Abstract
Background
Studies have observed associations between the gut microbiome and obesity. O-desmethylangolensin (ODMA) and equol are gut bacterial metabolites of daidzein, a compound found in high amounts in soy foods. Approximately 80–95% and 25–60% of individuals harbor gut microbial communities capable of producing ODMA or equol, respectively. Given that other phenotypes of gut bacterial metabolism of dietary compounds have been associated with obesity, we hypothesized that daidzein-metabolizing phenotypes would be associated with obesity.
Objective
To compare the prevalence of ODMA-producer and equol-producer phenotypes in obese, overweight, and normal weight individuals.
Methods
Adults aged 18 to 95 years (n=297) provided a first-void urine sample after a three-day soy challenge, and urinary ODMA and equol concentrations were used to classify individuals as producers or non-producers. Body mass index was calculated from self-reported weight and height.
Results
There were 60 ODMA non-producers and 173 equol non-producers. Obese individuals were 2.8-times more likely to be ODMA non-producers (OR=2.8, 95% CI: 1.2, 6.2) compared to normal weight individuals, when adjusted for age, race (white vs. non-white), and gender and menopausal status (male, premenopausal female, and postmenopausal female). Obesity was not associated with equol-producer phenotype (OR=1.1, 95% CI: 0.5, 2.2). Stronger associations with obesity were observed in the ODMA non-producers who were also equol producers than equol non-producers.
Conclusions
Results from this analysis suggest that the ODMA-producer phenotype, but not equol-producer phenotype, is associated with obesity in adults. These results support further work to replicate these findings and evaluate mechanisms of the observed associations.
Keywords: gut, obesity, microbiome, daidzein, equol, O-desmethylangolensin
INTRODUCTION
The host gut microbiome is an extensive community of microorganisms, including bacteria, fungi, and viruses 1. The overall effect of this microbiome is a complex system of microorganism-microorganism and host-microorganism interactions. Interindividual differences in gut microbial profiles exist 2, and these differences translate to differences in functional capabilities of the microbiome across individuals and have the potential to influence host health. Evidence from both human and animal studies supports that the microbiome is related to obesity 2.
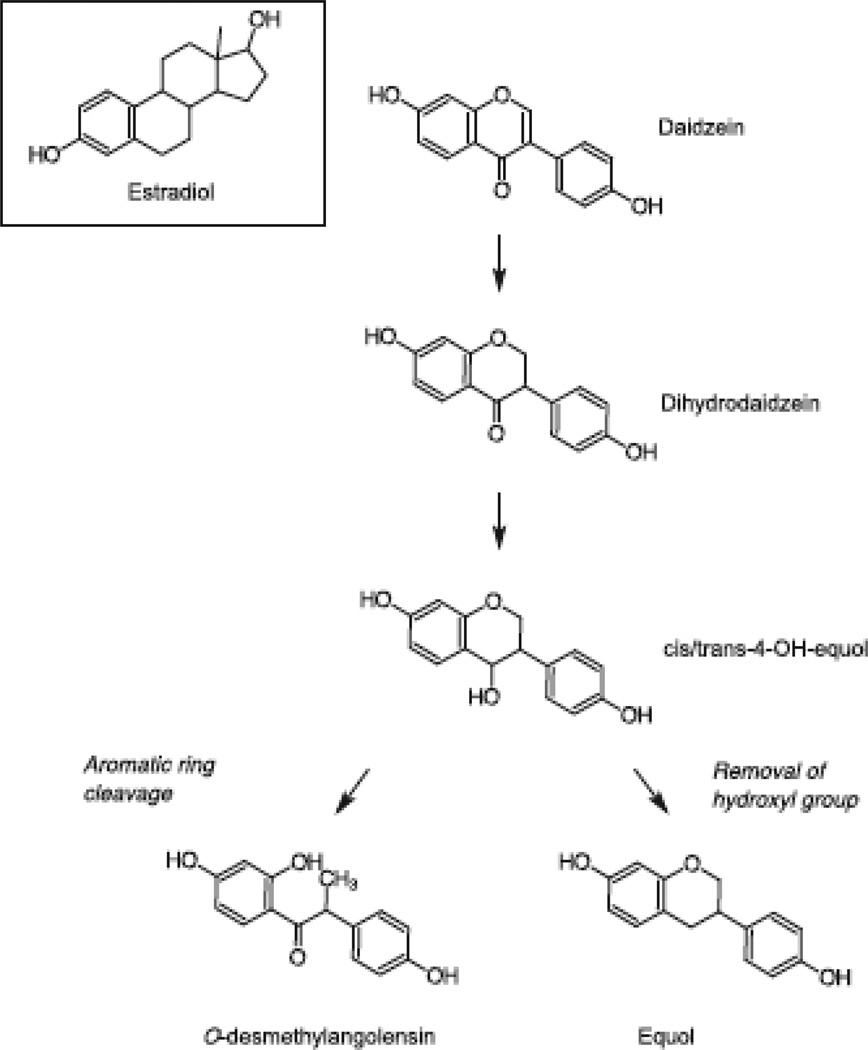
Daidzein, an isoflavone present in soy foods 3, can be metabolized by gut bacteria to O-desmethylangolensin (ODMA) and equol (Figure 1). Daidzein and equol are structurally similar to mammalian estrogens. Many studies have evaluated the estrogenic and anti-estrogenic effects of equol; in vitro and in vivo anti-estrogenic effects of equol include binding to estrogen receptors, inhibition of stimulatory effects of dihydrotestosterone, and scavenging free radicals 4. The structure of ODMA is less similar to estrogen than daidzein and equol because one of the phenolic rings is cleaved 5, 6. However, in vitro evidence suggests that ODMA exerts androgen receptor antagonistic activity and agonistic activity towards estrogen receptor (ER)-α and ERβ.
