Highlights
-
•
The colonic IgG4-RD is rare.
-
•
We report the case of a74-year-old female with IgG4-RD of the ileocecal region.
-
•
The patient was diagnosed asmalignant lymphoma and underwent right-hemi colectomy.
-
•
Postoperative pathologicalexamination revealed IgG4-RD of the ileocecal region.
-
•
Surgical resection for IgG4-RDis necessary for cases with concerns of malignancy.
Keywords: IgG4-related disease, Colon, Resection
Abstract
INTRODUCTION
Immunoglobulin G4-related disease (IgG4-RD) is a systemic disease characterized by chronic fibrosing inflammation with abundant IgG4-positive plasma cells, and responds well to steroids. Previous reports of IgG4-RD have focused on pancreatic and extrapancreatic including the gastrointestinal tract, however, the colonic IgG4-RD is rare.
PRESENTATION OF CASE
We herein report the case of a 74-year-old female with edematous wall thickening of the terminal ileum to the lower ascending colon confirmed by several preoperative imaging studies, who underwent right hemi-colectomy for suspected malignant lymphoma. The resected specimen showed an irregular wall thickness with subserosal sclerosis, and the lesion was 10 cm in length from the terminal ileum to the ascending colon. The patient was diagnosed with IgG4-RD by pathological examinations, which demonstrated an increased number of IgG4-positive plasma cells (150/HPF), and an elevated IgG4/IgG ratio (50%).
DISCUSSION
Gastrointestinal IgG4-RD appears to be difficult to diagnose prior to surgical resection because of its rarity, and the similarity of its features to malignancy.
The measurement of the serum IgG4 levels, immunohistochemical examination of biopsy specimens and use of several imaging modalities might help us to diagnose the disease without surgical resection, and this disease can generally be treated with steroid therapy. However, surgical resection for IgG4-RD may still be also necessary for patients with concerns regarding malignancy or with intractable gastrointestinal obstruction caused by this disease.
CONCLUSION
Gastrointestinal IgG4-RD often mimics malignancy, and we should therefore consider this disease in the differential diagnosis of colonic lesions in order to optimize the treatment.
1. Introduction
Immunoglobulin G4-related disease (IgG4-RD) is a systemic disease characterized by chronic fibrosing inflammation with abundant IgG4-positive plasma cells and elevated serum IgG4 levels; it tends to be mistaken for malignancy and responds well to steroids.1 Clinical manifestations are common in the pancreas, salivary glands, hepatobiliary tract, orbit, lymph nodes, and retroperitoneum but are rare in the gastrointestinal tract.2 We herein report a case of IgG4-RD of the ileocecal region diagnosed after surgical resection performed for a suspected malignancy. We also provide a review of the literature, with an emphasis on the treatment of this disease.
2. Presentation of case
A 74-year-old female presented with a two-month history of right lower abdominal pain and a slight fever. An approximately 5 cm mass in her right lower abdomen was detected by abdominal ultrasonography (US), and she was admitted for further examination. Her medical history included appendectomy. Colonoscopy revealed moderate stenosis caused by edematous wall thickening of the lower ascending colon, with reddening of the mucosa (Fig. 1A). Since the ileocecal valve was also swollen, the scope could not be inserted into the terminal ileum (Fig. 1B).
Fig. 1.

(A, B) Colonoscopy revealed moderate stenosis caused by edematous wall thickening of the lower ascending colon with reddening of the mucosa (A) and a swollen ileocecal valve (B; arrow). (C) A radiographic contrast enema indicated the presence of edematous asymmetrical stenosis, with erosion of the terminal ileum and lower ascending colon (arrow, ascending colon; dotted arrow, terminal ileum). (D, E) Abdominal CT scans indicated edematous wall thickening of the lower ascending colon (D; arrow) and terminal ileum (E; arrow). (F) FDG-PET revealed increased FDG uptake in the ascending colon (SUV max: 13.3; black dotted arrow), terminal ileum (SUV max: 6.9; white dotted arrow), spleen (SUV max: 5.9; black arrow) and paraaortic lymph node (SUV max: 5.3; white arrow).
A colonic biopsy from the swollen wall showed a nonspecific inflammation with lymphoid aggregates, with no evidence of malignancy. A radiographic contrast enema indicated edematous asymmetric stenosis with erosion from the terminal ileum to lower ascending colon (Fig. 1C). An abdominal computed tomography (CT) scan indicated edematous wall thickening from the terminal ileum to the lower ascending colon (Fig. 1D and E). Positron emission tomography with 18F-fluorodeoxyglucose (FDG-PET) revealed increased FDG uptake in the ascending colon (SUV max: 13.3), terminal ileum (SUV max: 6.9), and also in the spleen (SUV max: 5.9) and paraaortic lymph node (SUV max: 5.3) (Fig. 1F).
