Abstract
OBJECTIVE
To delineate adverse obstetric and neonatal outcomes as well as indications for cesarean delivery by maternal age in a contemporaneous large national cohort.
METHODS
This was a retrospective analysis of electronic medical records from 12 centers and 203,517 (30,673 women aged 35 years or older) women with singleton gestations stratified by maternal age. Logistic regression was performed to investigate maternal and neonatal outcomes for each maternal age strata (referent group, age 25.0–29.9 years), adjusting for race, parity, body mass index, insurance, pre-existing medical conditions, substance and tobacco use, and site. Documented indications for cesarean delivery were analyzed.
RESULTS
Neonates born to women aged 25.0–29.9 years had the lowest risk of birth weight less than 2,500 g (7.2%; P<.001), admission to neonatal intensive care unit (11.5%; P<.001), and perinatal mortality (0.7%; P<.001). Hypertensive disorders of pregnancy were higher in women aged 35 years or older (cumulative rate 8.5% compared with 7.8%; 25.0–29.9 years; P<.001). Previous uterine scar was the leading indication for cesarean delivery in women aged 25.0 years or older (36.9%; P<.001). For younger women, failure to progress or cephalopelvic disproportion (37.0% for those younger than age 20.0 years and 31.1% for those aged 20.0– 24.9-years; P<.001) and nonreassuring fetal heart tracing (28.7% for those younger than 20.0 years and 21.2% for those aged 20.0–24.9-years; P<.001) predominated as indications. Truly elective cesarean delivery rate was 20.2% for women aged 45.0 years or older (adjusted odds ratio 1.85 [99% confidence interval 1.03–3.32] compared with the referent age group of 25.0–29.9 years).
CONCLUSIONS
Maternal and obstetric complications differed by maternal age, as did rates of elective cesarean delivery. Women aged 25.0–29.9 years had the lowest rate of serious neonatal morbidity.
Adverse pregnancy outcomes have been linked to maternal age.1–5 Women younger than age 20 years are at increased risk for low birth weight, pre-term delivery, and neonatal death, even after adjusting for socioeconomic factors. Women aged 35 and older have an increased risk of miscarriage, chromosomal abnormalities, congenital anomalies, gestational diabetes, placenta previa, cesarean delivery, and hypertensive disorders of pregnancy.5–7 These risks are important at a population level because 9.2% of births occurred in women younger than 20 years in 2010, and an increasing number of U.S. women are delaying childbearing, with 14.9% of all births occurring for women of advanced maternal age the same year.8
For women aged 40–54 years, the cesarean delivery rate increased from 30.2% in 1996 to 49.5% in 2010, and it was twice as high compared with women younger than age 20 years.8 In addition, advanced maternal age has been associated with increased duration of labor, and both prelabor and intrapartum cesarean delivery rates may be higher with advancing maternal age.4,9–13 Possible explanations may include intrinsic alteration in the labor process compared with a lower threshold for provider intervention in an older parturient. There are limited data regarding the timing and indications for cesarean delivery related to maternal age and few studies pertaining to women aged 40 years or older.4,12
We sought to delineate adverse obstetric and neonatal outcomes as well as to explore the timing and indications for cesarean delivery by maternal age by using a large contemporaneous obstetric cohort of U.S. women.
MATERIALS AND METHODS
The Consortium on Safe Labor was a retrospective cohort study of all deliveries at 23 weeks of gestation or later (as recorded in the medical record, n=228,562) to 208,695 women from 2002 to 2008, from 19 hospitals across 9 states and the District of Columbia.13 All clinical sites obtained Institutional Review Board approval, and the MedStar Health Research Institute Institutional Review Board approved current analysis of the data presented here.
Maternal and neonatal information, as recorded in the electronic medical records, included maternal demographics, reproductive and medical history, prenatal history of current pregnancy, labor and delivery information, and newborn outcomes. Discharge diagnoses for every delivery were coded using International Classification of Diseases, 9th Revision, Clinical Modification. Validation studies evaluating four key diagnoses (cesarean delivery for nonreassuring fetal heart tracing, shoulder dystocia, neonatal intensive care unit admission for respiratory conditions, and asphyxia) indicated a high concordance between the electronic medical records and medical charts for these variables.13
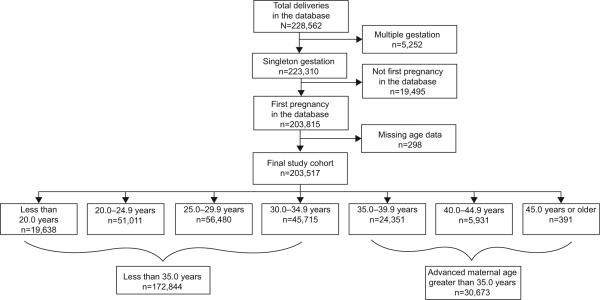
The final analyzed study cohort was limited to singleton gestations (n=223,310), and only the first recorded pregnancy per woman was included to avoid intraperson correlation (final analyzed cohort, n=203,517) (Fig. 1). Maternal age was stratified into the following age categories for evaluation of possible nonlinear associations: younger than 20.0 years; 20.0–24.9 years; 25.0–29.9 years; 30.0–34.9 years; 35.0–39.9 years; 40.0–44.9 years; and 45.0 years or older. Coexisting medical conditions analyzed included pregestational diabetes, chronic hypertension, asthma, and cardiac, renal, or neurologic disease. Pregnancy complications evaluated included gestational diabetes and hypertensive disorders of pregnancy (gestational hypertension, preeclampsia, eclampsia, and superimposed preeclampsia). Analyses of onset of labor, mode of delivery, and indications as well as the timing of cesarean deliveries were limited to 11 sites reporting these outcomes. Gestational age categories at delivery were defined as less than 28 weeks, 28–33 weeks, 34–36 weeks, 37–40 weeks, and 41 weeks or more of gestation. Neonatal outcomes included gestational age at birth, birth weight, Apgar score less than 7 at 5 minutes, neonatal intensive care unit admission and length of stay, and perinatal mortality.
Fig 1.
Patient selection diagram.
Timofeev. Pregnancy Outcomes and Maternal Age. Obstet Gynecol 2013.
