Diffusion tensor imaging (DTI) with tractography is an MRI technique that can visualize nerve pathways by taking advantage of anisotropy of water diffusion in tracts with longitudinally oriented fibers. To date, no study in humans has shown that DTI and tractography can follow regeneration of axons during recovery from traumatic peripheral nerve injury. We show that tractography is able to identify regenerating axons advancing through a graft repair of an injured nerve, and demonstrate correlation with evidence of recovery on serial clinical, electrodiagnostic, and muscle MRI assessments.
Case report.
A 25-year-old man lacerated his popliteal fossa with a concrete saw, resulting in a common peroneal neuropathy, with paralysis of ankle dorsiflexion and toe extension and 4/5 eversion weakness. Electrodiagnostic studies performed 4 months after injury showed no innervation of tibialis anterior (TA) and extensor hallucis longus (EHL), supplied by the deep peroneal nerve. Muscles supplied by the superficial peroneal nerve, such as peroneus longus, were only partially denervated.
Methods.
DTI and tractography of the peroneal nerve were performed on a 3T scanner (MR750, GE Healthcare, Milwaukee, WI) 2 months after injury. DTI was performed using FOCUS, a reduced field-of-view diffusion method that applies a 2D spatially selective RF excitation in single-shot echoplanar imaging sequence (FOCUS, 28 directions, repetition time 3,200 ms, echo time 55 ms, filed of view 18 × 9 cm, acquisition matrix 256 × 144, slice thickness 3.0 mm, voxel size 1.31 mm3, b value 600 s/mm2).1,2 The scan was acquired over 3 averages to improve signal to noise ratio. Fractional anisotropy (FA) maps were calculated at each slice from a region of interest tracing the outline of the common peroneal nerve proximal to the injury, the site of transection/grafting, and the distal deep peroneal nerve (figure, C). Tractography was performed using FiberTrak software (GE Healthcare) by placing seed points along the nerve visualized on a corresponding axial T1-weighted image, and calculated using FA minimum threshold of 0.18 and maximum turning angle threshold of 45°.
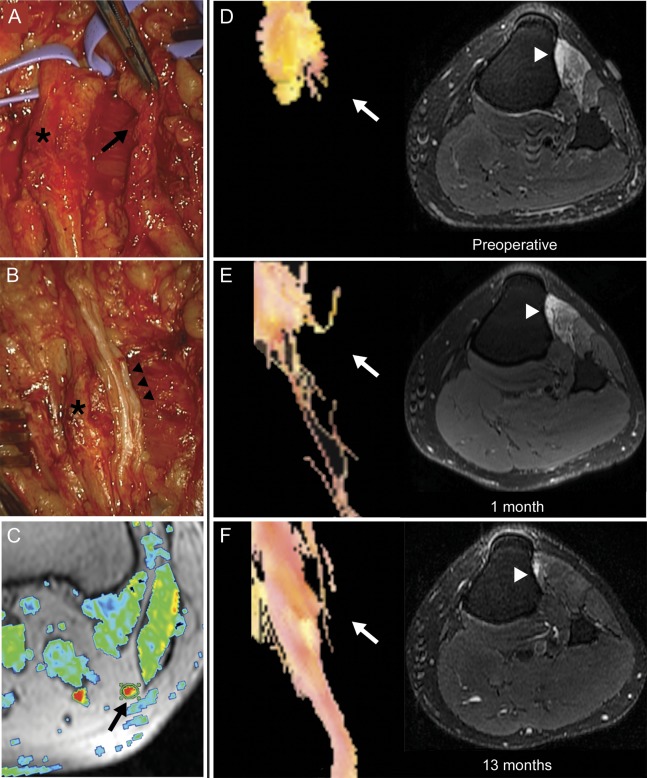
Figure. Magnetic resonance tractography demonstrating axonal regeneration.
Operative exploration of the left peroneal nerve identified a damaged, scarred deep peroneal nerve branch (black arrow, A) and relatively preserved superficial peroneal nerve branch (asterisk, A and B). The deep peroneal nerve branch was resected and the resulting defect bridged with 3 sural nerve grafts (black arrowheads, B). A thresholded fractional anisotropy (FA) map (C) is shown fused to the corresponding axial T1-weighted image. The proximal common peroneal nerve is identified as a region of increased FA signal (arrow), and is encircled by a region of interest used to calculate average FA values. Preoperative magnetic resonance (MR) tractography demonstrates the proximal stump of the deep peroneal nerve branch with no axons identified distal to the point of transection (white arrow, D), and T2-weighted, fat-suppressed imaging of the proximal left leg identifies denervation edema in deep-peroneal innervated muscles (tibialis anterior and extensor digitorum longus, white arrowhead). Repeat MR tractography performed 1 month after surgical repair identifies sparse, disorganized axons distal to the proximal graft neurorrhaphy (white arrow), and persistent muscle edema (E). Tractography performed 13 months after nerve graft repair demonstrates an organized bundle of fibers traversing the nerve grafts and resolution of muscle edema (F).
Results.