Blood tests revealed high levels of C-reactive protein (27.48 mg/dl), elevated LDH (252 U/l) and elevated ALP (561 U/l). The soluble interleukin-2 receptor (sIL-2R) level was elevated (2305 U/ml, normal range: 122–496 U/ml), but none of the other tumor marker levels were remarkable (CEA: 1.0 ng/ml, CA19-9: 7.1 U/ml). Although a pathological diagnosis was not obtained preoperatively, she was diagnosed to have malignant lymphoma based on these findings. It seemed that passage of stool would be completely obstructed by tumor growth in the near future. Consequently, she underwent a right hemi-colectomy in order to obtain a pathological diagnosis, and to avoid ileus caused by tumor growth.
Grossly, the segmental bowel resection included 25 cm of ascending colon and 125 cm of ileum (Fig. 2A). There was irregular wall thickening with submucosal sclerosis, which was 10 cm in length from the terminal ileum to the ascending colon that accompanied the sclerosis of the mesentery of the ileum. Since the sclerosing mesentery needed to be extirpated, the long ileum that received its blood supply from the mesentery was also resected. Microscopically, the lesion was composed of the ileal, cecal and colonic wall with lymphoplasmacytic infiltration, lymphoid follicles and fibrosis in the subserosa and surrounding mesenteric adipose tissue (Fig. 2B and C). Atypical lymphoid cells were not aggregated in these specimens. The Elastica van Gieson (EVG) staining showed obliterative phlebitis, which is one of the characteristic histological features of IgG4-RD (Fig. 2D).3,4 Immunohistochemical staining revealed an increased number of IgG4-positive plasma cells at 150/HPF (400×), and the IgG4/IgG ratio was 50% (Fig. 2E). The serum IgG4 level was postoperatively found to be normal level at 102 mg/dl (normal range: 6–121 mg/dl).
Fig. 2.
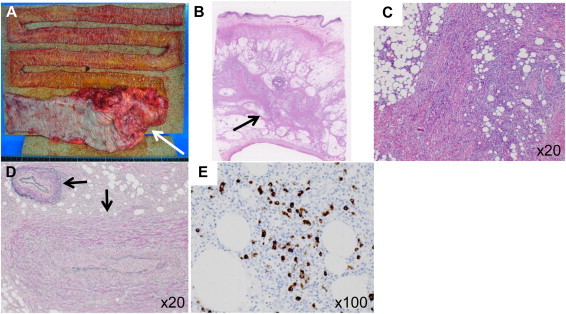
(A) The segmental bowel resection included 25 cm of ascending colon and 125 cm of ileum. There was an irregular wall thickness with submucosal sclerosis, which was 10 cm in length from the terminal ileum to the ascending colon, accompanied by sclerosis of the mesentery of the ileum (arrow). (B, C) Hematoxylin–eosin staining showed lymphoplasmacytic infiltration, lymphoid follicles and fibrosis in the subserosa and surrounding adipose tissue (arrow). (D) Elastica van Gieson (EVG) staining showed obliterative phlebitis (arrow). (E) Immunohistochemical staining revealed an increased number of IgG4-positive plasma cells at 150/HPF (400×), and an increased IgG4/IgG ratio (50%).
The patient's postoperative course was uneventful, and she was discharged home on postoperative day 18 tolerating a diet by mouth. She is currently doing well 10 months after the operation. During the preoperative FDG-PET examination, increased FDG uptake had been seen not only in the resected ileocecal region, but also in the spleen and paraaortic lymph node. While IgG4-RD might remain in these sites, the patient has had no symptoms or abnormal findings postoperatively. Thus, we decided to perform careful follow-up without any additional treatment, such as steroid therapy.
