Abstract
Objective:
We assessed salience of subjective memory complaints (SMCs) by older individuals as a predictor of subsequent cognitive impairment while accounting for risk factors and eventual neuropathologies.
Methods:
Subjects (n = 531) enrolled while cognitively intact at the University of Kentucky were asked annually if they perceived changes in memory since their last visit. A multistate model estimated when transition to impairment occurred while adjusting for intervening death. Risk factors affecting the timing and probability of an impairment were identified. The association between SMCs and Alzheimer-type neuropathology was assessed from autopsies (n = 243).
Results:
SMCs were reported by more than half (55.7%) of the cohort, and were associated with increased risk of impairment (unadjusted odds ratio = 2.8, p < 0.0001). Mild cognitive impairment (dementia) occurred 9.2 (12.1) years after SMC. Multistate modeling showed that SMC reporters with an APOE ε4 allele had double the odds of impairment (adjusted odds ratio = 2.2, p = 0.036). SMC smokers took less time to transition to mild cognitive impairment, while SMC hormone-replaced women took longer to transition directly to dementia. Among participants (n = 176) who died without a diagnosed clinical impairment, SMCs were associated with elevated neuritic amyloid plaques in the neocortex and medial temporal lobe.
Conclusion:
SMC reporters are at a higher risk of future cognitive impairment and have higher levels of Alzheimer-type brain pathology even when impairment does not occur. As potential harbingers of future cognitive decline, physicians should query and monitor SMCs from their older patients.
Subjective memory complaints (SMCs) may signal increased risk of progression into clinically recognized states of impairment, namely, mild cognitive impairment (MCI) and dementia.1–5 Although prior studies use incident dementia as an endpoint, there is a need to consider the effect of SMC on the occurrence of MCI as well.6 Little is known about risk factors associated with the occurrence of SMCs other than depression or poor psychological well-being.7 SMCs may reflect aspects of metamemory, as older adults monitor their own forgetting.8 Persons with SMC may show MRI-based hippocampal atrophy, a finding also seen in MCI.9 Finally, while Alzheimer disease (AD)-type neuropathologic changes occur years before clinical symptoms are detectable, only a few studies link SMC to neuropathology10,11 or to biomarkers.2
We hypothesized that baseline cognitively intact subjects who declared an SMC would be at a higher risk of a cognitive impairment and may harbor AD-type brain pathology even in the absence of observable impairment. We analyzed data from a longitudinal cohort study (Biologically Resilient Adults in Neurological Studies [BRAiNS]).12,13 We assessed risk factors associated with incident SMCs, MCI, dementia, or death without dementia, as well as the time to each endpoint using data from 531 volunteers. Because approximately half of this cohort has come to autopsy, we also investigated the relationship between each endpoint and AD-type brain pathology.
METHODS
Subjects.
BRAiNS is a cohort of more than 1,100 older individuals aged at least 60 years enrolled in a longitudinal study of aging and cognition at the University of Kentucky's Alzheimer's Disease Center.12 Participants agree to postmortem brain donation. Enrollment criteria exclude persons younger than 60 years, and those with active infectious diseases, neurologic disorders, dementing illness, psychiatric disorders including depression, or disabling medical disorders.12 Subjects included in the current analysis (n = 531) had at least 2 study visits, had APOE genotyping available, and enrolled before the initiation of the National Alzheimer’s Coordinating Center Uniform Data Set in 2005. These participants have been the subject of previous reports.14 All subjects were cognitively intact at entry.
Standard protocol approvals, registrations, and patient consents.
The University of Kentucky institutional review board approved all research activities, and each participant gave written informed consent.
Cognitive assessments.
The authors classified participants into 4 mutually exclusive cognitive states based on the results of the annual assessments, which included multiple measures of memory, language, executive, and visuospatial function12: no serious impairment (NSI), SMC, clinical MCI, or dementia. Before administration of the neurocognitive battery, participants were systematically asked if they had noticed any change in memory since their last visit. Participants entered the SMC state based on the date of their first “yes” response, provided no clinical impairment had yet been diagnosed. The diagnosis of clinical MCI or dementia was by a consensus team review; for detailed diagnostic criteria, see Abner et al.15
Cognitive states.
The NSI state encompasses participants who did not die or transition into SMC, MCI, or dementia between assessments (figure 1). Hence, persons in the NSI state may perform below expectation on some cognitive assessments but fail to qualify for a more severe diagnosis.15 Transitions from more impaired to less impaired states are prohibited in the model. Although 19 subjects made an apparent recovery from clinical MCI to NSI at individual assessments, a review determined these to be artifacts resulting from either underlying comorbidities or diagnostic misclassification; we recoded these diagnoses accordingly so that all transitions flow consistently from less impaired to more impaired.12
Figure 1. Flow diagram and frequency of transitions among states.
