Abstract
Background
Lamivudine monotherapy is effective in suppressing hepatitis B virus (HBV) replication to undetectable levels by PCR, in ameliorating liver disease and to some extent in achieving HBsAg seroconversion. This study aimed at assessing the virological and biochemical responses as well as breakthrough in HBeAg-negative chronic HBV (CHB) Egyptian patients receiving lamivudine therapy.
Methods
This retrospective study included 140 CHB patients with positive serum HBV-DNA by quantitative PCR assays and negative HBeAg who had never received prior anti-viral therapy for HBV. According to duration of lamivudine therapy (100 mg/day) patients were grouped into: group I (n=59) who received lamivudine for 1 year, group II (n=50) who received lamivudine for 2 years, and group III (n=31) who received lamivudine for 3 years.
Results
In group I, 76.3% patients had virologic response but this was reduced in group II and group III to 72% and 67.7% respectively. None of the patients in group I developed virologic breakthrough, whereas 12% and 25.8% in groups II and III respectively developed breakthrough. In group I, 25% of patients having high pre-treatment viremia showed virologic response compared to 84.6% and 83.3% having mild and moderate viremia respectively (P<0.01). However, in groups II and III, there was no significant relationship between pre-treatment viremia and virologic response. No significant relationship was found between pre-treatment viral load and incidence of breakthrough within each group.
Conclusion
Lamivudine remains one of the antiviral therapies for HBeAg negative CHB patients. The rates of maintained virologic and biochemical responses to lamivudine decrease in time due to selection of drug-resistant mutants and, hence, breakthrough.
Keywords: Chronic HBV, lamivudine, HBeAg, virologic response, virologic breakthrough
Introduction
Approximately one third of the world’s population has serological evidence of past or present infection with hepatitis B virus (HBV) [1]. An estimated 350 million persons worldwide are chronically infected with HBV [2]. The global prevalence of HBsAg varies greatly and countries can be defined as having high, intermediate and low prevalence of HBV infection based on prevalence of HBsAg carriers of >8%, 2-7%, and <2% respectively [3].
Studies in the Middle East showed that the prevalence of HBsAg ranges from 3% to 11% in Egypt and genotype D is the most prevalent genotype [4,5]. A decrease in HBV incidence is expected among children in intermediate-endemicity countries (3-5% HBsAg prevalence), such as Egypt, where 90% immunization coverage has been achieved [6].
The aim of treatment of chronic HBV (CHB) patients is to achieve sustained suppression of HBV replication and remission of liver disease. The ultimate goal is to prevent cirrhosis, hepatic failure and hepatocellular carcinoma (HCC). Parameters used to assess treatment response include normalization of serum alanine aminotransferase (ALT), decrease or disappearance of serum HBV DNA level, loss of HBeAg with or without detection of anti-HBe, and improvement in liver histology. In patients with treatment maintained viral suppression, necroinflammation is reduced and decrease in fibrosis score as well as regression of cirrhosis was observed [7].
Approved antiviral therapies for CHB patients include standard interferon (IFN), Peg IFN and nucleoside/nucleotide analogues (NUCs) including lamivudine, adefovir dipivoxil, tenofovir, entecavir and telbivudine [8]. A major concern with long-term NUCs treatment is the occurrence of antiviral-resistant mutations. The rate at which resistant mutants occur is related to pretreatment serum HBV DNA level, rapidity of viral suppression, type and duration of treatment, and prior exposure to NUCs therapies [9].
Emergence of antiviral-resistant mutations (virologic breakthrough) can lead to negation of the initial response, and in some patients to hepatitis flares and hepatic decompensation. Antiviral-resistant mutations can be detected months and sometimes years before biochemical breakthrough [10].
The aim of our study was to assess the virological and biochemical responses as well as breakthrough rates in HBeAg-negative CHB Egyptian patients receiving lamivudine therapy.
Patients and methods
This is a retrospective study that included 140 CHB patients, diagnosed by persistent seropositivity for HBsAg more than 6 months with positive serum HBV-DNA by quantitative PCR assays and negative HBeAg and who had never received prior antiviral therapy for HBV.
