Abstract
Recently, Xue, Atallah, and Scanziani reported that excitation/inhibition ratios across cortical pyramidal neurons are equalized by activity-dependent modulations of parvalbumin-neuron mediated feedforward inhibition. Their results raise questions about the developmental formation of this excitation-inhibition balance and the potential activity-dependent synaptic plasticity rules that mediate this process.
Across various neural networks, excitatory and inhibitory synaptic inputs are found to be tightly coupled. For example, in sensory cortical neurons, simple stimuli such as an oriented bar elicit an increase in excitatory synaptic conductance along with a concomitant increase in inhibitory conductance [1,2]. Concurrence of synaptic excitation and inhibition is also observed during spontaneous activity, network oscillations, and “up-state” persistent activity [1,3,4]. A prominent feature of this relationship is that inhibition appears in balance with excitation. That is, afferent activity induces inhibition, usually following excitation after a brief temporal delay, and this inhibition is somewhat proportional to the excitation generated by either afferent or local activity. Ultimately, this results in a relatively constant excitation/inhibition (E/I) ratio across different sensory stimuli and approximate co-tuning of excitation and inhibition for sensory attributes [1,2,5,6]. Such proportionality tightly controls neural excitability, prevents output saturation, and increases operational ranges [7].
On a network level, balanced inhibition allows a progressive recruitment of firing neurons as the number of active afferents increases, so that a broad range of afferent activity can be differentially represented by neuronal populations. Additionally, delayed and balanced inhibition restricts the spatial and temporal spread of activity, preventing epileptiform discharges and excitotoxicity. Finally, balanced inhibition contributes to sharpening the tuning of neurons to specific sensory features [1,2]. Thus, it is conceivable that disrupting this excitation-inhibition (E-I) balance could impair brain function, possibly contributing to neurological disorders such as autism and schizophrenia.
While E-I balance has been reported for various types of principal neuron, how neural circuits are adjusted to achieve this balance is not well understood. Inhibition, which is delayed relative to excitation, is provided by inhibitory neurons through feedforward or feedback circuits. In the cortex, inhibitory neurons contact nearby excitatory neurons rather indiscriminately [8]. Do these excitatory neurons, which receive excitatory inputs of variable strengths, receive inhibitory inputs of similar amplitudes, or does the inhibitory input vary in amplitude in accordance with the strength of excitation onto each individual cell? In a recent study [9], Scanziani’s group set out to address this question in visual cortical slices by simultaneously recording from multiple nearby layer (L) 2/3 pyramidal neurons while optogenetically stimulating L4 excitatory cells, utilizing a L4-specific Cre driver mouse line. In each recorded cell, stimulation generated both an excitatory and an inhibitory response, with the amplitudes of these conductances varying greatly among cells. Surprisingly, the E/I ratio varied much less compared to the synaptic amplitudes, providing initial evidence that E/I ratios are somewhat equalized across pyramidal cells. To exclude the possibility that this is a slice artifact, they utilized a mouse line where the promoter of the activity-dependent gene Fos drives the expression of Fos fused to enhanced green fluorescence protein (EGFP). The EGFP-positive (EGFP+) pyramidal neurons received significantly stronger excitation and also stronger inhibition than their EGFP-negative (EGFP−) neighbors. Nonetheless, E/I ratios were similar between the two groups of pyramidal cells, further confirming that E/I ratio is kept relatively constant across the pyramidal cell population. Next, by optogenetically stimulating parvalbumin-positive (PV) and somatostatin-positive (SOM) inhibitory neuron populations, they demonstrated that inhibitory inputs from PV neurons were stronger in EGFP+ than EGFP− cells, while those from SOM neurons were similar between the two pyramidal-cell groups. Therefore, it is PV neurons that contribute to the observed equalization of E/I ratios across pyramidal cells.