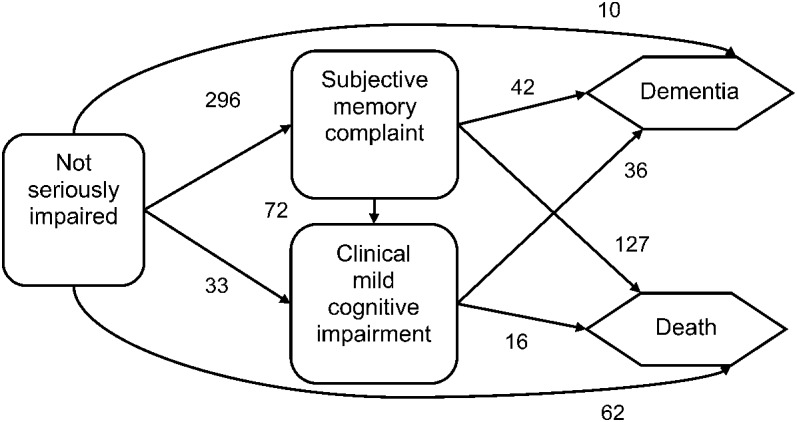
Risk factors.
A literature review identified potential risk factors for transitions among the clinical states (figure 1): APOE ε4 carrier status (ε4 allele positive/negative), female sex, low education (high school or less), overweight (body mass index >25 kg/m2), family history of dementia among first-degree relatives, smoking (never, former, or current smoker), high blood pressure, use of estrogen hormone replacement therapy (HRT), and presence of type II diabetes. Self-reports determine the latter 4 variables. Age is the time scale in the semi-Markov model and not a risk factor otherwise.
While procedures currently include annual neurocognitive, physical, and neurologic examinations, during the first 6 years of the study, only neurocognitive measures were collected annually, and medical history was collected during the baseline interview only. Accordingly, all risk factor exposures were fixed at baseline and coded as indicator variables.
Neuropathologic analysis.
The autopsy rate for participants who died during follow-up is 84.7%. University of Kentucky's Alzheimer's Disease Center neuropathologists performed autopsy assessments including lesion counts using previously described methods.16,17 Briefly, brain regions sampled include middle frontal gyrus (Brodmann area 9), superior and middle temporal gyri (areas 21 and 22), inferior parietal lobule (areas 39 and 40), occipital lobe including primary visual area (areas 17 and 18), amygdala, entorhinal cortex, and hippocampal CA1 and subiculum. Then mean neuritic plaques (NPs) (number/2.35 mm2) and neurofibrillary tangles (NFTs) (number/0.586 mm2) were generated per brain region based on the 5 most involved fields (subjectively determined). We averaged counts into 2 brain regions for data analysis: the medial temporal lobe (MTL) and neocortex. In addition, Consortium to Establish a Registry for Alzheimer's Disease (CERAD) ratings were derived from NP counts.18
Statistical analysis.
A semi-Markov model describes the movement of participants through the 5 clinical states (figure 1). Participants contribute up to 3 transitions to the analysis. Each transition involves 2 quantities: the probability of making that transition and, independently, the time required for that transition to occur (i.e., age of the first transition from NSI to another state or the time spent in SMC/MCI). Assuming a polytomous logistic model determines the probability of a transition, the analysis generated estimates of odds ratios (ORs) associated with risk factors affecting these probabilities; the logistic model depends on the participant's current state: NSI, SMC, or MCI. In all cases, the OR compares the odds of a transition to the state of interest vs the odds of a transition to death. Assuming each holding time follows a Weibull distribution that depends on both the current state and the state being entered, the analysis determines how the risk factors affect the mean holding time. Kryscio et al.14 describe the strategy for identifying significant risk factors associated with these probabilities and holding times. In this application, we assumed the transition to SMC occurred on the date the participant first admitted to an SMC, but the transitions to MCI and/or dementia were interval censored. Generalizing the equations discussed in Foucher et al.19 for a 4-state model to account for the SMC state, we fit the model to the data using PROC NLIN in PC-SAS/STAT 9.3 (equations and program available upon request).
We assessed NP and NFT count distributions in the 4 groups: (1) both SMC and impaired negative, (2) SMC positive but impaired negative, (3) SMC negative but impaired positive, and (4) both SMC and impaired positive. Here, impaired refers to a diagnosis of MCI or dementia. We used Wilcoxon rank sum tests to compare groups 1 to 2, 1 to 3, 2 to 4, and 3 to 4 in each brain region.