Indications for cesarean delivery, as recorded in the operative report, were mapped to the following prespecified categories: malpresentation; chorioamnionitis; emergency; failed induction; failed trial of forceps or vacuum; failed vaginal birth after cesarean delivery; failure to progress (arrest of dilation or descent) or cephalopelvic disproportion (combined as a single variable); nonreassuring fetal heart tracing; fetal indication or anomaly; macrosomia; human immunodeficiency virus and herpes simplex virus; placental abruption; placenta previa or vasa previa; maternal hypertensive disease (chronic hypertension, gestational hypertension, or preeclampsia); previous uterine scar; shoulder dystocia; history of shoulder dystocia; elective; or other. Because deliveries may have had more than one indication, the same delivery could have been included in more than one indication category. The “elective” category included cesarean deliveries with the following indications: maternal request; advanced maternal age; multiparity; sterilization request; diabetes mellitus; human papilloma virus; fetal demise; polyhydramnios; group B streptococcus; pregnancy remote from term; postterm or postdates gestation; and social or religious preferences. Any other reasons listed as cesarean indication not in any of these categories were classified as “other.” Three hierarchical mutually exclusive categories were then used to describe the indication for cesarean as “clinically indicated,” “mixed,” and “truly elective,” as described by Zhang et al.13 The clinically indicated category included cesarean deliveries performed for nonreassuring fetal heart tracing or fetal distress, failure to progress or cephalopelvic dispro-portion (if the last dilation was 6 cm or larger but less than 10 cm; or, in nulliparous women, if the last dilation was 10 cm and duration of full dilation was 180 minutes or longer; or, in multiparous women, the last dilation was 10 cm and duration of full dilation was 120 minutes or longer; or failure to progress or cephalopelvic disproportion and failed trial of forceps or vacuum were documented for the same delivery), failed operative vaginal delivery, uterine rupture, cord prolapse, placental abnormalities (placenta previa or vasa previa, placental abruption), shoulder dystocia or history of shoulder dystocia, or other obstetric emergency. Cesarean deliveries in the mixed indications category included those for failure to progress or cephalopelvic disproportion categories not meeting inclusion criteria for the clinically indicated category, failed induction, suspected fetal macrosomia, fetal anomaly, previous uterine scar, human immunodeficiency virus or herpes simplex virus (viral load or active outbreak status unknown), or fetal malpresentation. Truly elective cesarean deliveries included births in the “elective” category as defined. Hierarchy was maintained if more than one indication for cesarean delivery was listed for a particular gravid woman, with the highest-order indication prioritized to assign the classification of delivery as clinically indicated, mixed, or truly elective.
Missing data for specific variables are presented in Tables 1–4. For logistic regression analysis, only sites reporting on the variable of interest were included in the model. Characteristics and outcomes of the groups were compared using Student t test or ×2 as appropriate, with significance determined as P<.01. Adjusted odds ratios (ORs) with 99% confidence intervals (CIs) and P values for labor, delivery, and neonatal outcomes for maternal age categories were estimated using logistic regression adjusting for maternal race, parity, body mass index (BMI, calculated as weight (kg)/[height (m)]2), insurance type, pre-existing medical conditions (pregestational diabetes, chronic hypertension, cardiac disease, asthma, renal disease, and neurologic disease), substance abuse, tobacco use, and clinical site. The maternal age category with the lowest rates of neonatal morbidity was chosen as the reference group (age 25.0–29.9 years). Analyses were conducted using SAS 9.1.3.
Table 1.
Maternal and Obstetric Characteristics by Maternal Age Category
| Characteristic | Younger Than 20.0 y (n = 19,638) | 20.0–24.9 y (n = 51,011) | 25.0–29.9 y (n = 56,480) | 30.0–34.9 y (n = 45,715) | 35.0–39.9 y (n = 24,351) | 40.0–44.9 y (n = 5,931) | 45.0 y or Older (n = 391) |
|---|---|---|---|---|---|---|---|
| Age (y) | 17.8±1.3 | 22.1±1.4 | 27.0±1.4 | 31.9±1.4 | 36.6±1.4 | 41.2±1.2 | 46.1±1.8 |
| Race* | |||||||
| Non-Hispanic white | 5,266 (26.8) | 22,515 (44.1) | 29,801 (52.8) | 25,483 (55.7) | 13,516 (55.5) | 3,085 (52.0) | 214 (54.7) |
| Non-Hispanic black | 8,090 (41.2) | 14,264 (28.0) | 10,791 (19.1) | 7,310 (16.0) | 4,058 (16.7) | 1,166 (19.7) | 83 (21.2) |
| Hispanic | 4,721 (24.0) | 9,593 (18.8) | 9,550 (16.9) | 7,101 (15.5) | 3,708 (15.2) | 958 (16.2) | 50 (12.8) |
| Asian or Pacific Islander | 166 (0.85) | 1,183 (2.3) | 2,573 (4.6) | 2,760 (6.0) | 1,405 (5.8) | 322 (5.4) | 17 (4.4) |
| Other or unknown | 1,395 (7.1) | 3,456 (6.8) | 3,765 (6.7) | 3,061 (6.7) | 1,664 (6.8) | 400 (6.7) | 27 (6.9) |
| Gravidity* | 1 (1, 2) | 2 (1, 3) | 2 (1, 3) | 3 (2, 4) | 3 (2, 4) | 4 (2, 5) | 4 (2, 6) |
| Parity and previous uterine scar* | |||||||
| Nulliparous, no scar | 16,702 (85.1) | 28,829 (56.5) | 21,080 (37.3) | 14,184 (31.0) | 6,434 (26.4) | 1,478 (24.9) | 117 (29.9) |
| Multiparous, no scar | 2,467 (12.6) | 17,955 (35.2) | 28,215 (50.0) | 23,717 (51.9) | 12,793 (52.5) | 3,106 (52.4) | 173 (44.3) |
| Multiparous, previous scar | 469 (2.4) | 4,227 (8.3) | 7,185 (12.7) | 7,814 (17.1) | 5,124 (21.0) | 1,347 (22.7) | 101 (25.8) |
| BMI at admission,* (kg/m2) (missing data = 42,017) | 29.9±5.9 (n = 15,540) | 30.8±6.3 (n = 40,316) | 31.1±6.3 (n = 45,355) | 31.0±6.2 (n = 36,178) | 31.0±6.3 (n = 19,138) | 31.0±6.0 (n = 4,675) | 31.3±6.0 (n = 298) |
| Insurance* | |||||||
| Private | 5,648 (28.8) | 22,349 (43.8) | 34,480 (61.1) | 30,753 (67.3) | 15,966 (65.6) | 3,634 (61.3) | 227 (58.1) |
| Public or self-pay | 12,044 (61.3) | 24,297 (47.6) | 16,828 (29.8) | 9,420 (20.6) | 4,557 (18.7) | 1,178 (19.9) | 84 (21.5) |
| Other or unknown | 1,946 (9.9) | 4,365 (8.6) | 5,172 (9.2) | 5,542 (12.1) | 3,828 (15.7) | 1,119 (18.9) | 80 (20.5) |
| Tobacco use* | 1,615 (8.2) | 4,483 (8.8) | 3,771 (6.7) | 2,238 (4.9) | 1,232 (5.1) | 360 (6.1) | 18 (4.6) |
| Alcohol use* | 349 (1.8) | 1,022 (2.0) | 995 (1.8) | 774 (1.7) | 502 (2.1) | 131 (2.2) | 7 (1.8) |
| Drug use* (missing data = 21,205) | 500 (2.9) (n = 17,175) | 1,126 (2.5) (n = 45,640) | 979 (1.9) (n = 50,743) | 689 (1.7) (n = 40,914) | 414 (1.9) (n = 21,993) | 139 (2.5) (n = 5,484) | 5 (1.4) (n = 363) |
| Pre-existing medical conditions | |||||||
| Pregestational diabetes* | 182 (0.9) | 617 (1.2) | 1,142 (2.0) | 1,317 (2.9) | 972 (4.0) | 280 (4.7) | 21 (5.4) |
| Chronic hypertension* | 431 (2.2) | 1,049 (2.1) | 1,406 (2.5) | 1,532 (3.4) | 1,171 (4.8) | 456 (7.7) | 37 (9.5) |
| Cardiac disease* (missing data = 7,321) | 127 (0.7) (n = 18,346) | 307 (0.6) (n = 48,521) | 352 (0.6) (n = 54,583) | 368 (0.8) (n = 44,644) | 212 (0.9) (n = 23,871) | 71 (1.2) (n = 5,847) | 9 (2.3) (n = 384) |
| Asthma* | 2,018 (10.3) | 3,927 (7.7) | 3,544 (6.3) | 2,413 (5.3) | 1,240 (5.1) | 284 (4.8) | 16 (4.1) |
| Renal disease* (missing data = 7,321) | 193 (1.1) (n = 18,346) | 452 (0.9) (n = 48,521) | 359 (0.7) (n = 54,583) | 295 (0.7) (n = 44,644) | 118 (0.5) (n = 23,871) | 21 (0.4) (n = 5,847) | 2 (0.5) (n = 384) |
| Neurologic disease† (missing data = 23,022) | 33 (0.2) (n = 16,560) | 52 (0.1) (n = 44,780) | 43 (0.1) (n = 50,860) | 39 (0.1) (n = 41,254) | 18 (0.1) (n = 21,600) | 9 (0.2) (n = 5,095) | 0 (n = 346) |
BMI, body mass index.