Figure 1. Selected metabolic steps and notations for the biotransformation of daidzein to equol and O-desmethylangolensin. Adapted from Heinonen et al (1999)6.
The presence of ODMA or equol in urine after daidzein consumption serves as a marker of a gut microbial environment that supports ODMA- or equol-producing bacteria 7. The prevalence of equol producers in the population ranges from approximately 25–60%, with a higher prevalence observed in studies conducted in Asian populations, such as Japan and China 7, 8. Studies suggest that the prevalence of ODMA producers in the population is more than 80% 5, 7, 9, 10. Some evidence suggests that risk factor profiles for breast cancer, prostate cancer, and cardiovascular disease differ across producers and non-producers. Associations of health effects and each of the phentoypes have been comprehensively reviewed elsewhere 4, 5, 11, 12.
Gut bacteria also metabolize lignans, compounds found in plant foods such as grains, legumes, and seeds, to enterolignans (enterolactone and enterodiol). High urinary concentrations of enterolactone or enterodiol, adjusted for diet, suggest gut microbial environments capable of high levels of lignan biotransformation. A difference in obesity prevalence in relation to these lignan-metabolizing phenotypes was observed in a recent study of US adults and children 13. Obese individuals were 42% less likely to have high urinary enterodiol concentrations. Overweight and obese individuals were also 34% and 64% less likely to have high urinary enterolactone concentrations, respectively. Given this observation, we hypothesized that other microbial phenotypes would be associated with obesity.
In earlier work on daidzein-metabolizing phenotypes, we collected data on weight and height, but did not specifically analyze anthropometry in relation to the phenotypes 14. At the time of the parent study, evaluating obesity in relation to the microbiome was in its infancy as a line of research, and evaluating obesity per se in relation to the phenotypes was not part of the original study objectives. The size of the study and manner in which the daidzein-metabolizing phenotypes were measured provides an excellent source of data for evaluating whether daidzein-metabolizing phenotypes are associated with obesity. The objective of this work was to build on our earlier observation and evaluate daidzein-metabolizing phenotypes in relation to categories of overweight and obese in adults.
MATERIALS AND METHODS
Participants were from a study that evaluated familial aggregation and segregation of daidzein-metabolizing phenotypes, and details are published elsewhere 14. Briefly, for this study we analyzed data from adults aged 18 to 95 years who had provided self-reported weight and height, and who had information on ODMA-producer and equol-producer phenotypes (see below). Taking antibiotics in the three months prior to the study was an exclusion criterion.
In order to the classify individuals as ODMA producers or equol producers, each individual consumed a commercial soy bar (Revival, Kernersville, NC) or one-third of a bag of soy nuts (Genisoy, San Francisco, CA) once per day for three days, and collected a spot urine sample on the morning of the fourth day. Information provided from the manufacturers indicated that the soy bars contained ~83 mg daidzein and the package of soy nuts contained ~10 mg daidzein. The difference in daidzein dose between the two foods did not bias phenotype determination because producers were identified based on the presence of equol or ODMA in urine, not a specific concentration. Urine samples were shipped to the laboratory for analysis. Prior testing demonstrated that daidzein and metabolite compounds were stable in urine for a two-week testing period (the stability was not tested longer than two weeks), as detailed elsewhere 14. Urine was stored at −20°C until analysis, and compounds were measured using gas chromatography-mass spectrometry, as detailed elsewhere 15.