RESULTS
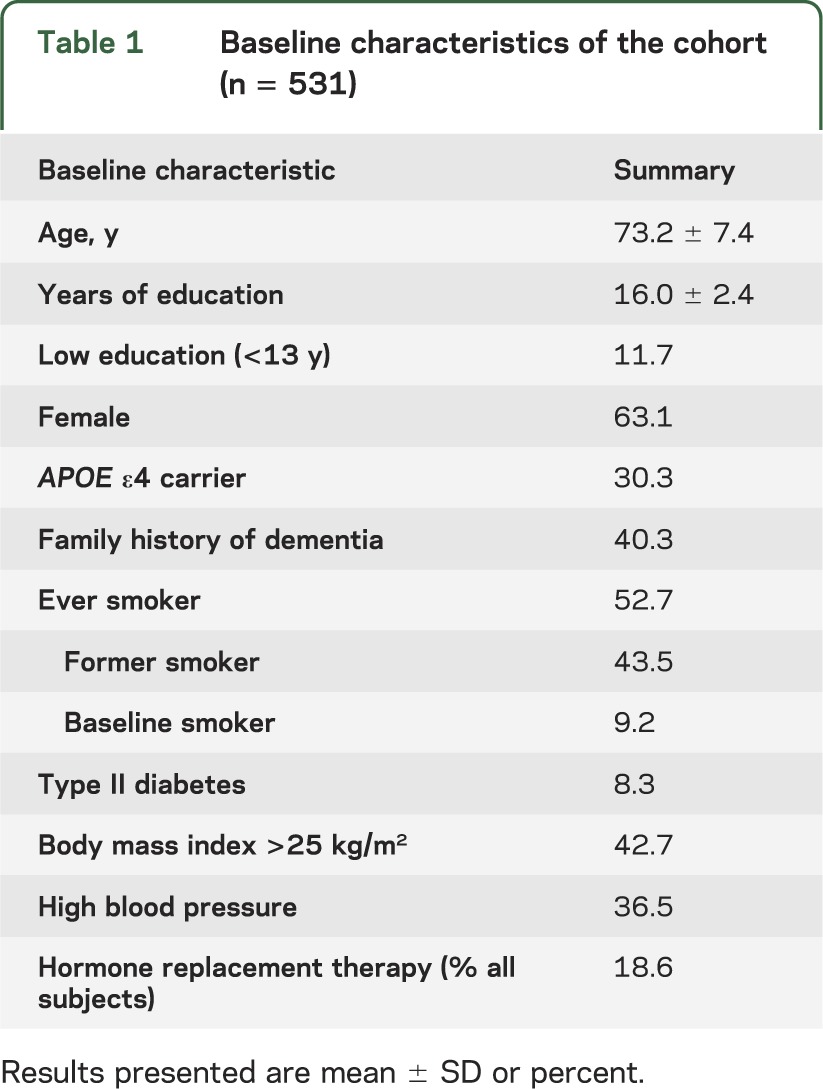
The participant group was relatively young at enrollment, majority female, had relatively high educational attainment, high risk of dementia (evidenced by high proportion of APOE ε4 carriers and family history of dementia), and a high proportion of current or former cigarette smokers (see table 1). The mean number of annual assessments per participant was 10.3 ± 4.1.
Table 1.
Baseline characteristics of the cohort (n = 531)
Probability of a transition.
Approximately 25% of the living participants remained in the NSI state at the last observed assessment. Of decedents, 11.7% were in the NSI state at last assessment, and another 23.9% had transitioned to the SMC state (figure 1). Almost 1 in 5 subjects (19.7%) met the clinical criteria for MCI during follow-up, and 68.6% of these entered the SMC state before this diagnosis. Approximately 1 in 6 subjects (16.6%) became demented during follow-up, and 79.6% of these first entered the SMC state. In total, the majority of the cohort entered the SMC state (296/531 or 55.7%). Those entering the SMC state had increased odds of subsequent impairment (either MCI or dementia) vs dying when compared with all others (OR = 2.8; 95% confidence interval: 1.9–4.2).