Data are mean±standard deviation, n (%), or median (interquartile range).
P<.001.
P=.003.
Table 4.
Indications and Timing of Cesarean Delivery by Maternal Age Categories*
| Younger Than 20.0 y (n = 4,094) | 20.0–24.9 y (n = 11,961) | 25.0–29.9 y (n = 14,874) | 30.0–34.9 y (n = 14,497) | 35.0–39.9 y (n = 9,484) | 40.0–44.9 y (n = 2,774) | 45.0 y or Older (n = 220) | |
|---|---|---|---|---|---|---|---|
| Timing of delivery (n = 57,903) | |||||||
| Primary cesarean delivery† | (n = 3,708) | (n = 8,400) | (n = 8,906) | (n = 7,979) | (n = 5,053) | (n = 1,544) | (n = 130) |
| Prelabor | 611 (16.5) | 1,519 (18.1) | 2,054 (23.1) | 2,275 (28.5) | 1,751 (34.7) | 571 (37.0) | 64 (49.2) |
| After spontaneous labor | 1,444 (38.9) | 3,220 (38.3) | 3,250 (36.5) | 2,548 (31.9) | 1,432 (28.3) | 389 (25.2) | 19 (14.6) |
| After induced labor | 1,653 (44.6) | 3,661 (43.6) | 3,602 (40.4) | 3,156 (39.6) | 1,870 (37.0) | 584 (37.8) | 47 (36.2) |
| Repeat cesarean delivery† | (n = 385) | (n = 3,561) | (n = 5,968) | (n = 6,518) | (n = 4,431) | (n = 1,230) | (n = 90) |
| Prelabor | 241 (62.6) | 2,224 (62.5) | 3,423 (57.4) | 4,096 (62.8) | 3,093 (69.8) | 887 (72.1) | 69 (76.7) |
| After spontaneous labor | 122 (31.7) | 1,148 (32.2) | 2,248 (37.7) | 2,108 (32.3) | 1,111 (25.1) | 276 (22.4) | 18 (20.0) |
| After induced labor | 22 (5.7) | 189 (5.3) | 297 (5.0) | 314 (4.8) | 227 (5.1) | 67 (5.5) | 3 (3.3) |
| Indications for cesarean delivery* (n = 53,947) (missing data = 3,956) | (n = 3,856) | (n = 11,303) | (n = 13,845) | (n = 13,342) | (n = 8,791) | (n = 2,602) | (n = 208) |
| Previous uterine scar† | 284 (7.4) | 2,695 (23.8) | 4,772 (34.5) | 5,020 (37.6) | 3,491 (39.7) | 951 (36.6) | 75 (36.1) |
| Failure to progress or cephalopelvic disproportion† | 1,427 (37.0) | 3,510 (31.1) | 3,463 (25.0) | 2,808 (21.1) | 1,488 (16.9) | 430 (16.5) | 29 (13.9) |
| Malpresentation | 791 (20.5) | 2,163 (19.1) | 2,734 (19.8) | 2,706 (20.3) | 1,759 (20.0) | 515 (19.8) | 50 (24.0) |
| Nonreassuring fetal heart tracing† | 1,107 (28.7) | 2,395 (21.2) | 2,235 (16.1) | 1,987 (14.9) | 1,306 (14.9) | 435 (16.7) | 37 (17.8) |
| Elective† | 326 (8.5) | 1,296 (11.5) | 1,637 (11.8) | 1,907 (14.3) | 1,538 (17.5) | 486 (18.7) | 38 (18.3) |
| Hypertensive disease† | 146 (3.8) | 289 (2.6) | 341 (2.5) | 362 (2.7) | 254 (2.9) | 83 (3.2) | 13 (6.3) |
| Macrosomia† | 50 (1.3) | 204 (1.8) | 325 (2.4) | 348 (2.6) | 253 (2.9) | 71 (2.7) | 4 (1.9) |
| Fetal indication or anomaly | 72 (1.9) | 178 (1.6) | 204 (1.5) | 198 (1.5) | 116 (1.3) | 34 (1.3) | 2 (1.0) |
| Failed induction† | 84 (2.2) | 192 (1.7) | 161 (1.2) | 135 (1.0) | 75 (0.9) | 21 (0.8) | 2 (1.0) |
| Placenta previa or vasa previa† | 11 (0.3) | 73 (0.7) | 135 (1.0) | 194 (1.5) | 158 (1.8) | 58 (2.2) | 5 (2.4) |
| Chorioamnionitis† | 44 (1.1) | 107 (1.0) | 97 (0.7) | 66 (0.5) | 41 (0.5) | 16 (0.6) | 0 |
| HIV, active herpes simples virus lesions† | 63 (1.6) | 112 (1.0) | 89 (0.6) | 87 (0.7) | 65 (0.7) | 15 (0.6) | 3 (1.4) |
| Placental abruption‡ | 43 (1.1) | 88 (0.8) | 87 (0.6) | 89 (0.7) | 67 (0.8) | 15 (0.6) | 3 (1.4) |
| Emergency | 21 (0.5) | 34 (0.3) | 55 (0.4) | 45 (0.3) | 38 (0.4) | 11 (0.4) | 0 |
| Failed trial of forceps or vacuum§ | 16 (0.4) | 27 (0.2) | 25 (0.2) | 26 (0.2) | 8 (0.1) | 6 (0.2) | 1 (0.5) |
| History of shoulder dystocia | 0 | 2 (0.02) | 13 (0.09) | 10 (0.07) | 6 (0.07) | 1 (0.04) | 0 |
| Failed VBAC | 1 (0.03) | 7 (0.06) | 7 (0.05) | 13 (0.10) | 6 (0.07) | 1 (0.04) | 0 |
| Shoulder dystocia | 0 | 3 (0.03) | 5 (0.04) | 7 (0.05) | 0 | 0 | 0 |
| Other or unknown† | 266 (6.9) | 639 (5.7) | 745 (5.4) | 772 (5.8) | 580 (6.6) | 221 (8.5) | 19 (9.1) |
| Hierarchical indication groups†∥ (n = 53,947, missing data = 3,956) | (n = 3,856) | (n = 11,303) | (n = 13,845) | (n = 13,342) | (n = 8,791) | (n = 2,602) | (n = 208) |
| Clinically indicated | 1,726 (44.8), referent | 3,916 (34.7), referent | 3,922 (28.3), referent | 3,568 (26.7), referent | 2,224 (25.3), referent | 675 (25.9), referent | 51 (24.5), referent |
| Mixed | 1,644 (42.6) | 6,127 (54.2) | 8,371 (60.5) | 8,133 (61.0) | 5,319 (60.5) | 1,486 (57.1) | 115 (55.3) |
| OR | 0.94 | 0.96 | Referent | 0.99 | 1.03 | 1.02 | 1.03 |
| 99% CI | 0.83–1.08 | 0.87–1.05 | 0.90–1.08 | 0.93–1.15 | 0.87–1.21 | 0.61–1.75 | |
| Truly elective | 486 (12.6) | 1,260 (11.2) | 1,552 (11.2) | 1,641 (12.3) | 1,248 (14.2) | 441 (17.0) | 42 (20.2) |
| OR | 0.97 | 1.01 | Referent | 1.04 | 1.23 | 1.47 | 1.85 |
| 99% CI | 0.81–1.17 | 0.89–1.15 | 0.93–1.18 | 1.08–1.42 | 1.21–1.80 | 1.03–3.32 |
HIV, human immunodeficiency virus; VBAC, vaginal birth after cesarean delivery; OR, odds ratio; CI, confidence interval.