They were scheduled for lamivudine (100 mg/day) in the Hepatology clinic of the National Hepatology and Tropical Medicine Research Institute (NHTMRI) in Cairo for at least one year up to 3 years during the period from August 2008 to July 2011 to assess the virological and biochemical responses. This study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Patients were randomly (computer based) grouped into: Group I: 59 patients who received lamivudine (100 mg/day) for 1 year; Group II: 50 patients who received lamivudine (100 mg/day) for 2 years; and Group III: 31 patients who received lamivudine (100 mg/day) for 3 years.
Inclusion criteria: 1) adult male or female patients (>18 years old); 2) positive serology for HBsAg for more than 6 months; 3) positive serum HBV- DNA by quantitative PCR assays; 4) negative serology for HBeAg; and 5) normal serum creatinine.
Exclusion criteria: 1) co-infection with hepatitis C virus (HCV); 2) positive serum anti-HBc IgM; 3) HBeAg positive patients; 4) patients who had received prior antiviral therapy for HBV; 5) patients having stigmata of liver cell failure e.g., ascites, encephalopathy; 6) pregnancy or breast feeding; 7) patients with organ transplants; and 8) patients receiving steroids and/or immunosuppressive drugs.
With approval of the ethics committee (after informed consent, history taking and complete physical examination) all patients were subjected to: 1) CBC, prothrombin time and INR; 2) liver biochemical profile: aspartate aminotransferase (AST), ALT, alkaline phosphatase (ALP), serum total and indirect bilirubin and serum albumin; 3) serum urea and creatinine; 4) anti-HCV antibodies (screened by 3rd generation EIA); 5) HBsAg, anti HBcAb IgG and IgM, HBeAg and anti HBeAb; 6) quantitative PCR of HBV-DNA; 7) abdominal ultrasound.
HBV DNA was assessed by sensitive quantitative PCR, using the COBAS Amplicor HBV Monitor test (Roche Diagnostics, Mannheim, Germany), according to the manufacturers’ instructions. Nucleic acid extraction was done from serum samples that were stored at -80°C. Prepared DNA was subjected to amplification using PCR. Serum HBV-DNA levels are expressed in IU/mL to ensure comparability between the assays. The lower limit of detection was 12 IU/mL. In general, an IU is equivalent to approximately 56 copies depending on the assay [11-13].
Evaluation of response to antiviral therapy and follow up was done (every six months) using the following parameters: 1) serum transaminases (AST and ALT every 3-6 months); 2) HBsAg and anti HBsAb; 3) quantitative HBV-DNA assay using PCR; 4) α-fetoprotein level; 5) serum urea and creatinine; and 6) abdominal ultrasound.
HBV DNA was done every six months from the start of therapy, as AASLD guidelines 2009 stated that “Primary non-response is decrease in serum HBV DNA by ≤2 log10 IU/mL after at least 24 weeks of therapy” [14].
Virologic response to NUCs (e.g. lamivudine) was defined as undetectable HBV-DNA by real-time PCR assay with therapy [15]. Virologic breakthrough was defined as increase in serum HBV DNA by >1 log10 (10-fold) above nadir (lowest value) after achieving virologic response, during continued treatment [1]. The patients responding to treatment continued to receive lamivudine, and patients who developed breakthrough were shifted to entecavir as it is available in the NHTMRI. Entecavir was given with a dose of 1 mg as they were not nucleoside naïve patients.
It is concluded that, after lamivudine resistance, the first option is to add adefovir or tenofovir, and the second choice is to stop lamivudine and start entecavir [14]. So, entecavir was chosen as it is the available drug in the NHTMRI.
Statistical analysis
Data were explored for normality using Kolmogorov-Smirnov test of normality. The results of Kolmogorov-Smirnov test indicated that some data were normally distributed (parametric data) and some were not normally distributed so both parametric and non parametric tests were used for comparisons accordingly.
Comparison between quantitative variables was carried out by the Student’s t-test of two independent samples. Repeated measures analysis of variance (ANOVA) test was used instead of t-test when comparing more than two groups of independent variables. Comparison between non parametric quantitative variables was carried out by Mann-Whitney U test. Kruskal-Wallis test was used when comparing between more than two groups of independent variables. The percentage change for ALT level was calculated as follows: level (after) - level (before) /level(before) × 100.