3. Discussion
Many previous reports of IgG4-RD have focused on pancreatic involvement, which is almost interchangeably called autoimmune pancreatitis (AIP),1,5 and it has been reported that infiltration of many IgG4-positive plasma cells was observed in the gastric mucosa, colonic mucosa and major duodenal papillae of some AIP patients.6–8 In addition, IgG4-positive plasma cell infiltration is sometimes detected in the colonic mucosa of patients with ulcerative colitis (UC).9 On the other hand, gastrointestinal IgG4-RDs without other lesions have also been reported in the esophagus10,11 and stomach.12–14 Colonic IgG4-RDs without other lesions, such as AIP or UC, have also been reported, but these have been quite rare. Chetty et al.15 reported two cases of solitary IgG4-RD at the cecum and sigmoid colon. In this case report, we presented a case of IgG4-RD of the ileocecal region without any evidence of AIP or UC based on the postoperative pathological examinations. The lesion was located at the subserosa of the ileum, cecum, ascending colon and mesenteric adipose tissue. A likely explanation for these findings is that the IgG4-RD of the present case might have originated at the mesentery and involved the gastrointestinal tract. In fact, some recent reports have presented IgG4-related sclerosing mesenteritis involving the gastrointestinal tract.16,17
Since gastrointestinal IgG4-RDs without other IgG4-RDs, such as AIP or UC, appear to be difficult to diagnose prior to surgical resection because of their rarity and features similar to malignancy, most of the solitary gastrointestinal IgG4-RDs in the previous reports were diagnosed after surgical resection, like the present case.10–13,15 On the other hand, a few cases that were successfully diagnosed as gastrointestinal IgG4-RD by the immunohistochemical staining of preoperative biopsy specimens have also been reported.14,18 In the present case, although a preoperative colonic biopsy was performed to rule out malignancy, we did not perform immunohistochemical examination for IgG4-positive plasma cells. It may be difficult to diagnose this disease based on biopsy specimens if there is sclerosis and an increased number of IgG4-positive plasma cells were spread predominantly in the subserosa like the present case.
According to the comprehensive clinical diagnostic criteria for IgG4-RD proposed in 2011,19 IgG4-RD is diagnosed when there is a characteristic diffuse/localized swelling or mass in a single or multiple organs with elevated serum IgG4 levels, or when there are histological findings of abundant infiltration of IgG4-positive plasma cells and lymphocytes, along with fibrosis. However, approximately 30% of patients have normal serum IgG4 levels, despite classic histopathological and immunohistochemical findings.20 In our present case, although histological features of IgG4-RD3 such as dense lymphoplasmacytic infiltration, fibrosis and obliterative phlebitis were observed, the serum IgG4 level was normal. One possible explanation for this finding is that the serum IgG4 level was decreased to the normal level at the point of measurement because it was measured postoperatively. Although some IgG4-RD patients might show false-negative serum IgG4 findings, the levels should be measured prior to surgical resection in case of gastrointestinal stenosis or obstruction of uncertain cause, and where this disease might be suspected.
As described above, measurement of the serum IgG4 levels, immunohistochemical examinations of biopsy specimens and the use of several imaging modalities might help to diagnose the condition without the need for surgical resection. If gastrointestinal IgG4-RD can be diagnosed without surgical resection, steroid therapy can be useful for the disease. However, if a malignancy cannot be ruled out completely after these examinations, surgical resection as a treatment for malignancy should be performed to obtain a definite diagnosis. In addition, many patients with gastrointestinal IgG4-RD may develop obstructive problems such as ileus. In such cases, surgical resection should be performed if the obstruction does not improve with conservative management.
4. Conclusions
Gastrointestinal IgG4-RD often mimics malignancy, and a preoperative diagnosis of this disease remains difficult. The most important thing to avoid unnecessary resection is that IgG4-RD in the gastrointestinal tract should be included in the differential diagnosis when marked wall thickening or a pseudotumor-like lesion is observed in the gastrointestinal region. However, surgical resection for IgG4-RD may still be necessary for patients with concerns regarding malignancy or intractable gastrointestinal obstruction caused by this disease.
Conflict of interest
None.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Eiji Oki, Yoko Zaitsu, Koji Ando, Hiroshi Saeki, Masaru Morita, Hideo Baba, Yoshihiko Maehara: writing.
Hidetaka Yamamoto: pathologist.
Key learning points.
-
•
The colonic IgG4-RD is rare.
-
•
We herein report the case of a 74-year-old female with IgG4-RD of the ileocecal region who underwent right hemi-colectomy for suspected malignant lymphoma.
-
•
The patient was diagnosed with IgG4-RD by postoperative pathological examinations.
-
•
The measurement of the serum IgG4 levels, immunohistochemical examination of biopsy specimens and use of several imaging modalities might help us to diagnose the disease without surgical resection, and this disease can generally be treated with steroid therapy.
-
•
Surgical resection for IgG4-RD may still be also necessary for patients with concerns regarding malignancy or with intractable gastrointestinal obstruction caused by this disease.