Data are n (%) unless otherwise specified.
Because more than one indication could have been listed, indications may add up to more than 100%.
P<.001.
P=.037.
P=.01.
Adjusted for maternal race, parity, body mass index, insurance type, pre-existing medical conditions, substance abuse, tobacco use, and reporting clinical site. The group aged 25.0–29.9 years was used as the referent category. Missing data for logistic regression = 10,650.
RESULTS
Maternal and obstetric characteristics by maternal age group are presented in Table 1. Of the cohort, 9.6% (n=19,638) of women were younger than 20.0 years and 15.1% (n=30,673) were aged 35.0 years or older (Fig. 1). The proportion of non-Hispanic white women increased with increasing maternal age and constituted the largest proportion of parturient women aged 35.0 years and older (54.8%). In contrast, non-Hispanic black women and Hispanic women tended to be younger than 25.0 years. Mean maternal BMI increased across maternal age (P<.001) (Table 1).
Chronic medical conditions increased with advancing maternal age, with pre-existing diabetes or chronic hypertension present in 5.4% and 9.5% of women aged 45.0 years and older, respectively (compared with 2.0% and 2.5% in women aged 25.0–29.9 years; P<.001) (Table 1). The risk of gestational diabetes increased with maternal age, with 1.6% of women younger than 20.0 years being affected compared with 14.3% of women aged 45.0 years and older (P<.001; adjusted OR 0.33 [99% CI 0.27– 0.40] and adjusted OR 3.33 [99% CI 2.09–5.30], respectively, compared with women 25.0–29.9 years of age; Table 2). Hypertensive disorders of pregnancy were increased for women older than those in the referent group as follows: 1.22-fold (99% CI 1.12– 1.33) for women aged 35.0–39.9 years; 1.63-fold for women aged 40.0–44.9 years (99% CI 1.42–1.88); and 1.89-fold (99% CI 1.21–2.96) for women aged 45.0 years or older (Table 2).
Table 2.
Labor and Delivery Outcomes by Maternal Age Category and Adjusted Odds Ratios
| Outcome | Younger Than 20.0 y (n = 19,638) | 20.0–24.9 y (n = 51,011) | 25.0–29.9 y (n = 56,480) | 30.0–34.9 y (n = 45,715) | 35.0–39.9 y (n = 24,351) | 40.0–44.9 y (n = 5,931) | 45.0 y or Older (n = 391) |
|---|---|---|---|---|---|---|---|
| Pregnancy complications | |||||||
| Gestational diabetes* | 316 (1.6) | 1,445 (2.8) | 2,727 (4.8) | 3,209 (7.0) | 2,315 (9.5) | 749 (12.6) | 56 (14.3) |
| OR | 0.33 | 0.59 | Referent | 1.48 | 2.03 | 2.81 | 3.33 |
| 99% CI | 0.27–0.40 | 0.53–0.65 | 1.36–1.61 | 1.84–2.23 | 2.43–3.25 | 2.09–5.30 | |
| Hypertensive disorders of pregnancy*† | 2,129 (10.8) | 4,347 (8.5) | 4,415 (7.8) | 3,337 (7.3) | 1,962 (8.1) | 611 (10.3) | 51 (13.0) |
| OR | 1.03 | 0.93 | Referent | 1.02 | 1.22 | 1.63 | 1.89 |
| 99% CI | 0.94–1.13 | 0.87–0.99 | 0.95–1.10 | 1.12–1.33 | 1.42–1.88 | 1.21–2.96 | |
| Superimposed preeclampsia* | 241 (1.2) | 490 (1.0) | 641 (1.1) | 663 (1.5) | 522 (2.1) | 194 (3.3) | 19 (4.9) |
| OR | 0.64 | 0.76 | Referent | 1.15 | 1.25 | 1.17 | 2.44 |
| 99% CI | 0.39–1.06 | 0.57–1.02 | 0.90–1.46 | 0.96–1.63 | 0.81–1.71 | 0.87–6.82 | |
| Onset of labor* | |||||||
| Spontaneous | 11,840 (60.3), referent | 29,812 (58.4), referent | 30,703 (54.4), referent | 22,685 (49.6), referent | 10,956 (45.0), referent | 2,353 (39.7), referent | 134 (34.3), referent |
| Induced | 6,946 (35.4) | 17,456 (34.2) | 20,300 (35.9) | 16,659 (36.4) | 8,551 (35.1) | 2,120 (35.7) | 124 (31.7) |
| OR | 0.79 | 0.87 | Referent | 1.03 | 1.03 | 1.08 | 1.10 |
| 99% CI | 0.75–0.84 | 0.83–0.90 | 0.99–1.07 | 0.98–1.08 | 0.98–1.19 | 0.76–1.60 | |
| Prelabor cesarean delivery | 852 (4.3) | 3,743 (7.3) | 5,477 (9.7) | 6,371 (13.9) | 4,844 (19.9) | 1,458 (24.6) | 133 (34.0) |
| OR | 0.43 | 0.69 | Referent | 1.26 | 1.70 | 2.26 | 4.31 |
| 99% CI | 0.37–0.49 | 0.64–0.75 | 1.17–1.35 | 1.56–1.85 | 1.98–2.59 | 2.83–6.57 | |
| Epidural* | 13,727 (69.9) | 35,112 (68.8) | 38,972 (69.0) | 30,505 (66.7) | 15,236 (62.6) | 3,543 (59.7) | 211 (54.0) |
| OR | 1.16 | 1.08 | Referent | 0.96 | 0.85 | 0.78 | 0.56 |
| 99% CI | 1.09–1.24 | 1.03–1.13 | 0.92–1.00 | 0.81–0.90 | 0.71–0.85 | 0.41–0.78 | |
| Oxytocin* | 8,251 (42.0) | 21,671 (42.5) | 24,265 (43.0) | 18,330 (40.1) | 8,789 (36.1) | 1,964 (33.1) | 116 (29.7) |
| OR | 0.94 | 0.97 | Referent | 0.99 | 0.93 | 0.81 | 0.60 |
| 99% CI | 0.88–1.01 | 0.92–1.01 | 0.95–1.04 | 0.87–0.98 | 0.73–0.90 | 0.41–0.87 | |
| Mode of delivery* | |||||||