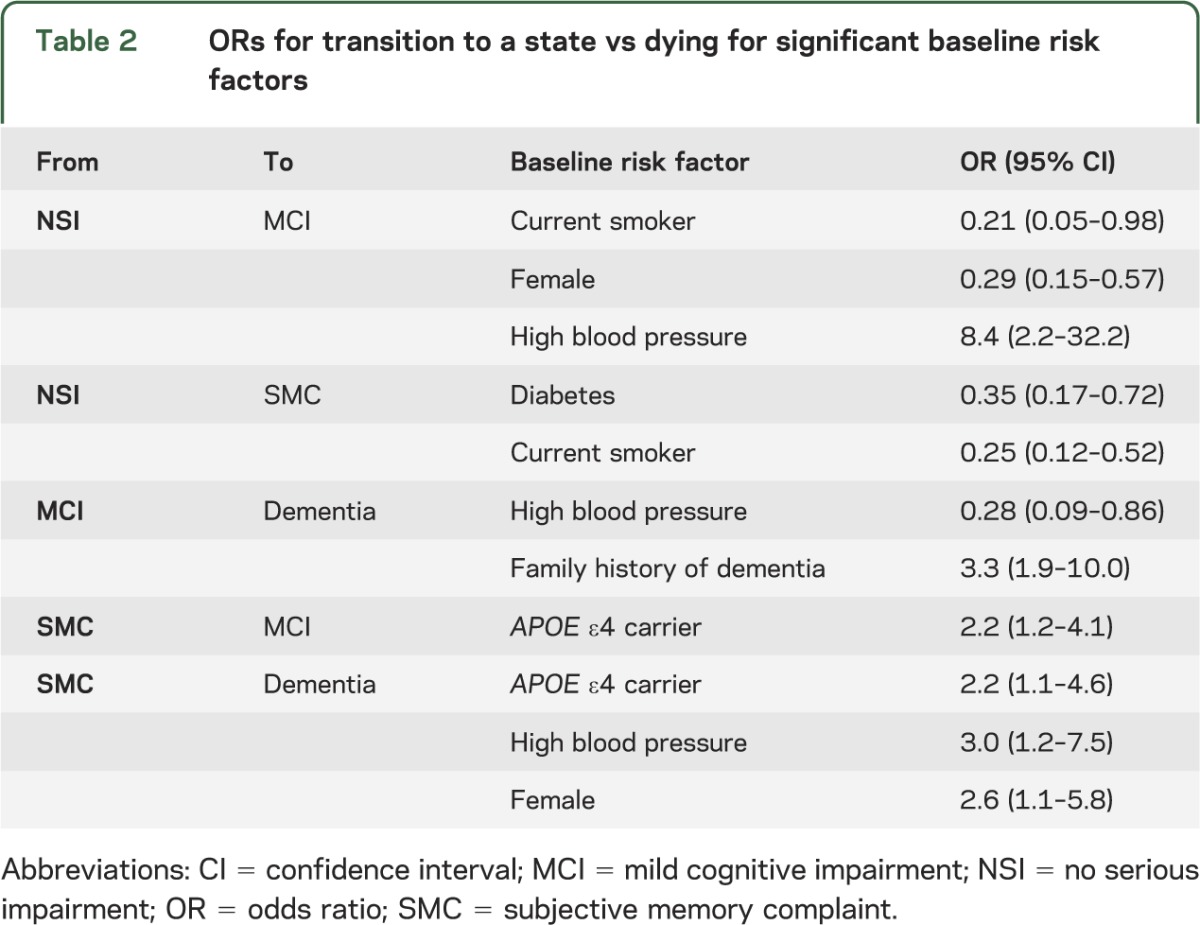
The presence of type II diabetes and current smoking decreased the odds of transition into the SMC state (table 2). This apparently counterintuitive result is because these risk factors promote mortality (a competing risk for cognitive impairment), thus decreasing the at-risk period for SMCs to occur. Current smoking and female sex reduced the risk of transition from NSI directly into MCI, while high blood pressure promoted this transition (OR = 8.4) (table 2). Once in the SMC state, an APOE ε4 carrier had increased odds of moving into MCI or dementia (OR = 2.2) when compared with a transition to death. Also, while in SMC, high blood pressure (OR = 3.0) and female sex (OR = 2.6) promoted transition directly into dementia vs death.
Table 2.
ORs for transition to a state vs dying for significant baseline risk factors
Mean time for a transition.
Mean holding times in each state, and risk factors affecting these means, varied little by destination state (table 3). For example, subjects entered the states of MCI, dementia, and death at an average age of 82.6, 84.2, and 90.0 years, respectively, meaning it took 9.4, 11.0, and 16.8 years for these events to occur from enrollment. The time to death, which again is a competing risk, was shortened by almost 5 years for former smokers and almost 9 years for current smokers. Of particular interest was the mean age for a transition from NSI into SMC (81.5 years), which implies that on average 8.3 years were required for participants to declare that they noticed a memory change since the last visit. This mean was abbreviated to 79.1 years for overweight subjects and to 79.6 years for subjects with a family history of dementia.
Table 3.
