Abstract
Background
The Essential Tremor (ET) Rating Assessment Scale (TETRAS) has shown excellent inter- and intra-rater reliability. To assess the scale’s ability to detect changes in tremor severity, we compared TETRAS performance with standard postural tremor accelerometry during a standardized ethanol challenge.
Methods
Fifteen adult ET patients received a single oral ethanol dose calculated to reach 0.05 g/dl breath alcohol content (brAC) on two different study days. Two investigators independently assessed the effects with accelerometry on one day and with TETRAS on another day. Measurements were taken at 8 time-points (2 time-points baseline and 6 time-points up to 2 hours post ethanol). Further outcome measures included brAC readings at the same time points.
Results
Because correlation between TETRAS and accelerometry revealed a logarithmic relation, for all comparisons, accelerometry data were log-transformed and a cumulative score logACC(R+L) was calculated. Correlation between logACC(R+L) and TETRAS was significant (r= 0.57, p<0.01). Repeated measures ANOVA for both TETRAS and accelerometry before and after ethanol showed a significant effect of time-point (F=34.6, p<0.01; F=13.5, p<0.01). Corrected post-hoc tests showed a difference between baseline and each of the following 6 time-points. TETRAS and brAC were significantly correlated (r=−0.29, p<0.01). Intra-rater test-retest analysis between baseline measures showed high correlation (ICC=0.974, p<0.001). The ethanol challenge showed excellent reproducibility.
Conclusion
We demonstrated sensitivity of the TETRAS performance scale to change after a therapeutic intervention. Our study provides responsiveness validity for TETRAS, further establishing its potential as a valid instrument for ET evaluation in both clinical and research settings.
Keywords: essential tremor, assessment scale, clinical rating, alcohol challenge, validation study
Introduction
The Tremor Research Group, because of several limitations of other existing scales, developed The Essential Tremor (ET) Rating Assessment Scale (TETRAS). TETRAS comprises two subscales, the ADL subscale (activities-of-daily-living) and the Performance subscale. Clinical rating with the latter applying objective metric anchors can be accomplished within minutes by using paper and pen1,2,3. TETRAS Performance has been shown to be a valid scale with excellent inter- and intra-rater reliabilities, which would make it ideal for the use in ET clinical trials3. Nevertheless, as in other tremor rating scales, a full evaluation of sensitivity to change, including estimates on minimum detectable change after drug intake, has not yet been reported. One recent study on temporal fluctuations in ET over six hours has demonstrated a good correlation of a quantitative motor assessment system with TETRAS for upper limb tremor, a subset of the TETRAS Performancescale4. Another study showed substantial changes of scores using the same subset after deep brain stimulation (DBS) in five patients5. Sensitivity to change after pharmacological intervention, either on the hand subset or of the whole TETRAS Performance scale, has not been reported to date. Given the relatively high prevalence of ET and the limited applicability and safety of drugs considered for its treatment, there is a demand for clinical trials to pursue new treatment options. To assess the impact of treatments it is important to ensure that rating scales used in these trials are appropriately sensitive
It is a characteristic observation in ET that many patients respond very well to small doses of ethanol6,7,8,9. Alcohol sensitivity has been tested recently in a small group of patients using instrumental measures such as tremor accelerometry and spirography, in combination with the modified Fahn-Tolosa-Marin Scale as a clinical measure10. Reproducibility of an alcohol challenge in essential tremor patients has not been validated to date.
In this study we aimed to assess the sensitivity of the TETRAS Performance scale to change in tremor severity after therapeutic intervention with alcohol. We used accelerometry, the gold standard test for assessing tremor severity, as an objective comparison. Data on test-retest and inter-rater reliability of TETRAS, as well as on the variability of a standardized alcohol challenge, are also provided.
Methods
Patients
Fifteen adult ET patients (eight female, mean age 68.7 ± 9.8) participating in two unrelated studies on the effects of octanoic acid and ethanol in ET, both of which utilized the same ethanol challenge, were included.
