Abstract
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal neoplasms of the gastrointestinal tract. Less than 1% occurs in the esophagus. Surgery is the primary treatment for patients with GISTs. We report a 29-year-old male was admitted after the detection of a posterior mediastinal mass during work-up with routine examination. He did not have any disease-related symptoms. The physical examination was unremarkable. Chest computed tomographic scan, the barium esophagogram and endoscopic esophageal ultrasound showed benign neoplasm. The patient was performed an enucleation surgery through the right posterolateral thoracotomy. The pathology revealed a 13.0 cm × 12.0 cm × 5.0 cm mass. The tumor was CD117 (C-kit), PDGFRA and DOG1 positive. These findings were consistent with a GIST of the esophagus. So the diagnosis of GIST of esophagus was confirmed. The pathological diagnosis of low grade of GIST of esophagus was confirmed. The patient has no evidence of recurrence and is in good clinical conditions up-to date, five years after surgery.
Keywords: Esophageal gastrointestinal stromal tumor, Long-term survival, Enucleation, Surgery, Follow-up
Core tip: We report a case of giant esophageal gastrointestinal stromal tumor in a 29-year-old male successfully treated with enucleation. The patient has no evidence of recurrence and is in good clinical conditions up-to date, five years after surgery.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal neoplasms of the gastrointestinal tract. The most frequent anatomic location of GIST is the stomach (60%-70%), followed by small intestine (20%-25%), colon and rectum (5%), and esophagus (< 1%)[1]. They typically present in older individuals. Surgery is the initial treatment for the esophageal GISTs. We present a rare case of long-term and good quality of life after enucleation of a giant esophageal GIST.
CASE REPORT
A 29-year-old male was admitted to department of thoracic surgery after the detection of a posterior mediastinal mass during work-up with routine examination. He did not have any disease-related symptoms, such as dysphagia, chest pain or dyspnea. The physical examination was unremarkable. Results of laboratory studies were all within normal limits. Chest computed tomographic (CT) scan revealed a 13 cm hyperdense soft-tissue mass, originating from the posterior wall of the esophagus. The barium esophagogram demonstrated a multilobulated protuberant lesion with a relatively smooth margin in the middle thoracic esophagus. Endoscopic esophageal ultrasound (EUS) demonstrated a hypoechoic, homogeneous, well-demarcated mass, arising from the muscularis propria of the esophagus at 24 cm from the incisors and extending to 35 cm (Figure 1).
Figure 1.
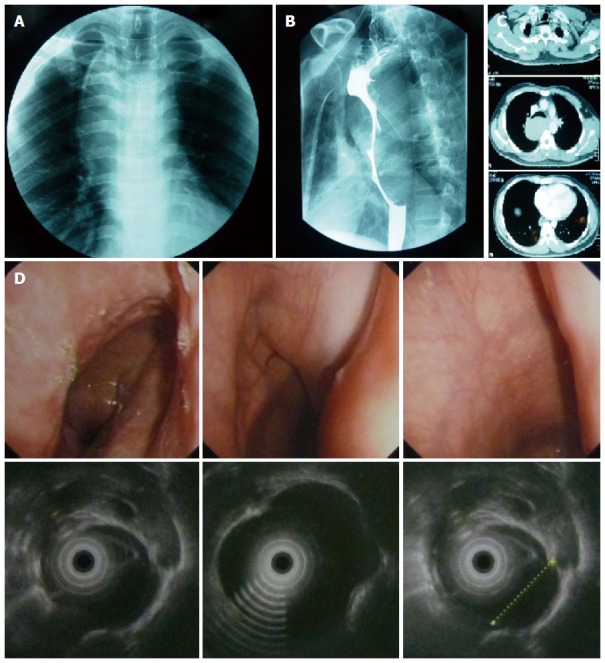
Mass showed before the surgery. A: The frontal chest radiograph showed a widened and undulated mediastinal contour; B: Barium esophagogram demonstrated a multilobulated protuberant lesion with a relatively smooth margin in the middle thoracic esophagus; C: Chest CT scan revealed a 13 cm × 5 cm × 6 cm hyperdense soft-tissue mass, originated from the posterior wall of the esophagus. The mass was well demarcated from the surrounding structures protruded into the esophageal lumen and compressed the trachea and the right main bronchus, and no metastases noted was found; D: ESU demonstrates a hypoechoic, homogeneous, well-demarcated mass, arising at 24 cm from the incisors and extending to 35 cm and arising from the muscularis propria of the esophagus.