| Vaginal, spontaneous | 14,419 (73.4), referent | 36,125 (70.8), referent | 38,742 (68.6), referent | 29,167 (63.8), referent | 13,927 (57.2), referent | 2,931 (49.4), referent | 160 (40.9), referent |
| Vaginal, operative (forceps or vacuum) | 1,126 (5.7) | 2,925 (5.7) | 2,864 (5.1) | 2,051 (4.5) | 940 (3.9) | 226 (3.8) | 11 (2.8) |
| OR | 0.84 | 0.91 | Referent | 1.09 | 1.12 | 1.48 | 0.90 |
| 99% CI | 0.75–0.94 | 0.84–0.98 | 1.00–1.19 | 1.00–1.26 | 1.21–1.82 | 0.33–2.48 | |
| Cesarean | 4,093 (20.8) | 11,961 (23.5) | 14,874 (26.3) | 14,497 (31.7) | 9,484 (39.0) | 2,774 (46.8) | 220 (56.3) |
| OR | 0.53 | 0.73 | Referent | 1.29 | 1.78 | 2.77 | 3.84 |
| 99% CI | 0.50–0.57 | 0.70–0.77 | 1.23–1.36 | 1.67–1.89 | 2.50–3.07 | 2.67–5.53 | |
| Gestational age at delivery* (wk) | |||||||
| Less than 28 | 284 (1.5) | 548 (1.1) | 491 (0.9) | 474 (1.0) | 322 (1.3) | 96 (1.6) | 7 (1.8) |
| OR | 0.94 | 0.95 | Referent | 1.23 | 1.44 | 1.84 | 2.34 |
| 99% CI | 0.74–1.21 | 0.79–1.16 | 1.01–1.49 | 1.15–1.81 | 1.30–2.61 | 0.79–6.96 | |
| 28–33 | 787 (4.0) | 1,516 (3.0) | 1,346 (2.4) | 1,243 (2.7) | 788 (3.2) | 245 (4.1) | 18 (4.6) |
| OR | 1.22 | 1.09 | Referent | 1.16 | 1.38 | 1.77 | 2.10 |
| 99% CI | 1.04–1.41 | 0.97–1.23 | 1.03–1.31 | 1.20–1.59 | 1.43–2.19 | 1.05–4.21 | |
| 34–36 | 1,752 (8.9) | 3,866 (7.6) | 4,123 (7.3) | 3,407 (7.5) | 1,998 (8.2) | 571 (9.6) | 40 (10.2) |
| OR | 1.11 | 1.01 | Referent | 1.02 | 1.13 | 1.28 | 1.42 |
| 99% CI | 1.00–1.23 | 0.94–1.09 | 0.95–1.11 | 1.03–1.23 | 1.11–1.48 | 0.86–2.34 | |
| 37–40 | 14,855 (75.6), referent | 40,516 (79.4), referent | 46,480 (82.3), referent | 37,579 (82.2), referent | 19,705 (80.9), referent | 4,668 (78.7), referent | 301 (77.0), referent |
| 41 or later | 1,960 (10.0) | 4,565 (9.0) | 4,040 (7.2) | 3,012 (6.6) | 1,538 (6.3) | 351 (5.9) | 25 (6.4) |
| OR | 1.04 | 1.06 | Referent | 0.97 | 0.96 | 0.94 | 1.00 |
| 99% CI | 0.94–1.15 | 0.99–1.14 | 0.89–1.04 | 0.87–1.06 | 0.79–1.12 | 0.54–1.83 | |
| Malpresentation* (missing data = 19,638) | 1,197 (6.7) (n = 17,982) | 3,248 (7.0) (n = 46,701) | 4,048 (8.0) (n = 50.833) | 3,825 (9.4) (n = 40,672) | 2,364 (10.8) (n = 21,899) | 680 (12.5) (n = 5,438) | 54 (15.3) (n = 354) |
| OR | 0.61 | 0.77 | Referent | 1.26 | 1.48 | 1.81 | 2.20 |
| 99% CI | 0.54–0.68 | 0.71–0.82 | 1.17–1.35 | 1.36–1.60 | 1.59–2.07 | 1.43–3.37 | |
| Placenta previa* | 52 (0.3) | 205 (0.4) | 305 (0.5) | 429 (0.9) | 335 (1.4) | 106 (1.8) | 12 (3.1) |
| OR | 0.52 | 0.68 | Referent | 1.57 | 2.11 | 2.70 | 5.73 |
| 99% CI | 0.33–0.82 | 0.51–0.90 | 1.25–1.96 | 1.65–2.70 | 1.90–3.82 | 2.53–12.98 | |
| Placental abruption | 323 (1.6) | 860 (1.7) | 900 (1.6) | 688 (1.5) | 425 (1.8) | 110 (1.9) | 9 (2.3) |
| OR | 0.88 | 0.96 | Referent | 1.03 | 1.25 | 1.44 | 2.02 |
| 99% CI | 0.71–1.08 | 0.83–1.10 | 0.89–1.20 | 1.05–1.50 | 1.07–1.93 | 0.83–4.93 | |
| Postpartum hemorrhage (missing data = 54,787) | 228 (1.5) (n = 15,229) | 669 (1.7) (n = 40,462) | 699 (1.6) (n = 43,041) | 463 (1.5) (n = 31,351) | 226 (1.5) (n = 15,022) | 56 (1.7) (n = 3,384) | 6 (2.5) (n = 241) |
| OR | 1.09 | 1.09 | Referent | 1.03 | 1.17 | 1.40 | 2.24 |
| 99% CI | 0.87–1.37 | 0.94–1.27 | 0.88–1.21 | 0.95–1.44 | 0.97–2.03 | 0.76–6.63 | |
| ICU admission* (missing data = 40,577) | 126 (0.8) (n = 15,913) | 207 (0.5) (n = 40,063) | 216 (0.5) (n = 45,643) | 196 (0.5) (n = 36,720) | 110 (0.6) (n = 19,606) | 55 (1.2) (n = 4,698) | 4 (1.4) (n = 297) |
| OR | 0.81 | 0.87 | Referent | 1.17 | 1.42 | 2.64 | 3.70 |
| 99% CI | 0.42–1.56 | 0.55–1.37 | 0.76–1.80 | 0.86–2.35 | 1.34–5.18 | 0.56–24.20 | |
| Endometritis* | 310 (1.6) | 449 (0.9) | 340 (0.6) | 236 (0.5) | 123 (0.5) | 34 (0.6) | 3 (0.8) |
| OR | 1.26 | 1.06 | Referent | 0.97 | 0.85 | 1.03 | 1.06 |
| 99% CI | 0.97–1.64 | 0.85–1.32 | 0.76–1.24 | 0.61–1.18 | 0.60–1.79 | 0.17–6.71 | |
| Wound separation or infection‡ | 67 (1.6) | 201 (1.7) | 268 (1.8) | 240 (1.7) | 135 (1.4) | 46 (1.7) | 5 (2.3) |
| OR | 1.24 | 1.07 | Referent | 1.01 | 0.91 | 1.31 | 1.80 |
| 99% CI | 0.83–1.86 | 0.82–1.39 | 0.79–1.30 | 0.67–1.24 | 0.85–2.03 | 0.54–5.97 | |
| Maternal thrombosis | 36 (0.2) | 135 (0.3) | 242 (0.4) | 318 (0.7) | 207 (0.9) | 65 (1.1) | 8 (2.1) |
| OR | 0.80 | 0.57 | Referent | 1.74 | 2.17 | 2.69 | 4.85 |
| 99% CI | 0.46–1.39 | 0.41–0.79 | 1.37–2.22 | 1.66–2.85 | 1.82–3.98 | 1.85–12.69 |
CI, confidence interval; OR, odds ratio; ICU, intensive care unit.