Male and female adults ages 18 to 95 (mean=48, SD=15) were included in this study, and analyses were adjusted for age. Age was considered as a continuous variable in regression models. BMI (kg/m2) was categorized as normal weight (BMI 18.5 to 24.9), overweight (BMI 25.0 to 29.9), and obese (BMI≥30.0), according to World Health Organization criteria 16. The number of individuals who were underweight (BMI<18.5) was too small to make meaningful comparisons (n=7), and underweight individuals were excluded from our analyses. Associations between phenotypes and BMI were modeled with logistic regression. Unadjusted models and models adjusted for gender and menopausal status (males, premenopausal females, and postmenopausal females), race (white vs. non-white), and age (years) were evaluated. Generalized estimating equations (GEE) were used to account for the correlative nature of the familial data. In regression analyses, the producer phenotype was considered as the reference category because of its higher frequency than the non-producer phenotype. Odds ratios are presented with 95% confidence interval (CI). Data analysis was conducted with Stata (StataCorp, version MP 11.2).
RESULTS
Twenty percent of participants were ODMA non-producers and 58% of participants were equol non-producers. Age and race did not differ across non-producers and producers for either phenotype (Table 1). A smaller proportion of males than females were ODMA producers. A smaller proportion of premenopausal females were equol non-producers, compared to postmenopausal females. Being an ODMA non-producer was positively, but not significantly, associated with being above average height (OR=1.90, 95% CI: 0.94, 3.83) and above average weight (OR=1.82, 95% CI: 0.98, 3.35). Equol-producer phenotype was not significantly associated with being above average height or weight (p>0.05). Equol-producer phenotype and ODMA-producer phenotype were not significantly associated with each other (χ2=2.40, p=0.12).
TABLE 1.
Mean and percentage1 of age, sex, race, height, and weight, in relation to ODMA-producer and equol-producer phenotypes in 297 adults
| Characteristics | ODMA producers (n=237) |
ODMA non-producers (n=60) |
Equol producers (n=124) |
Equol non-producers (n=173) |
|
|---|---|---|---|---|---|
| Sex | |||||
| Male | 78 (32.9) | 26 (43.3) | 42 (33.9) | 62 (35.8) | |
| Pre-menopausal female | 101 (42.6) | 17 (28.3) | 54 (43.6) | 64 (37.0) | |
| Post-menopausal female | 58 (24.5) | 17 (28.3) | 28 (22.6) | 47 (27.2) | |
| Race | |||||
| White | 215 (90.7) | 50 (83.3) | 111 (89.5) | 154 (89.0) | |
| Non-white | 22 (9.3) | 10 (16.7) | 13 (10.5) | 19 (11.0) | |
| Height2 | |||||
| Below or at population average | 72 (30.4) | 10 (16.7)* | 30 (24.2) | 52 (30.1) | |
| Above population average | 165 (69.6) | 50 (83.3)* | 94 (75.8) | 121 (69.9) | |
| Weight | |||||
| Below or at population average | 195 (82.3) | 41 (68.3)* | 103 (83.1) | 133 (76.9) | |
| Above population average | 42 (17.7) | 19 (31.7)* | 21 (16.9) | 40 (23.1) |
Column percentages
Height and weight were divided into two categories. Below or above average height and weight were calculated separately for females (average height=63.8 inches, average weight=166.2 lbs) and males (average height=69.3 inches and average weight=195.5 lbs(22)
p<0.05 compared to producers
Obese individuals were approximately three-times more likely to be ODMA non-producers than normal weight individuals (OR=2.8, 95% CI: 1.2, 6.2), adjusted for age, race, and gender and menopausal status (Table 2). Obesity was not associated with the equol-producer phenotype (OR=1.1, 95% CI: 0.5, 2.2).
TABLE 2.