Alcohol challenge
Oral ethanol at a total dose of 0.8 g/L of total body water (TBW) was administered with a sugarless, un-caffeinated drink (e.g., diet soda). Gender, age, height and weight were used for the calculation of TBW (for males, TBW = 2.447− (0.09516*age)+(0.1074*height [cm])+ (0.3362*weight [kg]), for females, TBW = −2.097+(0.1069*Height)+(0.2466*Weight)11. In each patient, the ethanol challenge was performed twice, once on two separate days, per the study procedures of two protocols on the effect of octanoic acid and ethanol in ET (clinicaltrials.gov identifiers: NCT01468948 and NCT01200966). The challenge was performed identically in both studies with respect to target breath alcohol content (brAC) and time-points of tremor measurement. In one study, enthanol response was rated using TETRAS, while the other used tremor accelerometry. Two independent investigators (BV, DH) performed TETRAS rating and accelerometry. During each ethanol challenge, subjects were instructed to drink their test dose within 5 minutes, and brAC was determined by Breathalyzer analysis (Drager Safety Inc., CO), before and 20, 40, 60, 80, 100, and 120 minutes after ethanol administration (± 5 min each).
TETRAS
The TETRAS Performance scale consists of several items for measurement of action tremor3. It is rated 0–4 in half-point intervals for the head, face including jaw, voice, upper limb, lower limb worse side, and while standing. The scale focuses on assessment of upper limb action tremor using the following subcategories: handwriting on the dominant side only; separate assessments on both sides for the following conditions: posture using arms forward outstretched and wing beat position, kinetic using finger to nose test, drawing of Archimedes spirals, and dot approximation in which a pen is held as close as possible to a dot on a piece paper without touching it. Before each ethanol challenge, tremor severity was measured twice, once during study screening and once before the ethanol was administered. We refer to these 2 time-points here as baseline 1 (BL1) and baseline 2 (BL2), to capture variability in tremor before a standardized treatment intervention. It was then repeated 20, 40, 60, 80, 100, and 120 min after ethanol intake (± 5 min for each time-point).
Accelerometry
Patients sat with their forearms resting on the arms of a comfortable chair; the hands and fingers were unsupported and extended parallel to the ground. Tremor was recorded using a triaxial piezo-resistive accelerometer (Kistler Instrument Corp, Amherst, NY) placed on the dorsum of each hand. Wrist oscillations in the vertical z-axis were recorded. EMG surface electrodes were placed over the extensor carpi radialis and flexor carpi radialis muscles of each arm. Tremor and EMG were recorded simultaneously for 2 min. Next, the recording was repeated for 2 min with 1 lb weights attached to the dorsum of each hand. Two baseline measurements were performed before administration of ethanol, 15 min apart. The assessment was then repeated 20, 40, 60, 80, 100, and 120 min after ethanol administration (± 5 min for each time-point).
Statistical analysis
We first tested the logarithmic relationship between TETRAS and postural accelerometry12,13 to allow for use of a cumulative log-transformed accelerometry score for both hands logACC(R+L) in a linear comparison. Correlation between log-transformed accelerometry and TETRAS scores was tested with Pearson’s correlation coefficient. Next, the effect of time-point after ethanol intake was tested using repeated measures ANOVA (rmANOVA) for both accelerometry and TETRAS, independently. Differences in TETRAS and brAC scores between the two baseline measures and between baseline and each following time point were calculated. These differences were then entered in a post hoc analysis (Bonferroni) to test the main hypothesis whether a change in tremor is equally seen after ethanol administration, the main hypothesis of this study. Regression analysis was used to calculate parameters for a fit-line α of the correlation. Applying Fechner’s law of psychophysics comparing visual rating scales with biomechanical measures, the equation logT= α*TETRAS+β was used for expressing the non-linear relationship, whereas logT was the tremor amplitude from accelerometry, α the fit-line and β a constant12,13. Test-retest analysis of TETRAS was performed with the baseline measurements using Pearson’s correlation coefficient and an uncorrected t-test as if one would look at differences during test-retest, independent from the following time course14. The minimal detectable change was computed based on the formula 1.96√2*SD√(1−ICC) using the intraclass correlation coefficient (ICC)14. The effect size of TETRAS from baseline to 60 minutes after alcohol intake in this study was also calculated. Also, for the alcohol challenge itself similar correlation and test-retest analyses were performed using brAC values after administration. Values were considered as significant at p<0.05.
