s0168-8278(98)80123-2. [DOI] [PubMed] [Google Scholar]
- 26.Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, Bernard PH, Boillot O, Boudjema K, Calmus Y, et al. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11:767–775. doi: 10.1002/lt.20418. [DOI] [PubMed] [Google Scholar]
- 27.Terzi E, Golfieri R, Piscaglia F, Galassi M, Dazzi A, Leoni S, Giampalma E, Renzulli M, Bolondi L. Response rate and clinical outcome of HCC after first and repeated cTACE performed “on demand”. J Hepatol. 2012;57:1258–1267. doi: 10.1016/j.jhep.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Herber S, Biesterfeld S, Franz U, Schneider J, Thies J, Schuchmann M, Düber C, Pitton MB, Otto G. Correlation of multislice CT and histomorphology in HCC following TACE: predictors of outcome. Cardiovasc Intervent Radiol. 2008;31:768–777. doi: 10.1007/s00270-007-9270-8. [DOI] [PubMed] [Google Scholar]
- 29.Ravaioli M, Grazi GL, Ercolani G, Fiorentino M, Cescon M, Golfieri R, Trevisani F, Grigioni WF, Bolondi L, Pinna AD. Partial necrosis on hepatocellular carcinoma nodules facilitates tumor recurrence after liver transplantation. Transplantation. 2004;78:1780–1786. doi: 10.1097/01.tp.0000145892.97114.ee. [DOI] [PubMed] [Google Scholar]