Comparison between qualitative variables was carried out by Chi-Square test (χ2). Fisher’s exact test was used instead of Chi-square test when one expected cell or more to be ≤5. Binary correlation was carried out by Pearson correlation test. Results were expressed in the form of correlation coefficient (R) and P-values. The results were assessed as P-value that was differentiated into: non-significant when P-value >0.05, significant when P-value ≤0.05, highly significant when P-value ≤0.01. Analysis of data was performed using SPSS 17 (Statistical Package for Scientific Studies) for Windows.
Results
This study included 120 males and 20 female patients with a male: female ratio of 6:1. Group I included 53 males and 6 females, group II included 40 males and 10 females and group III included 27 males and 4 females. The mean age in the whole studied population was 34.41±7.92 years. The mean age in group I was 34.69±7.50 years, in group II it was 33.66±8.57 years and in group III it was 35.06±7.78 years. The age or sex showed no significant difference among the 3 groups (P>0.05).
The mean pretreatment ALT was 56.36±36.18 IU/L in group I, 50.00±45.83 IU/L in group II and 59.39±54.79 IU/L in group III. While the mean post treatment ALT was 37.86±24.79 IU/L in group I, 37.24±26.28 IU/L in group II and 32.03±18.16 IU/L in group III. All our patients had elevated or fluctuating ALT levels as patients with persistently normal ALT are not indicated for treatment unless they have high HBV DNA and inflammation with or without fibrosis by liver biopsy according to guidelines [14].
On comparison between pre-treatment and post treatment ALT level within each group, the mean paired difference in group I and group III was 18.49±30.56 IU/L and 27.35±54.10 IU/L respectively which was statistically highly significant (P<0.001 and 0.009 respectively). Meanwhile, the mean paired difference in group II was 12.76±33.93 IU/L and it was statistically significant (P<0.011). This reflects biochemical response within each group as a result of lamivudine therapy.
There was highly significant positive correlation between pre-treatment and post treatment ALT levels within groups I and II while the correlation was non-significant between pre-treatment and post treatment ALT levels within group III (R 0.551, 0.681 and 0.204 respectively and P<0.001, <0.001 and 0.272 respectively).
The mean HBV-DNA viral load prior to lamivudine therapy was 6,524,873.5±44,470,000 IU/mL and there was no significant difference among the 3 groups (P>0.05) (Table 1).
Table 1.
Pre-treatment HBV-DNA among the studied groups
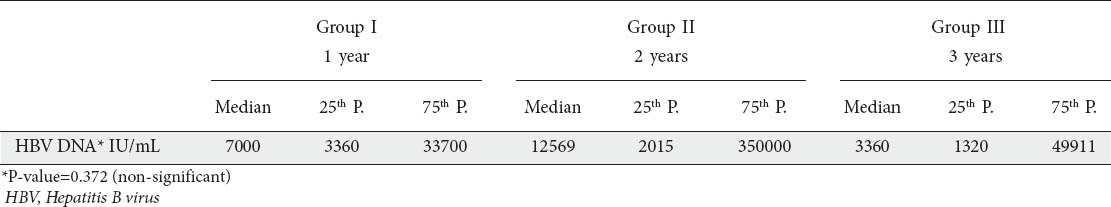
HBV-DNA assay following treatment showed that, in group I, 76.3% of patients had virologic response, compared to 23.7% patients who showed persistent viremia. Virologic response fell in group II and group III to 72% and 67.7% respectively. On comparison of virologic response among the 3 groups, there was no significant difference (P>0.05) (Table 2).
Table 2.
Post treatment virologic response and breakthrough among the three groups
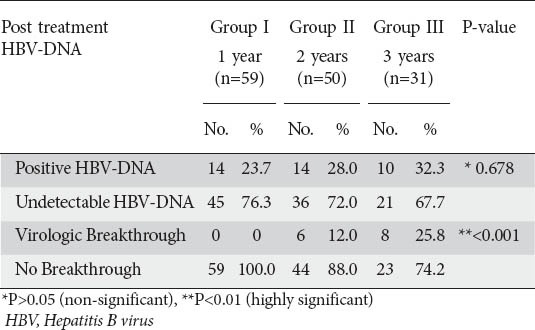
None of the patients in group I developed virologic breakthrough during lamivudine therapy, whereas 12% and 25.8% of patients in groups II and III respectively developed virologic breakthrough with continued treatment. This showed a highly significant difference among the three groups (P<0.001) (Table 2).