Preoperative examination and intraoperative findings showed benign neoplasm. The patient was underwent an enucleation surgery through the right posterolateral thoracotomy. The outer esophageal muscle was incised longitudinally. Careful dissection was done to separate and remove the mass from the underlying submucosa. After careful dissection, the mass was enucleated completely with unbroken pseudocapsule. During the operation, one piece of mucosa was inadvertently opened during dissection, which was repaired by interrupted suture. The underlying mucosa and longitudinal muscle were reapproximated. The muscularis propria and mediastinal pleural also were sutured. Intraoperative endoscopy with air insufflation was used to confirm mucosal integrity and safeguard against esophageal perforation.
The pathology revealed a 13.0 cm × 12.0 cm × 5.0 cm mass with an intact pseudocapsule. The tumor was CD117 (C-kit), PDGFRA, DOG1 and SMA positive, CD34 and calponin and weak positive, and negative for desmin. These findings were consistent with a GIST of the esophagus. The mass contained less than 5 mitoses per 50 high-power fields. So the diagnosis of low grade of GIST of esophagus was confirmed. We also found that exon 12 and exon 18 of PDGFRA and exon 9,11,13,17 of C-kit all were wild type (Figure 2).
Figure 2.
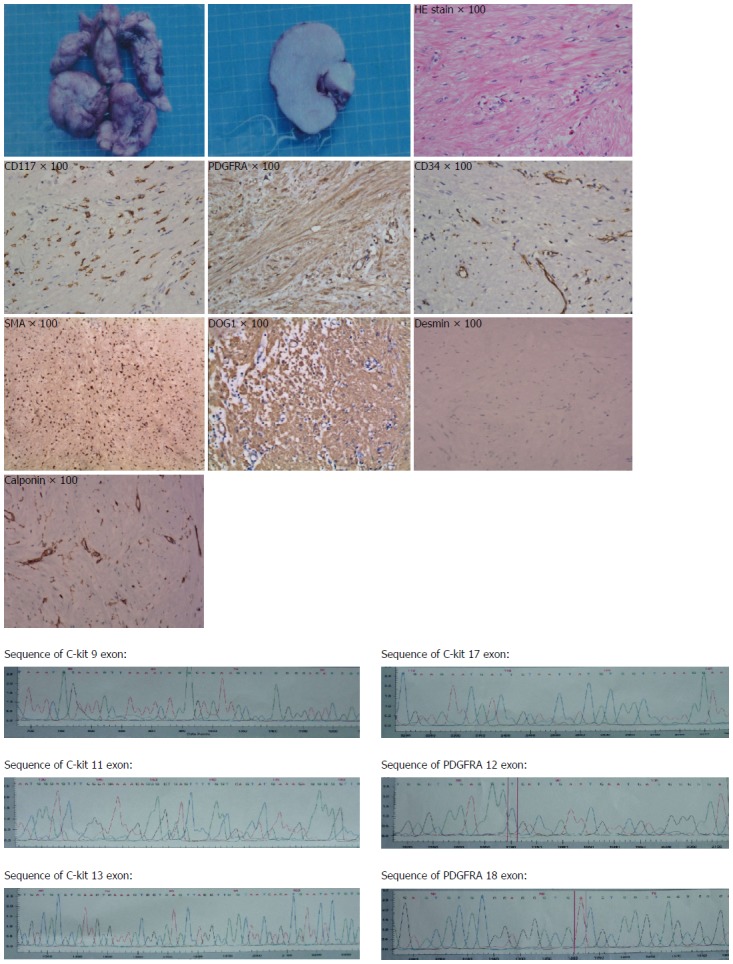
Pathology revealed a 13.0 cm × 12.0 cm × 5.0 cm mass with an intact pseudocapsule. The tumor was CD117 (C-kit), PDGFRA, DOG1 and SMA positive, CD34 and calponinand weak positive, and negative for desmin. Mutation sequencing found that exon 12 and exon18 of PDGFRA and exon 9,11,13,17 of C-kit all were wild type.
In one week postoperatively, the patient underwent an esophagogram that confirmed the absence of an esophageal leak or stricture. The patient had liquid diet one week after operation, swallowed most ordinary food with little difficulty and had no dysphagia two weeks later. At 5-year follow-up the patient is feeling well and CT scans have shown no evidence of recurrent disease.