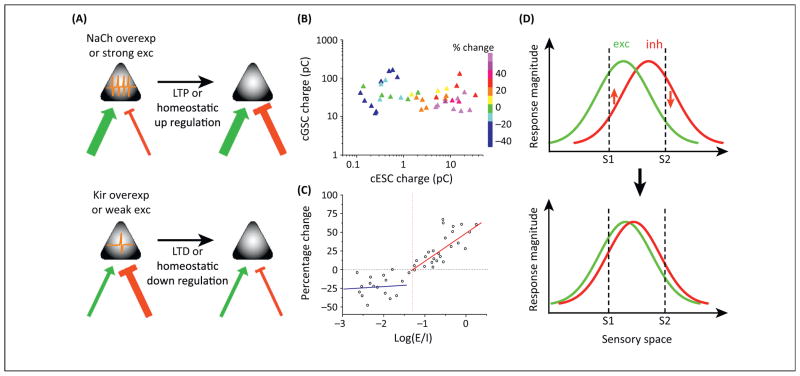
A prominent physiological difference between the EGFP+ and EGFP− neurons is that EGFP+ neurons fire more strongly than their EGFP− neighbors both spontaneously and in response to visual stimulation. This raised the question of whether the level of a cell’s spiking activity is used as a signal to instruct equalization of E/I ratios. For example, if a pyramidal cell receives strong inhibition but weak excitation, its spiking activity is low. This may be a signal for the cell to increase excitation or decrease inhibition until a specific E/I ratio is reached. To test this hypothesis, Xue and colleagues manipulated pyramidal cell activity by overexpressing an inward rectifying K+ channel (Kir2.1) to reduce activity, or a bacterial voltage-gated Na+ channel (NaChBac, which has a more negative activation threshold than endogenous Na+ channels) to increase activity. In Kir2.1-overexpressing pyramidal cells, inhibition was weakened compared with control cells, while excitation was not affected, resulting in a larger E/I ratio than control cells. The reduced inhibition was due to a selective decrease of PV-neuron mediated inhibition, achieved at least partially by changing the strength of the synaptic connection made by a single PV neuron onto its target cell. Conversely, enhancing cell activity resulted in a selective increase of PV-inhibition. In contrast to these changes in PV-mediated inhibition, SOM-neuron mediated inhibition was left unaffected. These results suggest that the equalization of E/I ratios is primarily achieved by adjusting PV-inhibition according to the level of a cell’s activity (Fig. 1A).
Fig. 1.
Plasticity rules underlying the formation of E-I balance. (A) High levels of spiking activity of the pyramidal cell resulting from strong excitation (green arrow) or overexpression of Na+ channels induce potentiation of its inhibitory input (red bar), decreasing the E/I ratio. Conversely, low levels of spiking activity resulting from weak excitation or overexpression of Kir channels induce depression of the inhibitory input, increasing the E/I ratio. (B) Repetitive visual stimulation induced changes of GABAergic input strength in relation to the strength of the GABAergic input and its co-activated gultamatergic input prior to the stimulation (adapted from [12]). (C) The magnitude of GABAergic plasticity as a function of E/I ratio (adapted from [12]). The vertical dash line indicates an “optimal” E/I ratio. Solid red and blue lines are linear regression lines for data points above and below this optimal value, respectively. (D) Matching of excitatory (green) and inhibitory (red) tuning. S1 and S2 are two different stimuli in the sensory space. In early circuits, S1 evokes much stronger excitation than inhibition, while S2 evokes stronger inhibition than excitation. Repetitive S1 and S2 stimulation would induce potentiation (upward arrow) of S1 activated inhibition, but depression (downward arrow) of S2 activated inhibition, leading to a progressive matching of excitatory and inhibitory tuning, as previously described [15].
Several interesting questions arise from these findings. For example, by ubiquitously activating L4 neurons, the Xue et al study does not directly address the issue of equalization of input-specific E/I ratios (i.e. approximately constant E/I ratios across different sensory stimuli, or co-tuning of excitation and inhibition) which may be even more relevant to the function of a single neuron. This is important, considering that in physiological conditions different sensory stimuli likely activate distinct subsets of L4 neurons. Perhaps by applying focal optic stimulation at different sites in L4 in future studies, it can be tested whether E/I ratios representing different input pathways are homogenously or heterogeneously modified after modification of cell excitability. An even more challenging follow-up experiment would be to manipulate activity not in a cell-wide but in a pathway-specific manner, and to test whether the E/I ratio specific to that pathway is modified selectively. Finally, it remains to be addressed whether E/I ratios across cells are equalized progressively in developing circuits, given the dramatic changes in activity during development.