Results
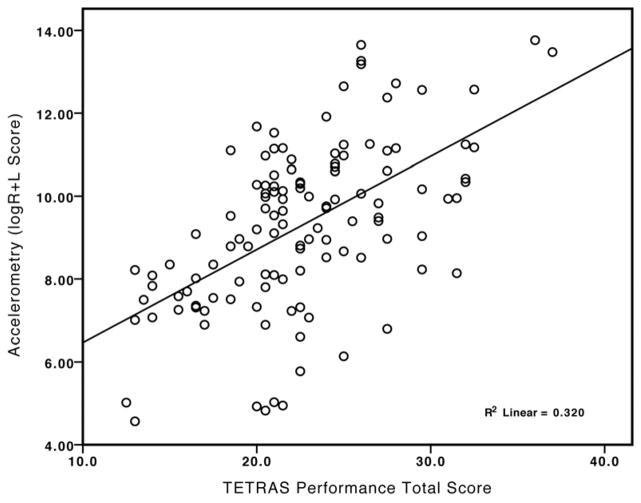
Correlation between TETRAS Performance total scores and accelerometry revealed a logarithmic relationship for all comparisons as predicted. Log-transformed accelerometry data was used to calculate a cumulative score logACC(R+L). The correlation between accelerometry and TETRAS was significant (Pearson’s r= 0.57, p<0.01) (Fig. 1). The correlation remained significant when analyzed separately for individual time points.
Figure 1.
Correlation between accelerometry (log transformed cumulative score of left and right accelerometry measures) and TETRAS Preformance total scores.(Pearson’s r= 0.57, p<0.01)
Both TETRAS Performance scores and the cumulative scores of right+left hand log-transformed accelerometry data [=logACC(R+L)]were reduced over time after ethanol, and rmANOVA after ethanol showed a significant effect of time-point (15 patients × 8 time-points, F=34.6, p<0.01; F=13.5, p<0.01) (Fig. 2).
Figure 2.
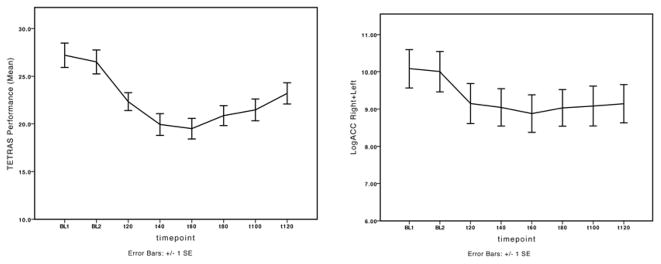
Change after alcohol intake following the same pattern is shown for total scores of the TETRAS Performance scale and the cumulative scores of both sides’ log-transformed accelerometry values, logACC(R+L).
Corrected post-hoc tests showed a difference between baseline and all of the time-points following ethanol for TETRAS Performance and accelerometry. There was, however, no difference between the two baseline measures. Parameters for the fit-line of correlation were logT= 0.23*TETRAS+4.21. TETRAS Performance total scores and brAC were significantly correlated (r=-0.29, p<0.01). Intra-rater test-retest analysis between the two baseline measurements (ICC=0.974, p<0.001). Comparing both TETRAS baseline total scores using an uncorrected t-test showed a difference (mean±SD: first baseline: 27.2±4.9; second baseline: 26.5±4.8; t=3.0, p<0.05), which was statistically significant.
The minimum detectable change of TETRAS Performance scale was 8.9% of the baseline measure. The effect size to demonstrate change between baseline and the time-point of maximum effect (60 min) was higher for TETRAS than for accelerometry (TETRAS: d=4.75 [95% CI 3.60–5.90]; accelerometry: d=2.36 [95% CI 1.21–2.50]), as expected from the error bars in figure 2.
Regarding the test-retest analysis and reproducibility of the alcohol challenge on brAC, the rmANOVA showed a significant effect for time-point (F=7.1, p<0.01), but not for the independent testing session (Figure 3). No interaction was found. At the time point of the maximum ethanol effect (60 min), there was no difference in brAC between sessions (uncorrected t-test).
Figure 3.