References
- 1.Kamisawa T., Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol: WJG. 2008;14(25):3948–3955. doi: 10.3748/wjg.14.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamano H., Arakura N., Muraki T., Ozaki Y., Kiyosawa K., Kawa S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol. 2006;41(12):1197–1205. doi: 10.1007/s00535-006-1908-9. [DOI] [PubMed] [Google Scholar]
- 3.Deshpande V., Zen Y., Chan J.K., Yi E.E., Sato Y., Yoshino T. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto H., Yamaguchi H., Aishima S., Oda Y., Kohashi K., Oshiro Y. Inflammatory myofibroblastic tumor versus IgG4-related sclerosing disease and inflammatory pseudotumor: a comparative clinicopathologic study. Am J Surg Pathol. 2009;33(9):1330–1340. doi: 10.1097/pas.0b013e3181a5a207. [DOI] [PubMed] [Google Scholar]
- 5.Hamano H., Kawa S., Horiuchi A., Unno H., Furuya N., Akamatsu T. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344(10):732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 6.Kamisawa T., Funata N., Hayashi Y., Tsuruta K., Okamoto A., Amemiya K. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52(5):683–687. doi: 10.1136/gut.52.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamisawa T., Egawa N., Nakajima H., Tsuruta K., Okamoto A., Hayashi Y. Gastrointestinal findings in patients with autoimmune pancreatitis. Endoscopy. 2005;37(11):1127–1130. doi: 10.1055/s-2005-870369. [DOI] [PubMed] [Google Scholar]
- 8.Deheragoda M.G., Church N.I., Rodriguez-Justo M., Munson P., Sandanayake N., Seward E.W. The use of immunoglobulin g4 immunostaining in diagnosing pancreatic and extrapancreatic involvement in autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2007;5(10):1229–1234. doi: 10.1016/j.cgh.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Raina A., Yadav D., Regueiro M., Krasinskas A.M., Saul M.I., Sapienza D.A. Mucosal IgG4 cell infiltration in ulcerative colitis is linked to disease activity and primary sclerosing cholangitis. Inflamm Bowel Dis. 2013;19(6):1232–1237. doi: 10.1097/MIB.0b013e318281344d. [DOI] [PubMed] [Google Scholar]
- 10.Lopes J., Hochwald S.N., Lancia N., Dixon L.R., Ben-David K. Autoimmune esophagitis: IgG4-related tumors of the esophagus. J Gastrointest Surg. 2010;14(6):1031–1034. doi: 10.1007/s11605-010-1172-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee H., Joo M., Song T.J., Chang S.H., Kim H., Kim Y.S. IgG4-related sclerosing esophagitis: a case report. Gastrointest Endosc. 2011;73(4):834–837. doi: 10.1016/j.gie.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 12.Kim do H., Kim J., Park do H., Lee J.H., Choi K.D., Lee G.H. Immunoglobulin G4-related inflammatory pseudotumor of the stomach. Gastrointest Endosc. 2012;76(2):451–452. doi: 10.1016/j.gie.2011.07.061. [DOI] [PubMed] [Google Scholar]
- 13.Rollins K.E., Mehta S.P., O’Donovan M., Safranek P.M. Gastric IgG4-related autoimmune fibrosclerosing pseudotumour: a novel location. ISRN Gastroenterol. 2011;2011:873087. doi: 10.5402/2011/873087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita T., Ando T., Sakakibara M., Hosoda W., Goto H. Refractory gastric ulcer with abundant IgG4-positive plasma cell infiltration: a case report. World J Gastroenterol: WJG. 2010;16(17):2183–2186. doi: 10.3748/wjg.v16.i17.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chetty R., Serra S., Gauchotte G., Markl B., Agaimy A. Sclerosing nodular lesions of the gastrointestinal tract containing large numbers of IgG4 plasma cells. Pathology. 2011;43(1):31–35. doi: 10.1097/PAT.0b013e328340e450. [DOI] [PubMed] [Google Scholar]
- 16.Nomura Y., Naito Y., Eriguchi N., Kume T., Itai N., Sonoda H. A case of IgG4-related sclerosing mesenteritis. Pathol Res Pract. 2011;207(8):518–521. doi: 10.1016/j.prp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Minato H., Shimizu J., Arano Y., Saito K., Masunaga T., Sakashita T. IgG4-related sclerosing mesenteritis: a rare mesenteric disease of unknown etiology. Pathol Int. 2012;62(4):281–286. doi: 10.1111/j.1440-1827.2012.02805.x. [DOI] [PubMed] [Google Scholar]
- 18.Fujita K., Naganuma M., Saito E., Suzuki S., Araki A., Negi M. Histologically confirmed IgG4-related small intestinal lesions diagnosed via double balloon enteroscopy. Dig Dis Sci. 2012;57(12):3303–3306. doi: 10.1007/s10620-012-2267-4. [DOI] [PubMed] [Google Scholar]
- 19.Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD). Research Program of Intractable Disease provided by the Ministry of Health, Labor, and Welfare of Japan. Nihon Naika Gakkai zasshi[nl]J Jpn Soc Intern Med. 2012;101(3):795–804. doi: 10.2169/naika.101.795. [DOI] [PubMed] [Google Scholar]
- 20.Sah R.P., Chari S.T. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr Opin Rheumatol. 2011;23(1):108–113. doi: 10.1097/BOR.0b013e3283413469. [DOI] [PubMed] [Google Scholar]