Relationship of body mass index to the ODMA-producer phenotype and equol-producer phenotype in 297 adults
| Phenotype | 18 to <25 kg/m2 | 25 to 29.9 kg/m2 | 30+ kg/m2 | p-trend |
|---|---|---|---|---|
| All | ||||
| ODMA producers, n (%) | 142 (59.9) | 71 (30.0) | 24 (10.1) | |
| ODMA non-producers, n (%) | 29 (48.3) | 17 (28.3) | 14 (23.3) | |
| OR1 | Reference | 1.1 (0.6, 2.2) | 3.0 (1.4, 6.5) | 0.014 |
| OR2 | Reference | 1.0 (0.5, 2.1) | 2.8 (1.2, 6.2) | 0.032 |
| All | ||||
| Equol producers, n (%) | 77 (62.1) | 32 (25.8) | 15 (12.1) | |
| Equol non-producers, n (%) | 94 (54.3) | 56 (32.4) | 23 (13.3) | |
| OR1 | Reference | 1.4 (0.8, 2.4) | 1.1 (0.6, 2.3) | 0.400 |
| OR2 | Reference | 1.3 (0.7, 2.2) | 1.1 (0.5, 2.2) | 0.629 |
Unadjusted odds ratios for the comparison of being a non-producer to a producer
Odds ratios adjusted for age (in years), race (non-white or white), and gender and menopausal status (male, premenopausal female, postmenopausal female)
The association of obesity and being an ODMA non-producer was stronger in the equol producers than in the equol non-producers, adjusted for age, race, and gender and menopausal status (Table 3). Individuals who were equol producers and obese had approximately 9-fold higher odds of being ODMA non-producers than ODMA producers (OR=8.7, 95% CI: 2.5, 30.8). Whereas, ODMA-producer phenotype was not associated with obesity within the equol non-producers (OR=1.5, 95% CI: 0.5, 4.7).
TABLE 3.
Relationship of body mass index to the ODMA-producer phenotype stratified by equol-producer phenotype in 297 adults
| Phenotype | 18 to <25 kg/m2 | 25 to 29.9 kg/m2 | 30+ kg/m2 | p-trend |
|---|---|---|---|---|
| Equol producers | ||||
| ODMA producers, n (%) | 63 (67.0) | 25 (26.6) | 6 (6.4) | |
| ODMA non-producers, n (%) | 14 (46.7) | 7 (23.3) | 9 (30.0) | |
| OR1 | Reference | 1.3 (0.5, 3.6) | 7.2 (2.2, 23.6) | 0.003 |
| OR2 | Reference | 1.1 (0.33, 3.3) | 8.7 (2.5, 30.8) | 0.001 |
| Equol non-producers | ||||
| ODMA producers, n (%) | 79 (55.2) | 46 (32.2) | 18 (12.6) | |
| ODMA non-producers, n (%) | 15 (50.0) | 10 (33.3) | 5 (16.7) | |
| OR1 | Reference | 1.1 (0.4, 2.6) | 1.6 (0.5, 4.9) | 0.455 |
| OR2 | Reference | 1.0 (0.4, 2.5) | 1.5 (0.5, 4.7) | 0.585 |
Unadjusted odds ratios for the comparison of being a non-producer to a producer
Odds ratios adjusted for age (in years), race (non-white or white), and gender and menopausal status (male, premenopausal female, postmenopausal female)
DISCUSSION
The gut microbiome is an important factor in food and nutrient metabolism, and interindividual differences in the composition of the gut microbiome influence nutrient exposure in the host. Interindividual differences in the microbiome are proposed to influence host factors such as obesity through, for example, an increase in energy extraction from non-digestible food components, alterations in gut permeability, gut hormone release in response to gut microbial metabolites, and an impact on host metabolism signaling molecules 2, 17. In other work, high urinary concentrations of enterolignans (gut microbial metabolites of phytoestrogen lignans) were associated with obesity 13. Specifically, after adjustment for demographics and intakes of energy, grain, vegetable, fruit, meat, and dairy, high urinary enterodiol concentration was associated with 18% and 42% lower likelihood of being overweight and obese, respectively. High urinary enterolactone concentration was associated with 24% and 64% lower likelihood of being overweight and obese, respectively. Daidzein is also a phytoestrogen that is metabolized to different extents between individuals, and we hypothesized that daidzein-metabolizing phenotypes (characterized by the ability to produce equol and / or ODMA) would be associated with obesity in adults. We observed that obese adults were more likely to be ODMA non-producers than normal weight adults, but there was no difference across equol-producer phenotype in obese, overweight, and normal weight individuals.
Associations between daidzein-metabolizing phenotypes and health may be related to microbial profiles associated with the ability or inability to produce daidzein metabolites. In the study sample used for this analysis, weekly servings of soy foods was less than two servings/week (previously published in Frankenfeld et al 200418). Given this low exposure to soy, the likely mechanism of influence on obesity is through microbial profiles associated with daidzein metabolism. Not all individuals harbor gut microbial profiles capable of metabolizing daidzein to equol or ODMA. The prevalence of equol producers in the population ranges from approximately 25–60%, with a higher prevalence observed in studies conducted in Asian populations, such as Japan and China 7, 8. The ODMA-producer phenotype has been less studied than the equol-producer phenotype, but studies suggest that the prevalence of ODMA producers in the general population is more than 80% 5, 7, 9, 10. There are several possible reasons for geographic variations in equol-producer phenotype prevalence, including study design differences (e.g., serum vs. urine measurements, absolute vs. relative amount of compounds) or the physical location influencing the gut microbial environment. Geographic variation in microbiota was observed in Japanese residents, both comparing urban and rural elderly Japanese 19 and comparing elderly Japanese with their Japanese-American counterparts 20. More recently, researchers have observed differences between residents of France, Germany, Italy and Sweden 21 as well as rural and urban areas of China 22.