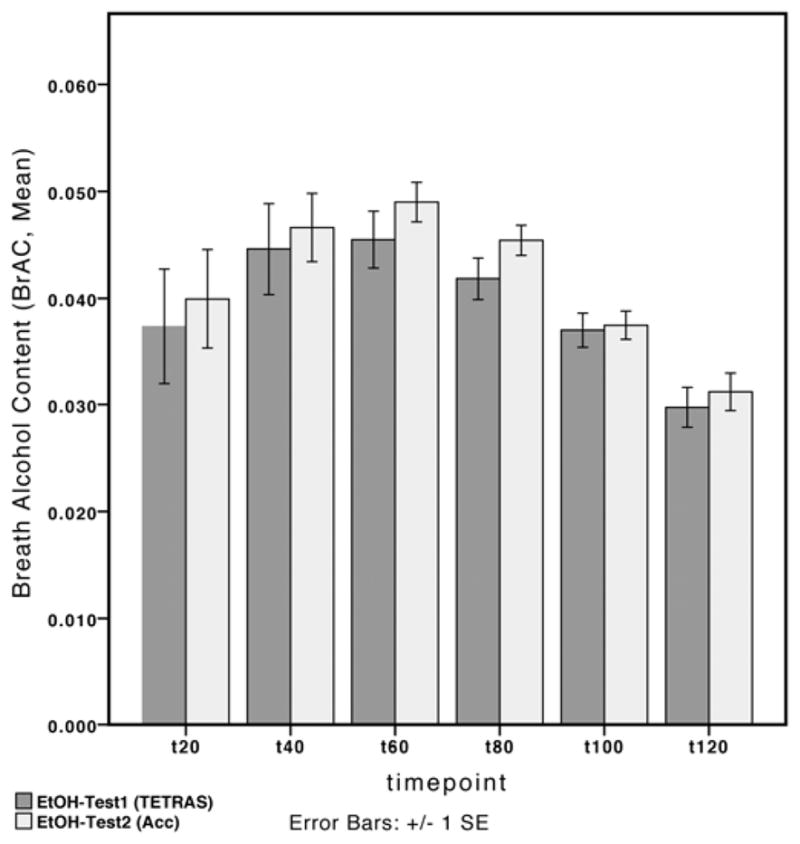
Alcohol challenge test. Breath alcohol content measured after ethanol challenge over time, on two different study days and by two different investigators, shows neither a significant difference over time nor at the time-point of maximum effect (60 minutes).
Discussion
TETRAS is a clinical rating scale developed by the members of the Tremor Research group3. So far, sensitivity to change of a clinical scale has only been shown for the daily variation of ET in upper limb tremor4 and in a case series of five patients after DBS5. Our results systematically show that a change of ET after intake of a standardized amount of ethanol is captured equally well by the TETRAS Performance scale, used clinically, as by accelerometry, used as the gold standard measurement for quantifying tremor amplitude12,15.
TETRAS is a cumulative measure of both hands and other body parts. Therefore, accelerometry measurements from both hands were combined in our study to improve comparability with the TETRAS scale. Accelerometry of the right hand only, which was the dominant hand in all of our patients, could also have been used for comparison, however this data would be comparable with only one sub-item of the total TETRAS Performance scale, i.e., postural tremor of the dominant hand. In addition, we demonstrated the log-relationship between TETRAS and accelerometry, which is consistent with prior studies comparing visual rating scales to instrumental measures12,13,16.
Our test-retest analysis shows a high correlation between the two baseline measures for TETRAS Performance, which is in good agreement with the intra-rater reliability analysis of a previous study3. The minimum detectable change from baseline is about 9% in our study, which is an improvement from the reported minimum detectable change of 30% in the Fahn-Tolosa-Marin scale17. However, this should be interpreted cautiously, as the interval between our ratings was rather short, in the range of one to two days, and the assessments were done by the same unblinded rater.
The slight difference between the baseline measurements with the t-test (uncorrected) might not be clinically relevant. Nevertheless, the second tremor rate is often better than the first as factors such as accommodation, e.g., less stress, come into effect. There might also be learning effects possibly reflected in tasks of the TETRAS Performance scale, which require manual dexterity, such as handwriting and spiral drawing. The logAcc (R+L) was not different between baseline measurements; therefore, another tremor component reflected by TETRAS is expected to be responsible for the difference, likely spiral drawing or handwriting. In our test-retest analysis only the TETRAS Performance total scores, but not the subscales, were significantly different. Of relevance, there was a trend towards slight improvement of the second measure with spiral drawing.