DISCUSSION
GISTs are uncommon mesenchymal tumors of the gastrointestinal tract and rare before the age of 40 years, and less than 1% occur in the esophagus[1,2]. Bülbül Doğusoy et al[3] reports a multicenter study of 1160 gastrointestinal stromal tumors and found 0.7% to be located in the esophagus.
The diagnosis of esophageal GIST is frequently not clear preoperatively. Half of patients with esophageal GIST are asymptomatic and the remaining ones present with dysphagia or atypical chest pain. A complete diagnostic evaluation includes esophagoscopy, EUS, CT scan, and barium esophagogram. The presence of a rounded filling defect on barium esophagogram, an endoscopically normal-appearing mucosa, a well-circumscribed, non-enhancing mass on contrast -enhanced CT scan, and a homogeneous, hypoechoic mass with smooth borders on EUS examination will help in the differential diagnosis with other malignant neoplasms but not with esophageal leiomyoma. CT can be helpful in the preoperative assessment of tumor extent and presence of distant metastases. A definite diagnosis of GIST depends on a comprehensive pathologic examination of the surgical specimen. In GIST cases, approximately 95% are KIT (CD117)-positive, CD34 is positivity in 95% and SMA is positive in 20%-30%, but often nonreactive with S-100 protein immnostains[4]. CD117 and DOG1 positive are specific in GIST as compared with smooth muscle and neural tumors. In the case represented here, we diagnosed the mass was GIST mainly because of positive staining for CD117 and DOG1.
There were few effective therapeutic options for patients with GISTs. For patients with primary localized and resectable GIST, surgical resection remains the only chance for cure, as chemotherapy and radiation are ineffective. The primary goal of surgical management of GISTs is to achieve a negative microscopic margin[5,6].
The surgery options available for esophageal GISTs are fairly controversial, esophageal resection or GIST enucleation.
Since a true tumor capsule does not exist, the surgeon must exercise additional care during the removal of a GIST not to rupture the tumor, as this complication is associated with an increased risk for the development of peritoneal metastases[7]. Much like with most other soft tissue sarcomas, GISTs rarely metastasize to lymph nodes, thus a regional lymphadenectomy is not necessary, unless locoregional lymph nodes are enlarged[8-10]. It is extremely important to avoid tumor rupture in the surgery because it is associated very poor outcomes. The tumor should not be held with forceps and should be handled gently. If there is any possibility of tumor rupture, an en bloc combined with a resection or even abandoning surgery and converting to neoadjuvant treatment should be considered. In the case present, we sutured and then draged the tumor through the stitch during the operation to avoid rupturing the tumor. All the procedures in the enucleation were out of pseudocapsule. We must make sure that the enucleated tumor with unbroken pseudocapsule negative margins.
Activating mutations of KIT or platelet-derived growth factor receptor alpha (PDGFRA) are found in the vast majority of GISTs. Two KIT and PDGFRA kinase inhibitors, imatinib and sunitinib, have proven clinical benefit in patients with recurrent or metastatic GISTs and have been received approval from the FDA for the treatment of gastrointestinal stromal tumours. More than half of patients unfortunately develop resistance to imatinib after about 2 years, eventually develop resistance to these agents, resulting in fatal disease progression[11,12]. Some other structurally distinct inhibitors of KIT and PDGFRA kinases have been developed, Demetri et al[13] showed that oral regorafenib could provide a significant improvement in progression-free survival compared with placebo in patients with metastatic GIST after failure of imatinib and sunitinib. Ajuvant tyrosine-kinase inhibitor treatment is debated as its survival benefit is controversial. Blay et al[5] reported that patients with completely resected GISTs who received adjuvant imatinib after surgery had not improvement the overall survival, although lower recurrence rates. Patients with wild-type GISTs are less responsive to imatinib-based therapies. In the case presented here, the patient was wild-type GIST, so he didn’t take the adjuvant treatment.