None of the patients in group I and group II had HBsAg clearance, while one patient in group III had HBsAg clearance without developing anti HBs, denoting no statistical significance among the 3 groups (P>0.05).
Abdominal ultrasound examination of patients at the end of treatment duration showed no significant difference among the three groups.
By studying the relationship between pre-treatment viremia and virologic response, we found that in group I, 25% of patients having high pre-treatment viremia (more than 20000 IU/mL) showed virologic response compared to 84.6% and 83.3% of patients having mild and moderate viremia respectively. This showed a highly significant relationship between pre-treatment high viremia and the incidence of viral resistance (P<0.01). However, in group II and group III, there was no significant relationship between the incidence of virologic response and pre-treatment viral load within each group (P>0.05) (Table 3).
Table 3.
Relation between pre-treatment viral load and virologic response
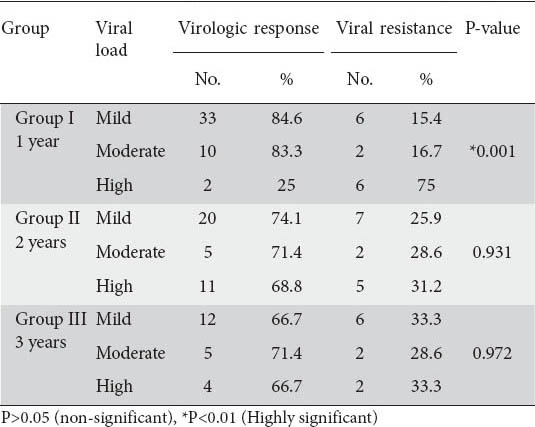
Also, there was no significant relationship between gender, age, pre-treatment ALT level, post treatment ALT level, percent reduction in ALT level and abdominal ultrasonographic findings in the incidence of virologic response within each group (P>0.05).
On the other hand, there was no significant relationship between pre-treatment viral load and the incidence of virologic breakthrough within each group (P>0.05). Also, there was no significant relationship between gender, age, pre-treatment ALT level, post treatment ALT level, percent reduction in ALT level and also abdominal ultrasonographic findings in the incidence of virologic breakthrough within each group (P>0.05).
There were no recorded signs of decompensation in patients with virologic breakthrough as they were shifted to entecavir with proper suppression of HBV replication. None of our cirrhotic patients developed virologic breakthrough. For other patients, the time between virologic breakthrough and biochemical breakthrough was three months as transaminases were done every 3-6 months, and once the patient developed elevation of HBV DNA by >1 log10 (10-fold) above nadir (lowest value) with or without elevation of ALT they were immediately shifted to entecavir 1 mg q.d. None of our patient developed deterioration of creatinine level, so, adjustment of the dose of lamivudine was not needed.
Discussion
HBV infection is a global health problem and is a major health problem in Egypt and the entire continent of Africa. Egypt is considered to be a region of intermediate prevalence for HBV infection with a reported figure of 4.5%. Nearly 2-3 million Egyptians are chronic carriers of HBV [16]. In Egypt, HBeAg negative variant state accounts for more than 80% among the older age group (22-45 years) [17].
The goal of therapy for HBV is to improve quality of life and survival by preventing progression of the disease. This goal can be achieved if HBV replication can be suppressed in a sustained manner, the accompanying reduction in histological activity of CHB lessening the risk of cirrhosis, end-stage liver disease and decreasing the risk of HCC in non-cirrhotic patients and probably also, but to a lesser extent, in cirrhotic patients [18]. However, HBV infection cannot be completely eradicated owing to the persistence of cccDNA in the nucleus of infected hepatocytes [19].
A male predominance was found, in our study, as there were 120 males compared to 20 female patients (male: female ratio is 6:1). This came close to El-Zayadi’s [17] study, who reported high prevalence of CHB among males with a male: female ratio of 9:1 and also, came closer to the study conducted by Osman [20], where there was male predominance with a male: female ratio of about 7:1.
The mean age in our studied population was 34.41±7.92 years. Similarly, in the study conducted by Osman [20], the mean age was 32±8 years. Also, El-Zayadi [17] stated that in Egypt, HBeAg negative variant state, as was our patients’ state, accounts for more than 80% among the 22-45 years age group.