Data are n (%) unless otherwise specified.
Adjusted odds ratios calculated from logistic regression and adjusted for maternal race, parity, body mass index, insurance type, pre-existing medical conditions, substance abuse, tobacco use, and reporting clinical site. The group aged 25.0–29.9 years was used as the referent category. Unless specified, missing data for logistic regression = 44,227. P<.001.
P<.001.
Includes gestational hypertension, preeclampsia, and eclampsia.
Includes only patients who had cesarean delivery.
Maternal complications in women aged 45.0 years or older increased with increasing maternal age when compared with women aged 25.0–29.9 years, including intensive care unit admission (1.4% compared with 0.5%; P<.001) and maternal thrombosis (2.1% compared with 0.4%; P<.001) (Table 2). Risk of intensive care unit admission was increased at older maternal age, with women aged 40.0–44.9 years having a 2.64-fold increased risk (99% CI 1.34–5.18), although the association was not significant for women aged 45.0 years or older (adjusted OR 3.70; 99% CI 0.56–24.20). Risk of thrombosis also increased, starting with the maternal age group of 30.0–34.9 years having 1.74-fold increased OR (99% CI 1.37–2.22) and women aged 45.0 years or older having up to 4.85-fold increased OR (99% CI 1.85–12.69). Complications such as placental abruption (P=.090), postpartum hemorrhage (P=.348), and wound separation or infection (P=.466) were not significantly different across maternal age groups. The rate of endometritis varied, with the highest rates observed in women younger than 20.0 years (1.6% compared with 0.6% in women aged 25.0–29.9; P<.001). This increased risk, however, was not statistically significant after adjusting for maternal demographics and medical complications (adjusted OR 1.26; 99% CI 0.97–1.64). Malpresentation increased with maternal age, occurring in 15.3% of women aged 45.0 years or older (compared with 8.0% in the referent group; P<.001; adjusted OR 2.20; 99% CI 1.43–3.37). The preterm birth rate was also increased at age extremes, with women aged 25.0–29.9 years having the lowest rates of delivery at less than 28 weeks, 28–33 weeks, and 34–36 weeks of gestation (P<.001 compared with all other ages).
Overall, 8.5% of all neonates weighed less than 2,500 g and 2.0% weighed less than 1,500 g. Women aged 25.0–29.9 years had the lowest rates (1.7%) of very low birth weight less than 1,500 g (P<.001 for most comparisons, except not significantly different compared with women aged 30.0–34.9 and 45 and older) and low birth weight less than 2,500 g (7.2%; P<.001 for all comparisons, except not significantly different compared with women aged 30.0–34.9 years). Birth weight more than 4,000 g paralleled the increase in gestational and pre-existing diabetes and maternal BMI observed with increasing maternal age. Neonatal intensive care unit admission occurred in 12.4% of all births, and 1.8% of neonates had Apgar scores less than 7 at 5 minutes, with the lowest rates in women aged 25.0–29.9 years (11.5% and 1.5%, respectively; P<.001 across age groups). The rate of perinatal mortality varied across age groups (P<.001 for most comparisons with women aged 25.0–29.9 years, except for those aged 30.0–34.9 years) and, although slightly increased for women younger than 20.0 years (1.0%; adjusted OR 1.10; 99% CI 0.82– 1.48) and women aged 45.0 years or older (1.8%; adjusted OR 2.16; 99% CI 0.66–7.04), was not statistically different from that of the referent group after adjustment.
The cesarean delivery rate was 28.5%, and primary cesarean delivery occurred in 17.6% of parturient women when indications for cesarean delivery were reported with complete data (n=53,947). Spontaneous labor onset decreased with increasing maternal age (P<.001) (Table 2). A concomitant increase in prelabor primary cesarean delivery rate was observed with increasing maternal age, with rates ranging from 16.5% for women younger than 20.0 years to 49.2% for women aged 45.0 years or older (P<.001) (Table 4). The rates of spontaneous vaginal delivery decreased as maternal age increased, from 73.4% in women aged younger than 20.0 years to 55.5% for women aged 35 years or older, whereas the cesarean delivery rate increased significantly from 20.8% in women younger than 20.0 years to 40.7% in women 35.0 years and older (P<.001) (Table 2).
Indications for cesarean delivery differed by maternal age (Table 4). Previous uterine scar comprised the most significant proportion of indications for women aged 25.0 and older (36.9%), whereas cesarean delivery for failure to progress or cephalopelvic disproportion and nonreassuring fetal heart tracing predominated as indications in the group younger than 20.0 years (37.0% and 28.7%, respectively) and in the group aged 20.0–24.9-years (31.1% and 21.2%, respectively). Using hierarchical classification, the proportion of truly elective cesarean deliveries increased from 12.6% of women aged younger than 20.0 years (adjusted OR 0.97; 99% CI 0.81–1.17) to 20.2% in women aged 45.0 years or older (adjusted OR 1.85; 99% CI 1.03–3.32) compared with those aged 25.0– 29.9 years.
DISCUSSION
The optimal maternal age with the least risks of maternal, pregnancy, and neonatal complications was 25.0–29.9 years. Higher rates of pre-existing medical conditions and obstetric complications were found with increasing maternal age, but the absolute risks were low. Notably, the overall risk of cesarean delivery was significantly higher in an older parturient woman (driven primarily by the indication of previous uterine scar). However, a higher proportion of truly elective cesarean deliveries also occurred in women aged 35.0 years and older.