The ODMA-producer phenotype has been associated with disease risk factors in some studies. For example, in a low-soy consuming population of overweight, postmenopausal women, ODMA non-producers, compared with ODMA producers, had lower bone mineral density (BMD), lower mammographic density, and lower 2-hydroxyestrone concentrations 15, 23, 24. However, in similar work with an also low-soy consuming normal weight, premenopausal women, associations with the ODMA-producer phenotype were null for BMD, urinary estrogen metabolites, and mammographic density 25–27. Here, we observed that obese individuals were approximately three-times more likely to be ODMA non-producers in a population that included a wide age range and males, premenopausal females, and postmenopausal females.
The combination of the ODMA-producer and equol-producer phenotypes may represent particular bacterial compositions. Bolca et al (2012) reviewed the role of polyphenol-metabolizing phenotypes (metabotypes), which includes the metabolism of isoflavones, flavonols, and other compounds, and highlighted that an overall metabotype may be an important contributor to the health effects of polyphenols 28. We observed that the relationship between obesity and ODMA non-producer phenotype was stronger in the equol producers. However, the number of individuals in some groups after stratification by equol-producer phenotype was small and these results should be interpreted cautiously.
The ODMA-producer phenotype (or, conversely, ODMA non-producer phenotype) may be a marker of harboring bacteria with particular functional capacity, and the particular bacterium or consortia of bacteria may be important for disease risk. Daidzein metabolism to ODMA or equol requires several steps 5, 7. For example, a C-ring cleavage step is required to biotransform daidzein to ODMA, but not to transform daidzein to equol. Bacteria that have been observed to biotransform daidzein to ODMA in vitro include Eubacterium ramulus 29 and Clostridium sp HGH 136 30. E. ramulus has also been observed to metabolize other polyphenolic compounds to phenolic acids in vitro 31. The presence of ODMA-producing bacteria may be relevant in the biotransformation of other polyphenolic compounds associated with human disease risk or in the production of other metabolic signaling molecules that may influence adiposity; this hypothesis could be evaluated in future studies. Microbial diversity has been associated with obesity in other studies 32. It is possible that the ODMA-producer phenotype reflects differences in microbial diversity across producers and non-producers, and it is this diversity that is related to obesity. Differences in microbial diversity across producers and non-producers have not been studied, but this could be considered in future work.
ODMA and equol are of interest in cancer and cardiovascular research because of their structural similarity to mammalian estrogens 5, 7. Many studies have evaluated the estrogenic and anti-estrogenic effects of equol, and several reviews detail in vitro and in vivo effects 4, 33–41. Compared to equol, the structure of ODMA is less similar to estrogen because one of the phenolic rings is cleaved. In vitro evidence suggests the ODMA exerts androgen receptor antagonistic activity, but does not exert agonistic or antagonistic activity toward glucocorticoid receptive, thyroid hormone (TR)-α1, or TRβ 42. Results of in vitro studies also suggests the ODMA exerts agonistic activity towards estrogen receptor (ER)-α and ERβ, as well as effects on cancer cell growth and integrity 43. However, evidence suggests that ODMA itself does not exert sufficient biological action in vivo to influence disease risk, due to low circulating concentrations or low activity 5. These observations, along with the low soy exposure in this population, support that associations of ODMA-producer phenotype and obesity are more likely related to gut microbial environments than circulating ODMA concentrations.
There are some limitations to the analysis. One, the study relied on self-reported height and weight, and a reporting bias may be present. However, because there is no clinical test for ODMA or equol producer status, individuals are unaware of their phenotypes and any reporting bias is unlikely to be related to their phenotype. The effect of such non-differential bias is that odds ratios tend to be biased towards the null and our observed point estimates may be conservative. Two, the study was cross-sectional in design, and temporal sequence cannot be determined. It is unknown whether the ODMA-producer phenotype preceded obesity or vice versa; however, evidence suggests that daidzein-metabolizing phenotypes may be established early in life and that the phenotype remains relatively stable in adults 9, 44. The study was also a family-study design, and the possibility of intrafamily correlations exists. This analysis statistically accounted for intrafamily correlations. The prevalence of ODMA and equol producer is similar to other published studies, suggesting the family study design did not bias selection based on phenotypes. If ODMA-producer phenotype precedes obesity, this study provides a foundation for evaluating if lack of ODMA-producing bacteria influences the onset of obesity. Another limitation of the study was the small sample size after creation of subgroups, and the association between the combined equol-producer and ODMA-producer metabotypes could be further evaluated in larger studies.