Virologic response had occurred in 76.3% of patients of group I but was reduced in group II and group III to 72% and 67.7% respectively. Likewise, Marcellin et al [21] reported virological response in 72% of HBeAg negative CHB patients at 1 year. Also, Papatheodoridis et al [22] detected decreased virologic remission from 73% at 12 months to 34% at 48 months.
However, cumulative rate of virologic response in our study appeared to be higher than that of the study conducted by Osman [20], who reported that HBV-DNA became undetectable in almost 50% of the patients. This could be explained by the presence of HBeAg-positive patients who are more likely to develop lamivudine resistance as reported by Silva et al [23]. They concluded that pretreatment HBeAg negativity could be considered as a positive predictive factor for response to lamivudine therapy, even in patients of high viremia. Also, it could be related to longer duration of treatment in Osman’s study [20] (up to 5 years) giving a chance for selection of drug resistant mutants as mentioned by Lok et al [24], who reported that rates of lamivudine resistance reach nearly 70% by year four of continuous therapy.
In our study, none of the patients showed HBsAg clearance in group I and group II. Similarly, EASL [25], reported that loss of HBsAg rates after one year were 0% with lamivudine, adefovir, entecavir, telbivudine or tenofovir. Also, in Osman’s [20] study none of the patients developed HBsAg seroconversion.
Only one case (3.23%) in group III showed post treatment HBsAg clearance. Fasano et al [26] found that in long-term responder patients, continuation of lamivudine monotherapy resulted in persistent viral suppression in most cases and 11.7% of these patients cleared HBsAg (after a 32-month median period).
Long-term lamivudine treatment was shown to decrease fibrosis [27] and in a randomized controlled trial, it was proved to decrease the incidence rate of hepatic decompensation and HCC [28]. In our study, the patients were not biopsied, but out of the 140 patients of the study only 3 patients showed liver cirrhosis (diagnosed by coarse liver with irregular surface by ultrasound with or without eosophageal varices by endoscopy), meanwhile none of them developed decompensated cirrhosis or HCC on follow up by ultrasound. Also, Osman [20], reported that none of the studied 85 patients developed cirrhosis or HCC on follow up and only one patient had presented with decompensated cirrhosis before the start of therapy and on follow up, showed improvement in serum billirubin and prothrombin activity.
There was highly significant positive correlation between pre-treatment and post treatment ALT levels within groups I and II. This indicates that lamivudine was effective in decreasing inflammation in liver tissue by effective viral suppression leading to significant decrease in ALT levels in comparison to pretreatment levels. However, the correlation was non-significant between pre-treatment and post treatment ALT levels within group III. This could be related to higher incidence of virologic breakthrough in this group.
The virologic breakthrough, in our study, was similar to the Chang et al study [29], as the cumulative rate of virologic breakthrough in the HBeAg-negative group was 0% and 7% at 12th and 24th months of lamivudine therapy, which was significantly lower than in the HBeAg-positive group that recorded 12% and 39% at 12th and 24th months respectively (P<0.01). Also, Osman [20], reported overall breakthrough rate of 35% in HBeAg-negative patients and this came close to our study results. However, Rizzetto [30] recorded higher rates. They detected virologic breakthrough in up to 57-64% in HBeAg-negative patients after 2 years. Also, Park et al [31] reported cumulative rates of 0%, 19.4%, 36%, and 48.5% in 6, 12, 18, and 24 months respectively. Similarly, Alam et al [32] reported breakthrough rates of 4.4, 22.8, 45.3, and 74% at 1, 2, 3, and 4 or more years, respectively.
The explanation of the rather low rates of virologic breakthrough in our study is unknown; it could be due to the genetics of Egyptian patients, the design of the study or viral genetics. This indicates that regional characteristics may have some major importance.
However, the fact that virologic breakthrough is less frequent in our study than in other populations together with the low cost of lamivudine treatment suggests that in some patients lamivudine can still be useful.
The highly significant difference among our 3 groups in the incidence of virologic breakthrough meant that longer duration of treatment was associated with an increased rate of lamivudine resistance and hence virologic breakthrough. This was reported also by Lok et al [33], who concluded that the longer the duration of treatment the higher rate of lamivudine resistance.
A highly significant relationship was found, in our study, between pre-treatment high viremia and the incidence of viral resistance. Lok and McMahon [34] also reported that a high pre-treatment serum HBV-DNA level is one of the factors associated with increased rate of lamivudine resistance. This was different from results in groups II and III where there was no significant relationship between pre-treatment HBV-DNA viral load and the incidence of viral resistance.