Published literature regarding the effect of maternal age on neonatal outcomes is controversial; some investigators2,5 report no increase in adverse neonatal outcomes, whereas others show higher risks of preterm delivery, low birth weight, and perinatal mortality for women older than age 45.10–12,14 There was variation in poor neonatal outcomes across maternal age strata, with the lowest neonatal morbidity observed in women aged 25.0–29.9 years. Some proposed mechanisms for increased neonatal morbidity in women younger than 20.0 years include maternal physiologic immaturity, sociodemographic factors with poor access or initiation of prenatal care, as well as the effect of concomitant pregnancy on nutritional demands of a still growing mother.6,7 In older women, the risk of adverse neonatal outcome may be related to the presence of chronic comorbidities, but other proposed mechanisms for increase in neonatal morbidity may include an unfavorable intrauterine environment because of poor placental function in light of progressive vascular changes occurring with increasing age and altered adaptation to the increased demands of pregnancy.2,5,15
The rates of cesarean delivery have increased in the United States, reaching 32.8% in 2011.16 In our study, women aged 35.0 years and older had progressively higher cesarean delivery rates compared with younger women. We also found an increase in mal-presentation with increasing maternal age. Potential explanations for this observation include nulliparity and a higher incidence of leiomyomas and uterine malformations, which may have been the reason for the original delay in childbearing.1,9,17
Several factors have been proposed as explanations for the increasing cesarean delivery trends, including advancing maternal age as women delay childbearing, higher rates of obesity and other medical comorbidities, medicolegal environment, and patient preferences. A concept of a more “valuable” pregnancy in the older parturient woman may also lead to elective delivery because of the perception of vaginal delivery as more dangerous.18 Adashek et al19 evaluated factors potentially contributing to the increased cesarean delivery birth rate and could not identify any controllable provider actions affecting the increased rate of cesarean in women older than age 35 years. Interestingly, patients who were able to achieve vaginal delivery required oxytocin for longer time periods and at higher doses, leading the authors to postulate that altered pelvic compliance and decreased uterine contractility may be a contributing factor.4,19 Similarly, Ecker et al1 noted higher rates of failure to progress and fetal distress as indications for cesarean delivery in women older than age 40 years. Our analysis also found that failure to progress and nonreassuring fetal heart tracing accounted for a high proportion of diagnoses at delivery, again raising the question of intrinsically altered biologic state in older parturients, altering labor progression, and, hence, contributing to a higher cesarean delivery rate.
Strengths of this study include using data from patient electronic medical records from multiple institutions across the United States. The large sample size of the cohort and detailed information from available medical records, as opposed to vital statistic data, allowed our analyses to account for important covariates. There are limitations of the study, including retrospective nature of the analysis, variability of definitions for various indications across clinical sites, and limited validation of variables. Presence of more than one indication for cesarean delivery, although potentially a limitation, is reflective of real-life practice because multiple factors may play into the provider's decision to proceed with cesarean delivery. This was the rationale for hierarchical classification of indications.
In summary, short-term neonatal outcomes were most favorable for women aged 25.0–29.9 years. There was an increased rate of adverse maternal outcomes at either extremes of maternal age, younger than 20 years and 35 years or older. Although the cesarean delivery indication of previous uterine scar increased with maternal age, so did the proportion of women undergoing elective cesarean delivery. This information is useful when providing preconception and pregnancy counseling to optimize both maternal and neonatal outcomes.
Supplementary Material
Table 3.
Neonatal Outcomes by Maternal Age Category and Adjusted Odds Ratios
| Outcome | Younger Than 20.0 y (n = 19,638) | 20.0–24.9 y (n = 51,011) | 25.0–29.9 y (n = 56,480) | 30.0–34.9 y (n = 45,715) | 35.0–39.9 y (n = 24,351) | 40.0–44.9 y (n = 5,931) | 45.0 y or Older (n = 391) |
|---|---|---|---|---|---|---|---|
| Gestational age at delivery* (wk) | 38.5±2.7 | 38.6±2.4 | 38.6±2.2 | 38.5±2.3 | 38.4±2.5 | 38.2±2.6 | 38.1±2.7 |
| Birth weight* (g), (missing data = 2,340) | 3,091 (610) (n = 19,458) | 3,196 (596) (n = 50,373) | 3,271 (585) (n = 55,836) | 3,294 (606) (n = 45,202) | 3,282 (646) (n = 24,072) | 3,240 (678) (n = 5,851) | 3,202 (708) (n = 385) |
| Birth weight less than 1,500 g* (missing data = 2,340) | 521 (2.7) (n = 19,458) | 1,045 (2.1) (n = 50,373) | 920 (1.7) (n = 55,836) | 839 (1.9) (n = 45,202) | 571 (2.4) (n = 24,072) | 160 (2.7) (n = 5,851) | 12 (3.1) (n = 385) |
| OR | 0.95 | 0.99 | Referent | 1.17 | 1.39 | 1.53 | 1.98 |
| 99% CI | 0.79–1.14 | 0.86–1.14 | 1.01–1.36 | 1.18–1.65 | 1.17–2.01 | 0.87–4.51 | |
| Birth weight less than 2,500 g* (missing data = 2,340) | 2,343 (12.0) (n = 19,458) | 4,505 (8.9) (n = 50,373) | 4,009 (7.2) (n = 55,836) | 3,373 (7.5) (n = 45,202) | 2,069 (8.6) (n = 24,072) | 633 (10.8) (n = 5,851) | 53 (13.8) (n = 385) |
| OR | 1.13 | 1.03 | Referent | 1.08 | 1.24 | 1.52 | 2.19 |
| 99% CI | 1.03–1.24 | 0.96–1.11 | 1.00–1.16 | 1.13–1.35 | 1.32–1.75 | 1.41–3.38 | |
| Birth weight more than 4,000 g* (missing data = 2,340) | 736 (3.8) (n = 19,458) | 2,880 (5.7) (n = 50,373) | 4,223 (7.6) (n = 55,836) | 3,928 (8.7) (n = 45,202) | 2,264 (9.4) (n = 24,072) | 539 (9.2) (n = 5,851) | 35 (9.1) (n = 385) |
| OR | 0.63 | 0.84 | Referent | 1.11 | 1.22 | 1.18 | 1.24 |
| 99% CI | 0.55–0.72 | 0.78–0.91 | 1.04–1.19 | 1.12–1.33 | 1.02–1.36 | 0.75–2.05 | |
| Apgar score less than 7 at 5 min* (missing data = 996) | 459 (2.4) (n = 19,511) | 932 (1.8) (n = 50,765) | 847 (1.5) (n = 56,247) | 743 (1.6) (n = 45,505) | 505 (2.1) (n = 24,214) | 141 (2.4) (n = 5,890) | 10 (2.6) (n = 389) |
| OR | 1.00 | 0.97 | Referent | 1.11 | 1.32 | 1.48 | 1.39 |
| 99% CI | 0.82–1.21 | 0.83–1.12 | 0.96–1.30 | 1.10–1.57 | 1.12–1.97 | 0.51–3.78 | |
| NICU admission* | 2,768 (14.1) | 6,445 (12.6) | 6,500 (11.5) | 5,358 (11.7) | 3,190 (13.1) | 896 (15.1) | 70 (17.9) |
| OR | 0.98 | 0.99 | Referent | 1.07 | 1.21 | 1.47 | 1.81 |
| 99% CI | 0.91–1.06 | 0.93–1.05 | 1.01–1.13 | 1.13–1.30 | 1.30–1.65 | 1.24–2.66 | |
| NICU length of stay* median (d) | 7.1 (1.0, 52.0) | 7.0 (1.0, 48.0) | 6.4 (1.0, 44.0) | 7.0 (1.0, 47.0) | 7.0 (1.0, 48.0) | 8.0 (2.0, 52.0) | 7.0 (2.0, 49.0) |
| Stillbirth* | 118 (0.6) | 232 (0.5) | 214 (0.4) | 182 (0.4) | 138 (0.6) | 41 (0.7) | 5 (1.3) |
| OR | 1.31 | 1.01 | Referent | 1.07 | 1.47 | 1.66 | 2.35 |
| 99% CI | 0.89–1.92 | 0.74–1.36 | 0.78–1.45 | 1.04–2.07 | 0.97–2.85 | 0.51–10.80 | |
| Neonatal death* | 74 (0.4) | 182 (0.4) | 161 (0.3) | 153 (0.3) | 86 (0.4) | 26 (0.4) | 2 (0.5) |
| OR | 0.84 | 0.86 | Referent | 1.06 | 0.82 | 1.22 | 1.95 |
| 99% CI | 0.53–1.34 | 0.61–1.22 | 0.75–1.50 | 0.52–1.29 | 0.63–2.37 | 0.31–12.49 | |
| Perinatal mortality*† | 192 (1.0) | 414 (0.8) | 375 (0.7) | 335 (0.7) | 224 (0.9) | 67 (1.1) | 7 (1.8) |
| OR | 1.10 | 0.95 | Referent | 1.06 | 1.16 | 1.44 | 2.16 |
| 99% CI | 0.82–1.48 | 0.76–1.19 | 0.84–1.33 | 0.89–1.53 | 0.95–2.19 | 0.66–7.04 |
CI, confidence interval; OR, odds ratio; NICU, neonatal intensive care unit.