The 5-year survival rate after complete resection of GISTs is approximately 50%-65%. Many factors including tumor size, mitotic rate, tumor location, kinase mutational status and occurrence of tumor rupture have been extensively studied and proposed to be predictors of survival outcomes. Tumor size and mitotic activity are the best prognostic features. Tumors are classified using a ranking system, grouping tumors into very low-, low-, intermediate-, and high-risk categories based on size (< 2 cm, 2-5 cm, 5-10 cm, and > 10 cm) and on number of mitoses within 50 high-power fields (HPFs); such measurements typically being reported as less than 5, 5 to 10, or greater than 10[14]. In the case represent here, the tumor size and the mitotic activity expressed per 50 HPFs were 13 cm and less than 5, respectively.
Katoh et al[15] reported 5 cases of GIST, and conclusion that if low malignancy GIST is completely removed by endoscopic resection, then no further treatment is necessary. In the case presented here, the patient did not receive any adjuvant therapy after surgery and no evidence of recurrent disease.
In the case presented here, the patient was diagnosed with a giant esophageal gastrointestinal stromal tumor in 29 years old. We also found that exon 12 and exon18 of PDGFRA and exon 9, 11, 13, 17 of C-kit all were wild type. These findings were consistent with a GIST of the esophagus. The mass contained less than 5 mitoses per 50 high-power fields. So the diagnosis of low grade of GIST of esophagus was confirmed. He did not receive any adjuvant therapy after surgery. At 5-year follow-up the patient is feeling well and CT scans have shown no evidence of recurrent disease.
COMMENTS
Clinical diagnosis
Computed tomographic (CT) the barium esophagogram; esophageal ultrasound (EUS) showed benign neoplasm from esophagus.
Differential diagnosis
In contrast to gastrointestinal stromal tumors (GISTs), leiomyoma and leiomyosarcoma are positive for SMA and desmin and negative for KIT and CD34.
Imaging diagnosis
Chest CT scan, the barium esophagogram and endoscopic EUS showed benign neoplasm in esophagus.
Pathological diagnosis
The authors do the immunohistochemistry for CD117, PDGFRA, DOG1, CD34, smooth muscle actin, desmin and calponin. We found that positive for CD117, PDGFRA, DOG1, CD34 and smooth muscle actin, negative for desmin and calponin.
Treatment
The authors enucleated a giant esophageal gastrointestinal stromal tumor in a 29-year-old male.
Related reports
Milman S reported enucleation of a giant esophageal gastrointestinal stromal tumor in an old man in 2009.
Experiences and lessons
The authors can enucleated a giant esophageal gastrointestinal stromal tumor and have good prognosis.
Peer review
The authors diagnosed the GISTs after the surgery, maybe we could diagnosed through biopsy or other methods before the surgery.
Footnotes
P- Reviewer: Gowrishankar S, Lee HW, Palazzo F S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 3.Bülbül Doğusoy G. Gastrointestinal stromal tumors: A multicenter study of 1160 Turkish cases. Turk J Gastroenterol. 2012;23:203–211. [PubMed] [Google Scholar]
- 4.Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11:728–734. [PubMed] [Google Scholar]
- 5.Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–578. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, Choi H, Corless CL, Debiec-Rychter M, DeMatteo RP, Ettinger DS, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5 Suppl 2:S1–S29; quiz S30. [PubMed] [Google Scholar]
- 7.Ng EH, Pollock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg. 1992;215:68–77. doi: 10.1097/00000658-199201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7:705–712. doi: 10.1007/s10434-000-0705-6. [DOI] [PubMed] [Google Scholar]
- 9.Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466–477. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 10.Agaimy A, Wünsch PH. Lymph node metastasis in gastrointestinal stromal tumours (GIST) occurs preferentially in young patients & lt; or = 40 years: an overview based on our case material and the literature. Langenbecks Arch Surg. 2009;394:375–381. doi: 10.1007/s00423-008-0449-5. [DOI] [PubMed] [Google Scholar]
- 11.Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 12.Gold JS, Gönen M, Gutiérrez A, Broto JM, García-del-Muro X, Smyrk TC, Maki RG, Singer S, Brennan MF, Antonescu CR, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045–1052. doi: 10.1016/S1470-2045(09)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M, Joensuu H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hueman MT, Schulick RD. Management of gastrointestinal stromal tumors. Surg Clin North Am. 2008;88:599–614, vii. doi: 10.1016/j.suc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Katoh T, Itoh Y, Mohri T, Suzuki H. Endoscopic enucleation of gastrointestinal stromal tumors of the stomach: report of five cases. World J Gastroenterol. 2008;14:2609–2611. doi: 10.3748/wjg.14.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]