Plasticity mechanisms previously shown to be active at GABAergic synapses could account for the activity-dependent equalization of E/I ratios observed by Xue and colleagues. For example, in dissociated hippocampal neurons, elevating individual neuron spiking upregulates GABAergic synaptic inputs, as reflected by the increases in frequency and amplitude of miniature inhibitory postsynaptic currents (mIPSCs) as well as in pre- and postsynaptic proteins of GABAergic synapses [10]. Elevating the excitability of individual adult-born granule cells (GCs) in the dentate gyrus by overexpressing Na+ channels also increases GABAergic inputs to these cells as compared with control GCs [11]. These changes are considered as homeostatic responses, since from hours to days of activity manipulations are required for their induction. Similarly, in the developing Xenopus retinotectal system, we previously discovered a form of rapidly induced GABAergic plasticity, the polarity and magnitude of which depends on the strength of co-activated glutamatergic input [12]. In tectal neurons, visual stimulation activates convergent glutamatergic and GABAergic inputs, with the E/I ratio varying over a broad range in an early developing stage. Repetitive visual stimulation results in long-term depression (LTD) of the GABAergic input if the co-activated glutamatergic input is weak (Fig. 1B) or if the E/I ratio is low (Fig. 1C). Conversely, long-term potentiation (LTP) of the GABAergic input is induced if the convergent glutamatergic input is strong and the E/I ratio is above a certain threshold (Fig. 1B,1C). More importantly, the magnitude of GABAergic LTP linearly correlates with E/I ratio (Fig. 1C), indicating that the more the E/I ratio deviates from (i.e., is larger than) an “optimal” value, the faster GABAergic input is modified. With ongoing bidirectional changes in GABAergic input induced by sensory stimulation, an optimal E-I balance may be reached in a faster time scale than via homeostatic regulations. In addition, since inhibition evoked by a sensory stimulus only listens to the excitation evoked by the same stimulus and is changed accordingly, pathway/stimulus specific E-I balance could be established, resulting in co-tuning of excitation and inhibition (Fig. 1D). Whether such excitatory input dependent GABAergic plasticity also contributes to the establishment of E-I balance in mammalian systems remains to be investigated.
Finally, what molecular mechanisms might underlie the equalization of E/I ratios reported by Xue et al? First, it is known that brain-derived neurotrophic factor (BDNF) plays an important role in promoting the maturation of inhibitory synapses [13]. Interestingly, the activity-dependent enhancement of inhibitory input also requires release of BDNF from the postsynaptic neuron and BDNF uptake by GABAergic synaptic terminals [10,12]. Thus, an immediate question is whether the equalization of E/I ratios would be impaired when BDNF expression is reduced or when BDNF release is disrupted. Secondly, it was recently found that an activity-dependent transcription factor, Npas4, is able to initiate a gene expression program upon excitatory input activity, which results in a selective increase in the number of somatic inhibitory synapses likely made by PV neurons [14]. Such an increase is mediated, at least partially, by BDNF, the expression of which is also regulated by Npas4 activity [14]. This result again highlights a potential role of BDNF in regulating the strength of PV-inhibition in response to changes of excitatory input strength. Further investigation of the function of activity-dependent regulators of inhibitory synapses will provide more insights into the mechanisms by which neural activity controls the E-I balance, and how disruption of this balance leads to neurological disorders.
Acknowledgments
H.W.T. is supported by National Institutes of Health (NIH) R01 EY019049. L.I.Z. is supported by NIH R01DC DC008983.
References
- 1.Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu GK, et al. From elementary synaptic circuits to information processing in primary auditory cortex. Neurosci Biobehav Rev. 2011;35:2094–2104. doi: 10.1016/j.neubiorev.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat Neurosci. 2008;11:535–537. doi: 10.1038/nn.2105. [DOI] [PubMed] [Google Scholar]
- 4.Shu Y, et al. Turning on and off recurrent balanced cortical acitivy. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- 5.Zhang LI, et al. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature. 2003;424:201–205. doi: 10.1038/nature01796. [DOI] [PubMed] [Google Scholar]
- 6.Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- 7.Liu BH, et al. Broad inhibition sharpens orientation selectivity by expanding input dynamic range in mouse simple cells. Neuron. 2011;71:542–554. doi: 10.1016/j.neuron.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karnani MM, et al. A blanket of inhibition: functional inferences from dense inhibitory connectivity. Curr Opin Neurobiol. 2014;26:96–102. doi: 10.1016/j.conb.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue M, et al. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 2014;511:596–600. doi: 10.1038/nature13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng YR, et al. Postsynaptic spiking homeostatically induces cell-autonomous regulation of inhibitory inputs via retrograde signaling. J Neurosci. 2010;30:16220–16231. doi: 10.1523/JNEUROSCI.3085-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sim S, et al. Increased cell-intrinsic excitability induces synaptic changes in new neurons in the adult dentate gyrus that require Npas4. J Neurosci. 2013;33:7928–7940. doi: 10.1523/JNEUROSCI.1571-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, et al. Heterosynaptic scaling of developing GABAergic synapses: dependence on glutamatergic input and developmental stage. J Neurosci. 2007;27:5301–5312. doi: 10.1523/JNEUROSCI.0376-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang ZJ, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 14.Bloodgood BL, et al. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature. 2013;503:121–125. doi: 10.1038/nature12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao HW, Poo MM. Activity-dependent matching of excitory and inhibitory inputs during refinement of visual receptive fields. Neuron. 2005;45:829–836. doi: 10.1016/j.neuron.2005.01.046. [DOI] [PubMed] [Google Scholar]