Data are mean±standard deviation, n (%), or median (10th, 90th percentiles) unless otherwise specified.
Adjusted odds ratios calculated from logistic regression and adjusted for maternal race, parity, body mass index, insurance type, pre-existing medical conditions, substance abuse, tobacco use, and reporting clinical site. The group aged 25.0–29.9 years was used as the referent category. Unless specified, missing data for logistic regression = 44,227.
P<.001.
Perinatal mortality defined as stillbirth and neonatal death.
Acknowledgments
The Consortium on Safe Labor was funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, through Contract No. HHSN267200603425C. Funded in part with Federal funds (grant # UL1RR031975) from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through the Clinical and Translational Science Awards Program, a trademark of Department of Health and Human Services, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise.”
The named authors alone are responsible for the views expressed in this article, which does not necessarily represent the decisions or the stated policy of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
For a list of institutions involved in the Consortium on Safe Labor, see the Appendix online at http://links.lww.com/AOG/A448.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Ecker JL, Chen KT, Cohen AP, Riley LE, Lieberman ES. Increased risk of cesarean delivery with advancing maternal age: Indications and associated factors in nulliparous women. Am J Obstet Gynecol. 2001;185:883–7. doi: 10.1067/mob.2001.117364. [DOI] [PubMed] [Google Scholar]
- 2.Bianco A, Stone J, Lynch L, Lapinski R, Berkowitz G, Berkowitz RL. Pregnancy outcome at age 40 and older. Obstet Gynecol. 1996;87:917–22. doi: 10.1016/0029-7844(96)00045-2. [DOI] [PubMed] [Google Scholar]
- 3.Ziadeh S, Yahaya A. Pregnancy outcome at age 40 and older. Arch Gynecol Obstet. 2001;265:30–3. doi: 10.1007/s004040000122. [DOI] [PubMed] [Google Scholar]
- 4.Paulson RJ, Boostanfar R, Saadat P, Mor E, Tourgeman DE, Slater CC, et al. Pregnancy in the sixth decade of life: obstetric outcomes in women of advanced reproductive age. JAMA. 2002;288:2320–3. doi: 10.1001/jama.288.18.2320. [DOI] [PubMed] [Google Scholar]
- 5.Cleary-Goldman J, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, et al. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105:983–90. doi: 10.1097/01.AOG.0000158118.75532.51. [DOI] [PubMed] [Google Scholar]
- 6.Chen XK, Wen SW, Fleming N, Demissie K, Rhoads GG, Walker M. Teenage pregnancy and adverse birth outcomes: a large population based retrospective cohort study. Int J Epidemiol. 2007;36:368–73. doi: 10.1093/ije/dyl284. [DOI] [PubMed] [Google Scholar]
- 7.Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. N Engl J Med. 1995;332:1113–7. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- 8.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Wilson EC, Mathews TJ. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1–71. [PubMed] [Google Scholar]
- 9.Gilbert WM, Nesbitt TS, Danielsen B. Childbearing beyond age 40: pregnancy outcome in 24,032 cases. Obstet Gynecol. 1999;93:9–14. doi: 10.1016/s0029-7844(98)00382-2. [DOI] [PubMed] [Google Scholar]
- 10.Simchen MJ, Yinon Y, Moran O, Schiff E, Sivan E. Pregnancy outcome after age 50. Obstet Gynecol. 2006;108:1084–8. doi: 10.1097/01.AOG.0000240139.46018.bd. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol. 2004;104:727–33. doi: 10.1097/01.AOG.0000140682.63746.be. [DOI] [PubMed] [Google Scholar]
- 12.Salihu HM, Shumpert MN, Slay M, Kirby RS, Alexander GR. Childbearing beyond maternal age 50 and fetal outcomes in the United States. Obstet Gynecol. 2003;102:1006–14. doi: 10.1016/s0029-7844(03)00739-7. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:326, e1–10. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delbaere I, Verstraelen H, Goetgeluk S, Martens G, De Backer G, Temmerman M. Pregnancy outcome in primiparae of advanced maternal age. Eur J Obstet Gynecol Reprod Biol. 2007;135:41–6. doi: 10.1016/j.ejogrb.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Crawford BS, Davis J, Harrigill K. Uterine artery atherosclerotic disease: histologic features and clinical correlation. Obstet Gynecol. 1997;90:210–5. doi: 10.1016/S0029-7844(97)00225-1. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2011. Natl Vital Stat Rep. 2012;61:1–18. [PubMed] [Google Scholar]
- 17.Rayl J, Gibson PJ, Hickok DE. A population-based case-control study of risk factors for breech presentation. Am J Obstet Gynecol. 1996;174:28–32. doi: 10.1016/s0002-9378(96)70368-x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Tanbo T, Abyholm T, Henriksen T. The impact of acvanced maternal age and parity on obstetric and perinatal outcomes in singleton gestations. Arch Gynecol Obstet. 2011;284:31–7. doi: 10.1007/s00404-010-1587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adashek JA, Peaceman AM, Lopez-Zeno JA, Minogue JP, Socol ML. Factors contributing to the increased cesarean birth rate in older parturient women. Am J Obstet Gynecol. 1993;169:936–40. doi: 10.1016/0002-9378(93)90030-m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.