Neither demographic characteristics (age, gender etc.) of the patients, ultrasound findings, nor pre or post treatment ALT levels had a significant relation with the incidence of virologic response or breakthrough. Similarly, Hongthanakorn et al [35] reported that an alarmingly high rate of virologic breakthrough is met in clinical practice with NUCs and failure to achieve undetectable HBV-DNA was the only factor significantly associated with virologic breakthrough. Also, Alam et al [32] showed that pretherapy ALT (P=0.698), HBeAg status (P=0.273), and age (P=0.059) were not associated with breakthrough, however, in their study, female sex was significantly associated with virologic breakthrough (P=0.01).
The explanation of absence of a relationship between high viremia and breakthrough in our patients is unknown. However, other studies showed a significant relationship between pretreatment high viremia and development of breakthrough after prolonged lamivudine therapy [36], after liver transplantation with HBIG and lamivudine prophylaxis [37] and after chemotherapy treatment with lamivudine prophylaxis [38].
In patients with cirrhosis and liver failure the breakthrough has a high risk for decompensation and death. This is well recognized by many authors and experts [25,39]. Also, Manolakopoulos et al [40], reported that decompensation develops rapidly without allowing enough time to deal with a new agent and they have concluded that the efficacy of any kind of therapeutic intervention is associated with the severity of liver disease before treatment. Fortunately, none of our three cirrhotic patients developed virologic breakthrough. Also, our patients had been closely monitored with LFT and HBV DNA by PCR to detect virologic breakthrough before clinical breakthrough and were immediately shifted to entecavir 1 mg q.d., as it is recorded that virologic breakthrough can be detected months before biochemical or clinical breakthrough (characterized by an increase in ALT levels) [14].
The switch from lamivudine to entecavir was not the best choice in our patients as entecavir is not highly effective for patients with YMDD mutation due to cross resistance. It is well known that, after lamivudine resistance, the first option is to switch to tenofovir or add adefovir if tenofovir is not available, and the second choice is to stop lamivudine and start entecavir [14,25,39]. But, entecavir was chosen as it is the available drug in the NHTMRI.
In conclusion, lamivudine therapy is effective in suppressing serum HBV-DNA to undetectable levels and in ameliorating liver disease in HBeAg-negative CHB. The rates of maintained virologic and biochemical responses to lamivudine therapy decrease with time due to selection of drug-resistant mutants and, hence, breakthrough. Whenever possible, the most potent NUCs with the lowest rate of genotypic resistance such as entecavir and tenofovir should be administered and compliance reinforced. However, the fact that virologic breakthrough is less frequent in this study together with the low cost suggests that in some patients lamivudine can still be useful.
Summary Box.
What is already known:
For the treatment of chronic hepatitis B (CHB):
Lamivudine monotherapy is currently considered a therapeutic option for patients with CHB irrespective of HBeAg status
Lamivudine achieves sustained suppression of hepatitis B virus (HBV) replication and remission of liver disease aiming at preventing cirrhosis (if not present), hepatic failure and hepatocellular carcinoma
Long-term lamivudine treatment leads to the occurrence of antiviral-resistant mutations (virologic breakthrough) which can lead to negation of the initial response, and in some patients hepatitis flares and hepatic decompansation
The lamivudine reported breakthrough rates are around 4.4%, 22.8%, 45.3%, and 74% at 1, 2, 3, and 4 or more years, respectively
What the new findings are:
Lamivudine monotherapy, in HBeAg-negative CHB, was effective in suppressing HBV replication and ameliorating liver disease
Virologic response to lamivudine was 76.3% in the first year and decreased to 72% and 67.7% at years 2 and 3 respectively