Mean age at first transition from the NSI state and mean holding time in the SMC and MCI states
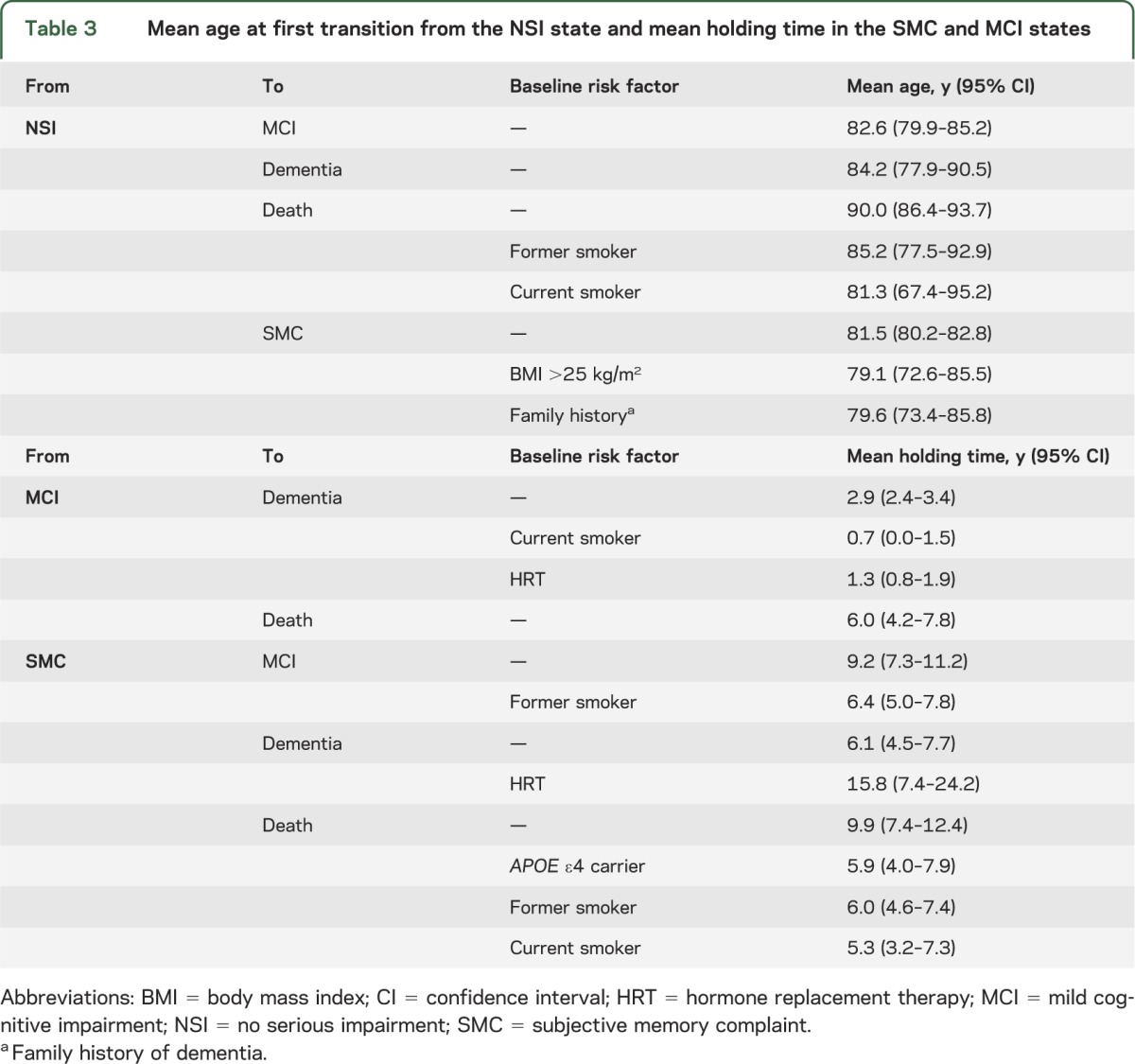
The transition from SMC to MCI took 9.2 years on average (this interval is abbreviated by 2.8 years for former smokers), and another 2.9 years on average was required to observe a conversion from MCI to dementia. Having at least one APOE ε4 allele doubles (OR = 2.2) the odds of a transition from SMC to either impairment vs dying. A small percentage (14.2%) of participants converted from SMC directly to dementia in 6.1 years on average; women and hypertensive individuals were most at risk for this latter transition. For HRT users, the transition from SMC directly to dementia was lengthened by 9.7 years. Finally, we note that the 127 of 296 subjects (42.9%) who entered SMC but died without a clinical impairment spent 9.9 years on average in SMC. This interval was shortened for APOE ε4 carriers and former smokers to near 6 years.
Neuropathology.
Almost half of the cohort has come to autopsy (243/531 or 45.7%); most others remain active in the cohort (244/288). To conduct our analysis, we classified the deceased participants with and without SMC by their impairment status (MCI or dementia), yielding 4 comparison groups: SMC negative and impaired negative (n = 56), SMC positive but impaired negative (n = 120), SMC negative but impaired positive (n = 17), and SMC positive and impaired positive (n = 50). The analyses focused on AD-type brain pathology.20 Boxplots of the NP and NFT counts for each group in the MTL and neocortex appear in figure 2.
Figure 2. Boxplots of neuritic plaque counts and neurofibrillary tangle counts in 2 brain regions.