On preoperative scans, the peroneal nerve was T2 hyperintense and enlarged. No nerve fibers were identified immediately distal to the site of nerve injury (figure, D). On MRI of the leg, TA and extensor digitorum longus (EDL) demonstrated diffuse T2 hyperintensity reflecting muscle denervation (figure, D).3
Surgical exploration of the nerve was performed 4 months after injury. The deep branch of the peroneal nerve was severely injured, did not transmit electrical impulses, and had a 7.5-cm segment of intraneural fibrosis. The superficial peroneal nerve was both in anatomical and electrophysiologic continuity (figure, A). The scarred segment was resected and the gap between proximal and distal stumps interposed with three 8.5-cm sural nerve grafts (figure, B).
One month after surgery, DTI showed a few axons extending past the repair site into the grafts and persistent muscle edema (figure, E). Thirteen months after surgery, the patient demonstrated significant recovery of foot dorsiflexion (TA 4+/5 strength) and toe extension (EHL 3/5 and EDL 4/5), EMG evidence of reinnervation of these muscles, and normal limb function. Repeat tractography identified regenerating nerve fibers extending through the grafts (figure, F). Muscle denervation changes seen on MRI had resolved.
FA values recorded on preoperative and postoperative scans were consistent with axonal regeneration. FA values increased with recovery in the grafts (preoperative 0, 1 month postoperative 0.20 ± 0.08, 13 months postoperative 0.41 ± 0.04) and deep peroneal nerve distal to the grafts (preoperative 0.23 ± 0.06, 1 month postoperative 0.19 ± 0.04, 13 months postoperative 0.29 ± 0.09). Values from the proximal common peroneal nerve remained relatively stable (preoperative 0.45 ± 0.13, 1 month postoperative 0.37 ± 0.07, 13 months postoperative 0.36 ± 0.11).
Discussion.
The feasibility of tractography to demonstrate peripheral nerve regeneration has been demonstrated in animal models,4 but unlike tractography of white matter tracts in the brain, tractography of peripheral nerves in human subjects has been limited by technical factors such as optimal scan plane selection, satisfactory image resolution, and prolonged scan time. Encouraging evidence emerged from a study of a single patient without clinical or EMG correlates of recovery.5
Successful management of traumatic nerve injury requires distinction between neurotmetic injuries, which require surgical intervention, and axonotmetic injuries, which may recover spontaneously through axonal regeneration. Presently, monitoring of recovery from peripheral nerve injury is based on EMG assessments, but EMG cannot detect axonal regeneration before nerve fibers have reached and reinnervated their target muscle. Magnetic resonance neurography may show evolution of signal changes with degeneration and regeneration following nerve injury.6,7 Magnetic resonance tractography, by visualizing growing nerve fibers, may allow distinction between axonotmetic and neurotmetic injuries earlier, thereby expediting necessary surgeries while reducing the need for exploratory nerve surgery.
Footnotes
Author contributions: Dr. Simon: study design, data acquisition, analysis and interpretation, drafting and revision of the manuscript. Dr. Narvid: data acquisition and analysis, critical revision of the manuscript for important intellectual content. Dr. Cage: critical revision of the manuscript for important intellectual content. Dr. Banerjee: study design and data acquisition, critical revision of the manuscript for important intellectual content. Dr. Ralph: data acquisition and analysis, critical revision of the manuscript for important intellectual content. Dr. Engstrom: data acquisition and analysis, critical revision of the manuscript for important intellectual content. Dr. Kliot: study design, data acquisition, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Chin: study design, data acquisition, analysis and interpretation, critical revision of the manuscript for important intellectual content.
Study funding: National Health and Medical Research Council of Australia and the Motor Neurone Disease Research Institute of Australia (grant 1039520) (N.G.S.).
Disclosure: The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1.Saritas EU, Cunningham CH, Lee JH, Han ET, Nishimura DG. DWI of the spinal cord with reduced FOV single-shot EPI. Magn Reson Med 2008;60:468–473 [DOI] [PubMed] [Google Scholar]
- 2.Zaharchuk G, Saritas EU, Andre JB, et al. Reduced field-of-view diffusion imaging of the human spinal cord: comparison with conventional single-shot echo-planar imaging. AJNR Am J Neuroradiol 2011;32:813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.West GA, Haynor DR, Goodkin R, et al. Magnetic resonance imaging signal changes in denervated muscles after peripheral nerve injury. Neurosurgery 1994;35:1077–1085; discussion 1085–1086 [DOI] [PubMed] [Google Scholar]
- 4.Takagi T, Nakamura M, Yamada M, et al. Visualization of peripheral nerve degeneration and regeneration: monitoring with diffusion tensor tractography. Neuroimage 2009;44:884–892 [DOI] [PubMed] [Google Scholar]
- 5.Meek MF, Stenekes MW, Hoogduin HM, Nicolai JP. In vivo three-dimensional reconstruction of human median nerves by diffusion tensor imaging. Exp Neurol 2006;198:479–482 [DOI] [PubMed] [Google Scholar]
- 6.Dailey AT, Tsuruda JS, Filler AG, Maravilla KR, Goodkin R, Kliot M. Magnetic resonance neurography of peripheral nerve degeneration and regeneration. Lancet 1997;350:1221–1222 [DOI] [PubMed] [Google Scholar]
- 7.Aagaard BD, Lazar DA, Lankerovich L, et al. High-resolution magnetic resonance imaging is a noninvasive method of observing injury and recovery in the peripheral nervous system. Neurosurgery 2003;53:199–203 [DOI] [PubMed] [Google Scholar]