There are also strengths to the analysis. Evidence regarding health conditions in relation to daidzein-metabolizing phenotypes is limited. One critical reason for limited evidence that the phenotypes needs to be determined in the presence of daidzein consumption, which can be done in populations that regularly consume soy or in study samples that have undergone a soy challenge. Secondary analyses of studies in low-soy consuming populations may be prone to false-negative non-producer phenotype classification. A strength of the present study is that daidzein-metabolizing phenotypes were measured after a soy challenge. Another reason is that the study was designed to evaluate the phenotypes, and individuals who were taking antibiotics were excluded. Antibiotics would alter the gut microbial environment and could lead to misclassification of producer phenotype. Three, the prevalence of ODMA producers in the general population is high (80–90%) 7, which creates a need for a large sample in order to obtain a sufficient number of ODMA non-producers. Despite that the sample size was too small to adequately evaluate subgroups of combined equol- and ODMA-producer phenotypes, the analysis was a relatively large sample for the individual phenotypes. This study population also included a broad distribution of age and representation from both genders and menopausal status. It is unlikely that sources of bias fully explain our observed relationship between the ODMA-producer phenotype and obesity.
As an extension of other work that observed obesity prevalence to differ across individuals with high and low urinary enterolignan concentrations 13, the hypothesis for this study was that daidzein-metabolizing phenotypes are related to obesity. We observed that obese individuals were more likely to be ODMA non-producers. However, a significant association of obesity and equol-producer phenotype was not observed. A hypothesis generated from this analysis is that there may specific components of the ODMA-producer phenotype or that ODMA non-producers are lacking related to underlying gut microbial community that are associated with obesity. The results of this study support further evaluation of obesity and other health outcomes in relation to ODMA-producer phenotype, as an overall phenotype or stratified by other phenotypes.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health Grants R03CA089785 and T32CA009168 (CLF). The work was carried out partially within the EU project PHYTOHEALTH QLRT-2001-02453. This study does not necessarily reflect the views of the commission and in no way anticipates the commission’s future policy in this area.
Footnotes
CONFLICT OF INTEREST
The authors have no competing financial interests in relation to this work.
AUTHOR CONTRIBUTIONS
CLF, CA, KW, and JWL contributed to the data acquisition. CLF drafted the article. All authors contributed to the concept and design, interpretation of data, revision of the article for important intellectual content, and approved the final version to be published.
REFERENCES
- 1.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4(11):430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 2.Hullar MA, Lampe JW. The gut microbiome and obesity. Nestle Nutr Workshop Ser. 2012;73:67–79. doi: 10.1159/000341288. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura Y, Tsuji S, Tonogai Y. Determination of the levels of isoflavonoids in soybeans and soy-derived foods and estimation of isoflavonoids in the Japanese daily intake. J AOAC Int. 2000;83(3):635–650. [PubMed] [Google Scholar]
- 4.Setchell KD, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr. 2010;140(7):1363S–1368S. doi: 10.3945/jn.109.119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankenfeld CL. O-Desmethylangolensin: The Importance of Equol's Lesser Known Cousin to Health Human. Adv Nutr. 2011;2:317–324. doi: 10.3945/an.111.000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinonen S, Wähälä K, Adlercreutz H. Identification of isoflavone metabolites dihydrodaidzein, dihydrogenistein, 6'-OH-O-dma, and cis-4-OH-equol in human urine by gas chromatography-mass spectroscopy using authentic reference compounds. Anal Biochem. 1999;274(2):211–219. doi: 10.1006/abio.1999.4279. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 2005;230(3):155–170. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- 8.Setchell KD, Clerici C. Equol: history, chemistry, and formation. J Nutr. 2010;140(7):1355S–1362S. doi: 10.3945/jn.109.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frankenfeld CL, Atkinson C, Thomas WK, Gonzalez A, Jokela T, Wähälä K, et al. High concordance of daidzein-metabolizing phenotypes in individuals measured 1 to 3 years apart. Br J Nutr. 2005;94(6):873–876. doi: 10.1079/bjn20051565. [DOI] [PubMed] [Google Scholar]