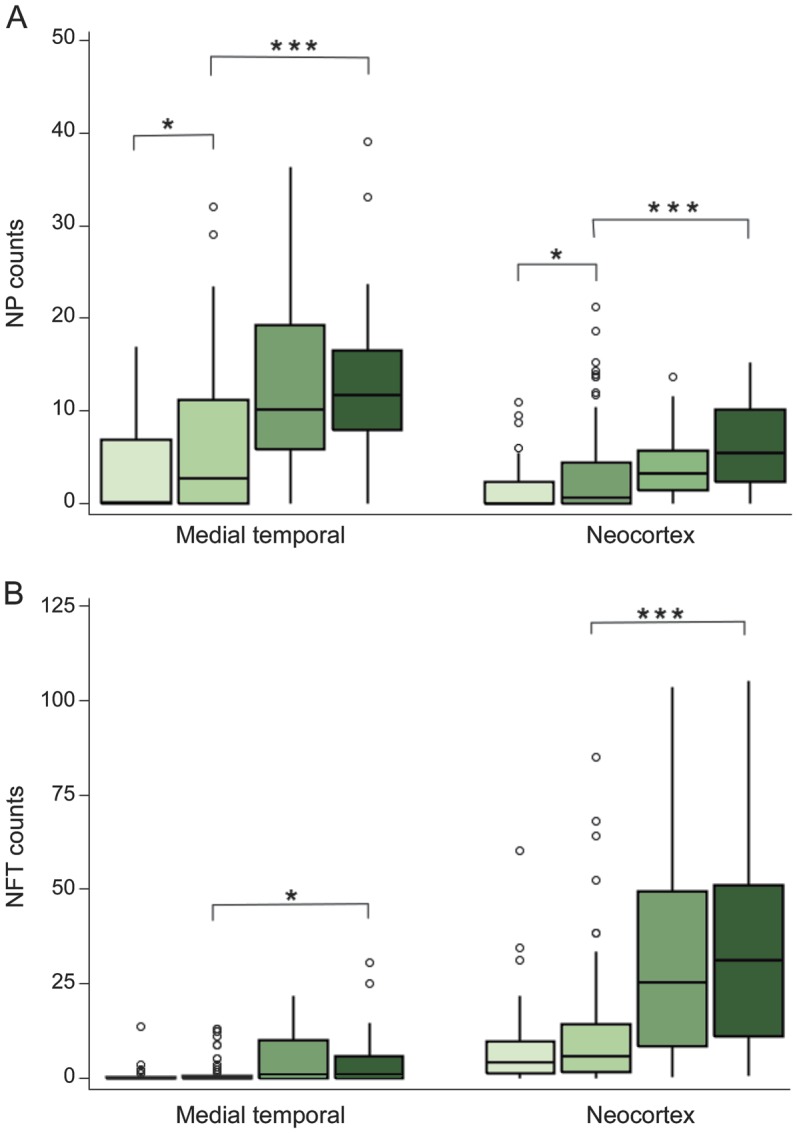
The boxplots provide neuritic plaque (NP) (A) and neurofibrillary tangle (NFT) (B) counts for each of 4 groups (in order and shaded light to dark): subjective memory complaint (SMC) negative and impaired negative; SMC positive and impaired negative; SMC negative and impaired positive; and SMC positive and impaired positive. *p < 0.05, ***p < 0.001.
Among unimpaired participants, mean NP counts in the MTL and neocortex were significantly higher for SMC positive compared with SMC negative (p < 0.03 in both regions; Wilcoxon test). However, among SMC positives, mean NP and NFT counts were still significantly lower in the unimpaired participants (p < 0.001 in both regions). In contrast, mean NFT counts in the unimpaired SMC-positive vs SMC-negative group were not different (p = 0.13 [MTL] and p = 0.27 [neocortex]). The percentage of autopsies blindly rated at level 3 or 4 (CERAD NP severity moderate or severe)18 increased monotonically: 23.2%, 36.7%, 58.8%, and 76.0% for the groups SMC and impaired negative, SMC positive but impaired negative, SMC negative but impaired positive, and SMC and impaired positive, respectively.
DISCUSSION
This study constitutes a thorough examination of the appearance of, and salient events following, self-declared memory complaints in a cohort of well-characterized older adults who volunteered for annual cognitive assessments and brain donation upon death. The majority of the subjects enrolled in the BRAiNS cohort (55.7%) declared SMCs, which is supported by results from the Nurses' Health Study, in which 56.4% of the participants declared a recent change in their ability to remember things.21
We found that SMC represented a higher risk of a subsequent criterion-based diagnosis of cognitive impairment, either clinical MCI or dementia, in agreement with much of the previous literature,1,4,6,22 although some studies did not observe SMC as a prognostic factor.5,23 Little has been reported, however, about the time required for such transitions to occur. The current study noted these changes occurred at age 81.5 years on average, approximately 8.3 years after enrollment into the cohort. Furthermore, the interval between the initial SMC and diagnosis of impairment was lengthened or shortened by multiple risk factors, which suggests that SMCs may signal an opportunity for intervention before clinical impairment manifests.
Recent research correlates the presence of SMC and biomarkers including brain region volumes,24–26 amyloid burden,26 brain atrophy,9 and gray matter atrophy,27 and is consistent with earlier neuropathologic reports.13,28 In this study, neuropathology was obtained on a large proportion of participants who died. It is important to note that while 42.9% of SMC subjects died with no observable clinical impairment, one-third of these had AD pathology by CERAD criteria at autopsy. The SMC-positive but unimpaired group had elevated NP counts in both the neocortex and MTL when compared with the 50 subjects in the cohort who died without SMC or clinical impairment.