Finally, the effect size between baseline and 60 minutes after ethanol intake was higher for TETRAS Performance than for accelerometry, due to higher variability of accelerometry. Breath alcohol content measurements after the standardized alcohol challenge showed an excellent reproducibility of the test, which corroborates the results on the comparison between TETRAS and accelerometry in this study. The standardized alcohol challenge could also be of importance for further studies on the effects of ethanol in ET patients.
The main results of this study provide responsiveness validity for the TETRAS Performance scale. The shown sensitivity to change is another advantage of the scale besides the ease of use for clinical assessment of ET. This further establishes the potential for use of the TETRAS Performance scale as a valid instrument for ET evaluation in both clinical and research settings.
Acknowledgments
Funding Sources for study
NINDS Intramural Research Program.
We thank David A Luckenbaugh, National Institutes of Mental Health (NIH), Bethesda, Maryland, USA for his assistance with the statistical analysis.
Footnotes
- Research project: A. Conception, B. Organization, C. Execution;
- Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
- Manuscript Preparation: A. Writing of the first draft, B. Review and Critique;
B Voller, 1ABC, 2B, 3A
E Lines, 1BC, 3B
G McCrossin, 1BC, 3B
A Artiles, 1C, 3B
S Tinaz, 1C, 3B
C Lungu, 1C, 2C, 3B
M Hallett, 1AB, 2AC, 3B
D Haubenberger, 1ABC, 2ABC, 3B
Financial Disclosure/Conflict of Interest
Dr. Voller and Ms. Lines were both sponsored by Manhattan Pharmaceuticals Inc.. Dr. Voller worked as a contractor and as a Special Volunteer at NIH/NINDS in accordance with the Cooperative Research and Development Agreement (CRADA). Funding was not related to the nature of the research of this study.
Ms. Lines worked through the Postbaccalaureate Intramural Research Training Award Program at NIH/NINDS.
Ms. McCrossin reports no disclosures.
Mr. Artiles reports no disclosures.
Dr. Tinaz reports no disclosures.
Dr. Lungu serves on the Medical Advisory Board of the Parkinson Foundation of the National Capital Area. He has received speaker fees from the American Academy of Physical Medicine and Rehabilitation. He is part of CTAs with The Kinetics Foundation and BCN Peptides, Inc.
Dr. Hallett serves as Chair of the Medical Advisory Board for and receives funding for travel from the Neurotoxin Institute; serves as Chair of the Medical Advisory Board of the Benign Essential Blepharospasm Foundation and Chair of the Medical Advisory Board of the International Essential Tremor Foundation; has received honoraria and/or funding for travel for lectures or educational activities not funded by industry; serves on Editorial Advisory Boards for Clinical Neurophysiology, Brain, Acta Neurologica Scandinavica, Journal of Clinical Neurophysiology, Italian Journal of Neurological Sciences, Medical Problems of Performing Artists, Annals of Neurology, Neurology and Clinical Neurophysiology, The Cerebellum, NeuroRx, Current Trends in Neurology, Faculty of 1000 Biology, Faculty of 1000 Medicine, Brain Stimulation, Journal of Movement Disorders (Korea), and World Neurology; may accrue revenue on US Patent #6,780,413 B2 (issued: August 24, 2004): immunotoxin (MAB-Ricin) for the treatment of focal movement disorders; US Patent #7,407,478 (issued: August 5, 2008): coil for magnetic stimulation and methods for using the same; receives royalties from publishing from Blackwell Publisher, Cambridge University Press, Springer Verlag, Taylor & Francis Group, Oxford University Press, John Wiley & Sons, and Elsevier; receives research support from Ariston Pharmaceuticals, NIH/NINDS (Intramural Program) and the US Department of Defense (Army); has received license fee payments from the NIH (from Brainsway) for licensing the patent for the H-coil. Dr. Hallett’s research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds come from the US Army via the Henry Jackson Foundation, Manhattan Pharmaceutical Company via a Cooperative Research and Development Agreement (CRADA) with the NIH, and the Kinetics Foundation via a Clinical Trials Agreement (CTA) with the NIH. Dr. Hallett is an inventor for patent applications of 1-octanol and octanoic acid held by NINDS/NIH.