The lamivudine breakthrough rates, in our study, were 0%, 12% and 25.8% at 1, 2, 3 years respectively
Our virologic breakthrough patients were immediately shifted to entecavir (although it is not the best first option) and fortunately none of them developed hepatitis flares and hepatic decompansation
The fact that virologic breakthrough is less frequent in this study together with the low cost suggests that, in some patients, lamivudine can be still useful
Biography
Cairo University, Egypt
Footnotes
Conflict of Interest: None
References
- 1.Lok AS, Zoulim F, Locarnini S, et al. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. J Hepatol. 2007;46:254–265. doi: 10.1002/hep.21698. [DOI] [PubMed] [Google Scholar]
- 2.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 3.McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25(Suppl 1):3–8. doi: 10.1055/s-2005-915644. [DOI] [PubMed] [Google Scholar]
- 4.Qirbi N, Hall AJ. Epidemiology of hepatitis B virus infection in the Middle East. East Medit Health J. 2001;7:1034–1045. [PubMed] [Google Scholar]
- 5.Khaled IA, Mahmoud OM, Saleh AF, Bioumie EE. Prevalence of HBV genotypes among Egyptian hepatitis patients. Mol Biol Rep. 2011;38:4353–4357. doi: 10.1007/s11033-010-0562-8. [DOI] [PubMed] [Google Scholar]
- 6.Zakaria S, Fouad R, Shaker O, et al. Changing patterns of acute viral hepatitis at a major urban referral center in Egypt. Clin Infect Dis. 2007;44:e30–e36. doi: 10.1086/511074. [DOI] [PubMed] [Google Scholar]
- 7.Dienstag JL, Goldin RD, Heathcote EJ, et al. Histological outcome during long-term lamivudine therapy. J Gastroenterol. 2003;124:105–117. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 8.Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. J Hepatol. 2007;45:1056–1075. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- 9.Bartholomeusz A, Locarnini SA. Antiviral drug resistance: clinical consequences and molecular aspects. Semin Liver Dis. 2006;26:162–170. doi: 10.1055/s-2006-939758. [DOI] [PubMed] [Google Scholar]
- 10.Fung SK, Chae HB, Fontana RJ, et al. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44:283–290. doi: 10.1016/j.jhep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Saldanha J, Gerlich W, Lelie N, et al. An international collaborative study to establish a World Health Organization international standard for hepatitis B virus DNA nucleic acid amplification techniques. Vox Sang. 2001;80:63–71. doi: 10.1046/j.1423-0410.2001.00003.x. [DOI] [PubMed] [Google Scholar]
- 12.Pawlotsky JM. Hepatitis B virus (HBV) DNA assays (methods and practical use) and viral kinetics. J Hepatol. 2003;39(Suppl 1):S31–S35. doi: 10.1016/s0168-8278(03)00136-3. [DOI] [PubMed] [Google Scholar]
- 13.Pawlotsky JM, Dusheiko G, Hatzakis A, et al. Virologic monitoring of hepatitis B virus therapy in clinical trials and practice: recommendations for a standardized approach. J Gastroenterol. 2008;134:405–415. doi: 10.1053/j.gastro.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AASLD practice guidelines Chronic hepatitis B: update 2009. Hepatology. 2009;50:1–36. [Google Scholar]
- 15.EASL Clinical Practice Guidelines. Management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Shaaban FA, Hassanin AI, Samy SM, Salama SI, Said ZN. Long-term immunity to hepatitis B among a sample of fully vaccinated children in Cairo, Egypt. East Medit Health J. 2007;13:750–757. [PubMed] [Google Scholar]
- 17.El-Zayadi A. Hepatitis B virus infection: the Egyptian situation. Arab J Gastroenterol. 2007;8:94–98. [Google Scholar]
- 18.Liaw YF, Chien RN, Yeh CT. No benefit to continue lamivudine after the emergence of YMDD mutations. Antivir Ther. 2004;9:145–148. [PubMed] [Google Scholar]
- 19.Pollicino T, Belloni L, Raffa G, et al. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. J Gastroenterol. 2006;130:823–837. doi: 10.1053/j.gastro.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Osman WS. Thesis for Master Degree in Hepatology. Menoufiya University; 2011. Incidence of genotypic resistance to lamivudine long term therapy in chronic hepatitis B. [Google Scholar]
- 21.Marcellin P, Bonino F, Lau GK, et al. Virological and biochemical response in patients with HBeAg-negative CHB treated with peginterferon alfa-2a (40 kD) + lamivudine: 3-year follow-up results. J Hepatol. 2007;46(Suppl 1):S25–S26. [Google Scholar]