While SMC has been shown to correlate with AD-type pathology regardless of impairment,11 the current study showed a strong impairment effect. Specifically, in the absence of clinical impairment, SMC-positive individuals had elevated NP counts when compared with SMC-negative individuals, but these counts were still significantly less when compared with SMC-positive and impaired participants, which is consistent with current concepts of preclinical AD.29 Also consistent with earlier reports, the NFT counts did reveal an SMC effect because these counts were not elevated unless the participant was impaired.17
The elevated neuritic amyloid plaque counts appear to be driven partially by the presence of an APOE ε4 allele. APOE ε4 carriers had significantly higher NP counts in the neocortex compared with noncarriers (p = 0.003; Wilcoxon rank sum test). Because participants were blinded to their APOE status, these results suggest an interaction between the APOE ε4 genotype and SMC, such that carriers with SMC were more likely to show AD-type changes. This interaction hypothesis is supported by a recent study that shows that smaller hippocampal volumes are associated with SMC positives among carriers but larger volumes are associated with SMC positives among noncarriers.30 However, an SMC-positive APOE ε4 carrier had an abbreviated time to death without any cognitive impairment (mean time reduced from 9.9 to 5.9 years).
This study has some limitations. SMC assessment was measured by one simple question each year on memory changes since the last visit. No informant was involved, and no further probing of the participant with an SMC was conducted. This could partially explain why approximately one-third of the SMC subjects died without ever converting to a cognitive impairment, although we provide evidence that this subset of individuals was more likely to harbor NPs. Moreover, once an SMC is reported in the cohort, 70% of subsequent queries confirm the SMC. In addition, all participants were self-selected volunteers; APOE ε4 carriers and individuals with positive family history are overrepresented because of their concerns about their own risk of AD. Thus, selection bias could affect the results. Furthermore, SMCs are often associated with depressive symptoms,7,31,32 but objective ratings of depression were not available for this cohort. However, clinical depression is an exclusionary criterion for entry into the BRAiNS cohort. Hence, while participants may have developed depressive symptoms during follow-up, it is unlikely that clinical depression had a major role in this study. Finally, several risk factors, including smoking, body mass index, HRT, diabetes, and high blood pressure were recorded only at baseline and could have changed during the study. Moreover, because these factors were derived from self-reports, some cases may have been misclassified. These limitations are to be weighed against the substantial follow-up and high autopsy rate on these well-characterized study participants.
The present study adds strong evidence to the literature supporting the hypothesis that SMCs are common among older adults and are often prognostic of future cognitive impairment. Physicians should solicit and monitor SMCs from their older patients. However, as shown here, SMCs are not a cause for immediate alarm because impairments could be many years away or preempted by death. Also, older adults with subjective complaints do harbor increased NPs regardless of impairment, a finding that has implications for the design of dementia prevention studies.
GLOSSARY
- AD
Alzheimer disease
- BRAiNS
Biologically Resilient Adults in Neurological Studies
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease
- HRT
hormone replacement therapy
- MCI
mild cognitive impairment
- MTL
medial temporal lobe
- NFT
neurofibrillary tangle
- NP
neuritic plaque
- NSI
no serious impairment
- OR
odds ratio
- SMC
subjective memory complaint
AUTHOR CONTRIBUTIONS
R. Kryscio: study concept and design, drafting and revising the manuscript, statistical analysis. E. Abner: manuscript revision, data management, and statistical analysis. D. Fardo: statistical analysis and manuscript revision. G. Cooper and G. Jicha: participant recruitment and assessment. P. Nelson: neuropathology and manuscript revision. C. Smith: participant recruitment and assessment. L. Van Eldik: manuscript revision. L. Wan: statistical analysis. F. Schmitt: study design, participant assessment, neuropsychology, and manuscript revision.
STUDY FUNDING
This research was partially funded with support from the following grants to the University of Kentucky's Center on Aging: R01 AG038651, P30 AG028383, R01 AG019241, and K25 AG043546 from the National Institute on Aging, as well as a grant to the University of Kentucky's Center for Clinical and Translational Science, UL1TR000117, from the National Center for Advancing Translational Sciences.