Dr. Haubenberger received research support through the NINDS Intramural Research Program and the Austrian Science Fund FWF (Erwin Schroedinger Fellowship, Project number J2783-B09). Dr. Haubenberger serves as member of the Medical Advisory Board of the International Essential Tremor Foundation Dr. Haubenberger received honoraria and conference support from Ipsen and UCB.
References
- 1.Tintner R for the Tremor Research Group. The Tremor Rating Scale (TRS) Mov Disord. 2004;19:1131–1132. [Google Scholar]
- 2.Elble R, Comella C, Fahn S, et al. The essential tremor rating assessment scale (TETRAS) Mov Disord. 2008;23(Suppl 1):357. [Google Scholar]
- 3.Elble R, Comella C, Fahn S, et al. Reliability of a New Scale for Essential Tremor. Mov Disord. 2012;27:1567–1569. doi: 10.1002/mds.25162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mostile G, Fekete R, Giuffrida JP, et al. Amplitude fluctuations inessential tremor. Parkinsonism Relat Disord. 2012;18:859–863. doi: 10.1016/j.parkreldis.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Chang WS, Chung JC, Kim JP, Chang JW. Simultaneous Thalamic and Posterior Subthalamic Electrode Insertion With Single Deep Brain Stimulation Electrode for Essential Tremor. Neuromodulation. 2012 Sep 17; doi: 10.1111/j.1525-1403.2012.00503.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Lou JS, Jankovic J. Essential tremor: clinical correlates in 350 patients. Neurology. 1991;41:234–238. doi: 10.1212/wnl.41.2_part_1.234. [DOI] [PubMed] [Google Scholar]
- 7.Koller WC, Busenbark K, Miner K. The relationship of essential tremor to other movement disorders: report on 678 patients. Essential Tremor Study Group. Ann Neurol. 1994;35:717–723. doi: 10.1002/ana.410350613. [DOI] [PubMed] [Google Scholar]
- 8.Deuschl G, Wenzelburger R, Löffler K, Raethjen J, Stolze H. Essential tremor and cerebellar dysfunction clinical and kinematic analysis of intention tremor. Brain. 2000;123:1568–1580. doi: 10.1093/brain/123.8.1568. [DOI] [PubMed] [Google Scholar]
- 9.Zeuner KE, Molloy FM, Shoge RO, Goldstein SR, Wesley R, Hallett M. Effect of ethanol on the central oscillator in essential tremor. Mov Disord. 2003;18:1280–1285. doi: 10.1002/mds.10553. [DOI] [PubMed] [Google Scholar]
- 10.Knudsen K, Lorenz D, Deuschl G. A clinical test for the alcohol sensitivity in essential tremor. Mov Disord. 2011;26:2291–2295. doi: 10.1002/mds.23846. [DOI] [PubMed] [Google Scholar]
- 11.Watson PE. Total body water and blood alcohol levels: updating the fundamentals. In: Crow KE, Batt RD, editors. Human metabolism of alcohol. Boca Raton: CRC Press; 1989. pp. 53–54. [Google Scholar]
- 12.Elble RJ, Pullman SL, Matsumoto JY, Raethjen J, Deuschl G, Tintner R. Tremor amplitude is logarithmically related to 4- and 5-point tremor rating scales. Brain. 2006;129:2660–2666. doi: 10.1093/brain/awl190. [DOI] [PubMed] [Google Scholar]
- 13.Haubenberger D, Kalowitz D, Nahab FB, et al. Validation of digital spiral analysis as outcome parameter for clinical trials in essential tremor. Mov Disord. 2011:2073–2080. doi: 10.1002/mds.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19:231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 15.Zesiewicz TA, Elble RJ, Louis ED, et al. Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology. 2011;77:1752–1755. doi: 10.1212/WNL.0b013e318236f0fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mostile G, Giuffrida JP, Adam OR, Davidson A, Jankovic J. Correlation between Kinesia system assessments and clinical tremor scores in patients with essential tremor. Mov Disord. 2010;25:1938–1943. doi: 10.1002/mds.23201. [DOI] [PubMed] [Google Scholar]
- 17.Elble RJ, Lyons KE, Pahwa R. Levetiracetam is Not Effective for Essential Tremor. Clin Neuropharmacol. 2007;30:350–356. doi: 10.1097/WNF.0b013E31807A32C6. [DOI] [PubMed] [Google Scholar]