- 10.Song KB, Atkinson C, Frankenfeld CL, Jokela T, Wähälä K, Thomas WK, et al. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J Nutr. 2006;136(5):1347–1351. doi: 10.1093/jn/136.5.1347. [DOI] [PubMed] [Google Scholar]
- 11.Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr. 2009;89(5):1664S–1667S. doi: 10.3945/ajcn.2009.26736T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shor D, Sathyapalan T, Atkin S, Thatcher N. Does equol production determine soy endocrine effects? Eur J Nutr. 2012;51:389–398. doi: 10.1007/s00394-012-0331-7. [DOI] [PubMed] [Google Scholar]
- 13.Frankenfeld CL. Relationship of obesity and high urinary enterolignan concentrations in 6806 children and adults: analysis of National Health and Nutrition Examination Survey data. Eur J Clin Nutr. 2013;67(8):887–889. doi: 10.1038/ejcn.2013.107. [DOI] [PubMed] [Google Scholar]
- 14.Frankenfeld CL, Atkinson C, Thomas WK, Goode EL, Gonzalez A, Jokela T, et al. Familial correlations, segregation analysis, and nongenetic correlates of soy isoflavone-metabolizing phenotypes. Exp Biol Med (Maywood) 2004;229(9):902–913. doi: 10.1177/153537020422900906. [DOI] [PubMed] [Google Scholar]
- 15.Frankenfeld CL, McTiernan A, Tworoger SS, Atkinson C, Thomas WK, Stanczyk FZ, et al. Serum steroid hormones, sex hormone-binding globulin concentrations, and urinary hydroxylated estrogen metabolites in post-menopausal women in relation to daidzein-metabolizing phenotypes. J Steroid Biochem Mol Biol. 2004;88(4–5):399–408. doi: 10.1016/j.jsbmb.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO) Obesity and overweight. Fact sheet N°311. 2012 May
- 17.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26(1):5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 18.Frankenfeld CL, Atkinson C, Thomas WK, Goode EL, Gonzalez A, Jokela T, et al. Familial correlations, segregation analysis, and nongenetic correlates of soy isoflavone-metabolizing phenotypes. Exp Biol Med (Maywood) 2004;229(9):902–913. doi: 10.1177/153537020422900906. [DOI] [PubMed] [Google Scholar]
- 19.Finegold SM, Attebery HR, Sutter VL. Effect of diet on human fecal flora: comparison of Japanese and American diets. Am J Clin Nutr. 1974;27(12):1456–1469. doi: 10.1093/ajcn/27.12.1456. [DOI] [PubMed] [Google Scholar]
- 20.Benno Y, Endo K, Mizutani T, Namba Y, Komori T, Mitsuoka T. Comparison of fecal microflora of elderly persons in rural and urban areas of Japan. Appl Environ Microbiol. 1989;55(5):1100–1105. doi: 10.1128/aem.55.5.1100-1105.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72(2):1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Xu W, Ibrahim SA, Jin J, Feng J, Jiang J, et al. Effects of age and region on fecal microflora in elderly subjects living in Bama, Guangxi, China. Curr Microbiol. 2011;62(1):64–70. doi: 10.1007/s00284-010-9676-4. [DOI] [PubMed] [Google Scholar]
- 23.Frankenfeld CL, McTiernan A, Aiello EJ, Thomas WK, LaCroix K, Schramm J, et al. Mammographic density in relation to daidzein-metabolizing phenotypes in overweight, postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13(7):1156–1162. [PubMed] [Google Scholar]
- 24.Frankenfeld CL, McTiernan A, Thomas WK, LaCroix K, McVarish L, Holt VL, et al. Postmenopausal bone mineral density in relation to soy isoflavone-metabolizing phenotypes. Maturitas. 2006;53(3):315–324. doi: 10.1016/j.maturitas.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson C, Newton KM, Aiello Bowles EJ, Lehman CD, Stanczyk FZ, Westerlind KC, et al. Daidzein-metabolizing phenotypes in relation to mammographic breast density among premenopausal women in the United States. Breast Cancer Res Treat. 2009;116(3):587–594. doi: 10.1007/s10549-008-0199-7. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson C, Newton KM, Stanczyk FZ, Westerlind KC, Li L, Lampe JW. Daidzein-metabolizing phenotypes in relation to serum hormones and sex hormone binding globulin, and urinary estrogen metabolites in premenopausal women in the United States. Cancer Causes Control. 2008;19(10):1085–1093. doi: 10.1007/s10552-008-9172-3. [DOI] [PubMed] [Google Scholar]