DISCLOSURE
R. Kryscio is partially supported by grants from the NIA, NIDA, NCATS, and NINDS, and receives a consulting fee from the Alltech Corporation. E. Abner receives partial support by grants from the NIA and NINDS. G. Cooper is partially supported by grants from Eli Lilly and Company. D. Fardo is partially supported by a K award from the NIA. G. Jicha is partially supported by grants from the NIA, NINDS, and NICHD, and receives contract research support from Alltech, Baxter, Esai, and Lilly. P. Nelson receives partial support by grants from the NIA and NINDS. C. Smith is partially supported by grants from the NIA, NINDS, and Neuronetrix, Inc. L. Van Eldik is partially supported by grants from the NIA and NINDS. L. Wan reports no disclosures. F. Schmitt receives support on grants from the NIA and NICHD. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement 2010;6:11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Harten AC, Visser PJ, Pijnenburg YA, et al. Cerebrospinal fluid Aβ42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement 2013;9:481–487 [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Van Belle G, Crane PK, et al. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc 2004;52:2045–2051 [DOI] [PubMed] [Google Scholar]
- 4.Geerlings MI, Jonker C, Bouter LM, Adèr HJ, Schmand B. Association between memory complaints and incident Alzheimer's disease in elderly people with normal baseline cognition. Am J Psychiatry 1999;156:531–537 [DOI] [PubMed] [Google Scholar]
- 5.Jungwirth S, Zehetmayer S, Weissgram S, Weber G, Tragl KH, Fischer P. Do subjective memory complaints predict senile Alzheimer dementia? Wien Med Wochenschr 2008;158:71–77 [DOI] [PubMed] [Google Scholar]
- 6.Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry 2010;67:414–422 [DOI] [PubMed] [Google Scholar]
- 7.Benito-León J, Mitchell AJ, Vega S, Bermejo-Pareja F. A population-based study of cognitive function in older people with subjective memory complaints. J Alzheimers Dis 2010;22:159–170 [DOI] [PubMed] [Google Scholar]
- 8.Murphy MD, Sanders RE, Gabriesheski AS, Schmitt FA. Metamemory in the aged. J Gerontol 2011;26:631–635 [DOI] [PubMed] [Google Scholar]
- 9.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 2006;67:834–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes L, Schneider J, Boyle P, Bienias J, Bennett D. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology 2006;67:1581–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorm A, Masaki K, Davis D, et al. Memory complaints in nondemented men predict future pathologic diagnosis of Alzheimer disease. Neurology 2004;63:1960–1961 [DOI] [PubMed] [Google Scholar]
- 12.Schmitt FA, Nelson PT, Abner E, et al. University of Kentucky Sanders-Brown healthy brain aging volunteers: donor characteristics, procedures and neuropathology. Curr Alzheimer Res 2012;9:724–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt F, Davis D, Wekstein D, Smith C, Ashford J, Markesbery W. “Preclinical” AD revisited neuropathology of cognitively normal older adults. Neurology 2000;55:370–376 [DOI] [PubMed] [Google Scholar]
- 14.Kryscio RJ, Abner EL, Lin Y, et al. Adjusting for mortality when identifying risk factors for transitions to mild cognitive impairment and dementia. J Alzheimers Dis 2013;35:823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abner EL, Kryscio RJ, Cooper GE, et al. Mild cognitive impairment: statistical models of transition using longitudinal clinical data. Int J Alzheimers Dis 2012;2012:291920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol 2010;20:66–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson PT, Jicha GA, Schmitt FA, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol 2007;66:1136–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486 [DOI] [PubMed] [Google Scholar]
- 19.Foucher Y, Giral M, Soulilou JP, Daures JP. A flexible semi-Markov model for interval-censored data and goodness-of-fit testing. Stat Methods Med Res 2010;19:127–145 [DOI] [PubMed] [Google Scholar]
- 20.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc 2011;59:1612–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population‐based studies. Int J Geriatr Psychiatry 2000;15:983–991 [DOI] [PubMed] [Google Scholar]
- 23.Roberts J, Clare L, Woods R. Subjective memory complaints and awareness of memory functioning in mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord 2009;28:95–109 [DOI] [PubMed] [Google Scholar]
- 24.Copenhaver BR, Rabin LA, Saykin AJ, et al. The fornix and mammillary bodies in older adults with Alzheimer's disease, mild cognitive impairment, and cognitive complaints: a volumetric MRI study. Psychiatry Res 2006;147:93–103 [DOI] [PubMed] [Google Scholar]
- 25.Jessen F, Feyen L, Freymann K, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging 2006;27:1751–1756 [DOI] [PubMed] [Google Scholar]
- 26.Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 2012;50:2880–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peter J, Scheef L, Abdulkadir A, et al. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimers Dement 2014;10:99–108 [DOI] [PubMed] [Google Scholar]
- 28.Price JL, McKeel DW, Jr, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 2009;30:1026–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging–Alzheimer's Disease Association criteria for preclinical Alzheimer disease. Ann Neurol 2012;71:765–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Striepens N, Scheef L, Wind A, et al. Interaction effects of subjective memory impairment and ApoE4 genotype on episodic memory and hippocampal volume. Psychol Med 2011;41:1997–2006 [DOI] [PubMed] [Google Scholar]
- 31.Jessen F, Wiese B, Cvetanovska G, et al. Patterns of subjective memory impairment in the elderly: association with memory performance. Psychol Med 2007;37:1753–1762 [DOI] [PubMed] [Google Scholar]
- 32.Jorm A, Christensen H, Korten A, Jacomb P, Henderson A. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7–8 years. Psychol Med 2001;31:441–450 [PubMed] [Google Scholar]