- 22.Papatheodoridis GV, Dimou E, Dimakopoulos K, et al. Outcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with Lamivudine. Hepatology. 2005;42:121–129. doi: 10.1002/hep.20760. [DOI] [PubMed] [Google Scholar]
- 23.Silva LC, Fonseca LE, Carrilho FJ, et al. Predictive factors for response to lamivudine in chronic hepatitis B. Rev Inst Med Trop Sao Paulo. 2000;42:189–196. doi: 10.1590/s0036-46652000000400003. [DOI] [PubMed] [Google Scholar]
- 24.Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000 summary of a workshop. J Gastroenterol. 2001;120:1828–1853. doi: 10.1053/gast.2001.24839. [DOI] [PubMed] [Google Scholar]
- 25.EASL Clinical Practice Guidelines. Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Fasano M, Lampertico P, Marzano A, et al. HBV-DNA suppression and HBsAg clearance in HBeAg negative chronic hepatitis B patients on lamivudine therapy for over 5 years. J Hepatol. 2012;56:1254–1258. doi: 10.1016/j.jhep.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Poynard T, Zoulim F, Ratziu V, et al. Longitudinal assessment of histology surrogate markers (fibrotest–actitest) during lamivudine therapy in patients with chronic hepatitis B infection. Am J Gastroenterol. 2005;100:1970–1980. doi: 10.1111/j.1572-0241.2005.41957.x. [DOI] [PubMed] [Google Scholar]
- 28.Hann HL, Fontana RJ, Wright T, et al. A United States compassionate use study of lamivudine treatment in non-transplantation candidates with decompensated hepatitis B virus–related cirrhosis. Liver Transplant. 2003;9:49–56. doi: 10.1053/jlts.2003.50005. [DOI] [PubMed] [Google Scholar]
- 29.Chang YJ, Yim JY, Cho NY, Choi CW, Baek SJ. Viral breakthrough in HBeAg-negative chronic hepatitis B patients receiving lamivudine therapy. Taehan Kan Hakhoe Chi Dec. 2002;8:397–404. [PubMed] [Google Scholar]
- 30.Rizzetto M. Efficacy of lamivudine in HBeAg-negative chronic hepatitis B. J Med Virol. 2002;66:435–451. [PubMed] [Google Scholar]
- 31.Park HE, Lee DH, Heo J, et al. Correlation of HBV DNA level and viral breakthrough during lamivudine therapy for chronic hepatitis B. Korean J Hepatol. 2006;12:173–183. [PubMed] [Google Scholar]
- 32.Alam S, Azam G, Mostafa G, et al. Pretreatment and on-treatment predictors of viral breakthrough in lamivudine therapy for chronic hepatitis B. Hepatol Int. 2008;2:494–497. doi: 10.1007/s12072-008-9095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. J Gastroenterol. 2003;125:1714–1722. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 34.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:507–539. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 35.Hongthanakorn C, Chotiyaputta W, Oberhelman K, et al. Virological breakthrough and resistance in patients with chronic hepatitis B receiving nucleos(t)ide analogues in clinical practice. J Hepatol. 2011;53:1854–1863. doi: 10.1002/hep.24318. [DOI] [PubMed] [Google Scholar]
- 36.Yuen MF, Sablon E, Hui CK, et al. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology. 2001;34:785–791. doi: 10.1053/jhep.2001.27563. [DOI] [PubMed] [Google Scholar]
- 37.Chun J, Kim W, Kim BG, et al. High viremia, prolonged lamivudine therapy and recurrent hepatocellular carcinoma predict posttransplant hepatitis B recurrence. Am J Transplant. 2010;10:1649–1659. doi: 10.1111/j.1600-6143.2010.03162.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim IK, Kim BG, Kim W, et al. Clinical prediction of failure of lamivudine prophylaxis for hepatitis B virus-infected patients undergoing cytotoxic chemotherapy for malignancy. Antimicrob Agents Chemother. 2012;11:5511–5519. doi: 10.1128/AAC.00821-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.EASL Clinical Practice Guidelines. The International Liver Congress 2013 Abstract Book. J Hepatol. 2013;58:S229–S408. [Google Scholar]
- 40.Manolakopoulos S, Karatapanis S, Elefsiniotis J, et al. Clinical course of lamivudine monotherapy in patients with decompensated cirrhosis due to HBeAg negative chronic HBV infection. Am J Gastroenterol. 2004;99:57–63. doi: 10.1046/j.1572-0241.2003.04021.x. [DOI] [PubMed] [Google Scholar]