- 27.Atkinson C, Newton KM, Yong M, Stanczyk FZ, Westerlind KC, Li L, et al. Daidzein-metabolizing phenotypes in relation to bone density and body composition among premenopausal women in the United States. Metabolism. 2012;61(12):1678–1682. doi: 10.1016/j.metabol.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolca S, Van de Wiele T, Possemiers S. Gut metabotypes govern health effects of dietary polyphenols. Curr Opin Biotechnol. 2012;24(2):220–225. doi: 10.1016/j.copbio.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Schoefer L, Mohan R, Braune A, Birringer M, Blaut M. Anaerobic C-ring cleavage of genistein and daidzein by Eubacterium ramulus. FEMS Microbiol Lett. 2002;208(2):197–202. doi: 10.1111/j.1574-6968.2002.tb11081.x. [DOI] [PubMed] [Google Scholar]
- 30.Hur HG, Beger RD, Heinze TM, Lay JO, Jr, Freeman JP, Dore J, et al. Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of the isoflavonoid daidzein. Arch Microbiol. 2002;178:8–12. doi: 10.1007/s00203-002-0414-6. [DOI] [PubMed] [Google Scholar]
- 31.Schneider H, Blaut M. Anaerobic degradation of flavonoids by Eubacterium ramulus. Arch Microbiol. 2000;173(1):71–75. doi: 10.1007/s002030050010. [DOI] [PubMed] [Google Scholar]
- 32.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gil-Izquierdo A, Penalvo JL, Gil JI, Medina S, Horcajada MN, Lafay S, et al. Soy isoflavones and cardiovascular disease epidemiological, clinical and -omics perspectives. Current pharmaceutical biotechnology. 2012;13(5):624–631. doi: 10.2174/138920112799857585. [DOI] [PubMed] [Google Scholar]
- 34.Ishimi Y. Soybean isoflavones in bone health. Forum of nutrition. 2009;61:104–116. doi: 10.1159/000212743. [DOI] [PubMed] [Google Scholar]
- 35.Jackman KA, Woodman OL, Sobey CG. Isoflavones, equol and cardiovascular disease: pharmacological and therapeutic insights. Curr Med Chem. 2007;14(26):2824–2830. doi: 10.2174/092986707782360178. [DOI] [PubMed] [Google Scholar]
- 36.Jackson RL, Greiwe JS, Schwen RJ. Emerging evidence of the health benefits of S-equol, an estrogen receptor beta agonist. Nutr Rev. 2011;69(8):432–448. doi: 10.1111/j.1753-4887.2011.00400.x. [DOI] [PubMed] [Google Scholar]
- 37.Martin D, Song J, Mark C, Eyster K. Understanding the cardiovascular actions of soy isoflavones: potential novel targets for antihypertensive drug development. Cardiovascular & hematological disorders drug targets. 2008;8(4):297–312. doi: 10.2174/187152908786786214. [DOI] [PubMed] [Google Scholar]
- 38.Wiseman H. The therapeutic potential of phytoestrogens. Expert opinion on investigational drugs. 2000;9(8):1829–1840. doi: 10.1517/13543784.9.8.1829. [DOI] [PubMed] [Google Scholar]
- 39.Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora--implications for health. Mol Nutr Food Res. 2007;51(7):765–781. doi: 10.1002/mnfr.200600262. [DOI] [PubMed] [Google Scholar]
- 40.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132(12):3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 41.Lampe JW. Emerging research on equol and cancer. J Nutr. 2010;140(7):1369S–1372S. doi: 10.3945/jn.109.118323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeuchi S, Takahashi T, Sawada Y, Iida M, Matsuda T, Kojima H. Comparative study on the nuclear hormone receptor activity of various phytochemicals and their metabolites by reporter gene assays using Chinese hamster ovary cells. Biol Pharm Bull. 2009;32(2):195–202. doi: 10.1248/bpb.32.195. [DOI] [PubMed] [Google Scholar]
- 43.Pfitscher A, Reiter E, Jungbauer A. Receptor binding and transactivation activities of red clover isoflavones and their metabolites. J Steroid Biochem Mol Biol. 2008;112(1–3):87–94. doi: 10.1016/j.jsbmb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Lourdes M, Cruz A, Wong WW, Mimouni F, Hachey DL, Setchell KDR, et al. Effects of infant nutrition on cholesterol synthesis rates. Pediatr Res. 1994;35(2):135–140. doi: 10.1203/00006450-199402000-00001. [DOI] [PubMed] [Google Scholar]