Abstract
Background
To investigate whether chloroquine enhances the effect of antibiotics against Orientia tsutsugamushi, the causative organism of scrub typhus, we compared the effect of antibiotics in combination with chloroquine with the effect of antibiotics alone in vitro.
Materials and Methods
The Boryong or AFSC-4 strain was inoculated into ECV304 cells, and incubated in medium containing doxycycline (4 µg/mL), rifampin (4 µg/mL), azithromycin (0.5 µg/mL), chloroquine (1 µg/mL), and each of these antibiotics in combination with chloroquine for 7 d. Immunofluorescence (IF) staining for O. tsutsugamushi was performed 4 hr and 7 d after inoculation of the bacteria, and IF-positive foci were enumerated.
Results
Chloroquine inhibited the growth of O. tsutsugamushi by 15.5%. In combination with chloroquine, the antimicrobial effects increased by 4.4% for doxycycline (a 92.9% reduction of bacterial numbers for doxycycline versus a 97.3% reduction for doxycycline plus chloroquine), 4.6% for rifampin (90.0% versus 94.6%), and 8.3% for azithromycin (86.9% versus 95.2%). The antimicrobial effect of the antibiotics alone was significantly different compared to the combined effect of antibiotics and chloroquine (Wilcoxon signed-rank test, P = 0.001).
Conclusions
The combined use of chloroquine with an antibiotic for the treatment of O. tsutsugamushi infections may be useful for increasing the efficacy of the antibiotics.
Keywords: Azithromycin, Chloroquine, Combination drug therapy, Doxycycline, Drug synergism, Orientia tsutsugamushi, Rifampin, Scrub typhus
Introduction
Orientia tsutsugamushi causes scrub typhus, followed by a chronic latent infection irrespective of appropriate antibiotic therapy [1, 2, 3]; the infection sometimes relapses, manifesting as myocarditis or pneumonia [4, 5]. Several clinical studies have revealed that treatment with doxycycline and chloramphenicol presents acceptable clinical responses, even though these antibiotics are bacteriostatic to O. tsutsugamushi. In 1996, it was reported that scrub typhus exhibits a delayed response to doxycycline treatment in northern Thailand [6]. To prevent this persistent infection and to treat those cases of scrub typhus that are relatively resistant to antibiotic therapy, treatment regimens with more potent therapeutic efficacy, ideally bactericidal treatments, should be investigated. This requires the investigation of new antibiotics. Azithromycin and rifampin are relatively new antibiotics for the treatment of scrub typhus. These antibiotics have demonstrated high therapeutic efficacies and no cases with relapse; rifampin, in particular, has a bactericidal effect on certain bacteria [7, 8]. A previous in vitro experiment, however, indicated that these antibiotics are also bacteriostatic against O. tsutsugamushi [9]. A combination antibiotic therapy may also be considered for this purpose, although an in vitro experiment revealed that this modality is not more effective than a single antibiotic [10].
Chloroquine has been used as an anti-malarial agent for a long time. In addition, it has been shown to increase the antimicrobial efficacy of doxycycline for the treatment of Q fever [11] and Whipple's disease [12]. This activity is thought to be mediated by the alkalization of phagosomes [13]. Chloroquine enters cells, accumulates within the acidic components such as endosomes, phagosomes, and Golgi vesicles, and increases the pH of these vesicles. O. tsutsugamushi uses phagosomes to enter phagocytes and then escapes from phagosomes to release into the cytosol during the early stages of an infection. When these vesicles are alkalized by NH4Cl or bafilomycin A, an alkalizing agent for acidic vesicles, the escape from the endocytic pathway and the infectivity of the bacterium are markedly reduced [14]. Thus, it may be hypothesized that chloroquine inhibits replication of O. tsutsugamushi and increases the antimicrobial activity of anti-rickettsial antibiotics. To verify this hypothesis, we performed an in vitro study to evaluate the antimicrobial effect of doxycycline, rifampin, and azithromycin, both individually and in combination with chloroquine against O. tsutsugamushi.
Meterials and Methods
1. Infection of ECV304 cells with O. tsutsugamushi
The ECV304 cell line, which has many characteristics similar to endothelial cells [15], was used for this study. ECV304 cell monolayers were cultured on polyethylene terephthalate (PET) cover slips in a 6-well (9.08 cm2) PET culture plate (CorePath, Daegu, Korea). A PET cover slip was estimated to contain 1.5 × 106 ECV304 cells. The cell cultures were maintained in M199 medium (pH 7.2) (Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL) at 37℃ in a humidified atmosphere containing 6% CO2. The culture medium was changed every 3 to 4 days.
The Boryong and AFSC-4 strains of O. tsutsugamushi were used in this study. The former strain is the most prevalent genotype in Korea and is susceptible to doxycycline. The minimal inhibitory concentrations for this strain, which were measured by a flow cytometric method, ranged from 0.05-0.1 µg/mL for doxycycline, 0.025-0.05 µg/mL for rifampin, and 0.1-0.05 µg/mL for azithromycin [16, 17]. The latter strain was isolated from Thailand and reported to be insensitive to doxycycline [18]. Stock solutions of each strain were inoculated onto monolayers of ECV304 cells seeded in 75-cm2 culture flasks. When the infected ECV304 cells presented maximum cytopathic effects, the degree of infection was assessed by immunofluorescence (IF) staining. The cells were then disrupted with glass beads and centrifuged at 350 × g for 5 min. The resultant supernatant was divided equally into 9 aliquots, inoculated onto ECV304 cell monolayers grown on the PET cover slips, and incubated for 4 hr. At the end of the initial incubation period, the inocula were replaced with fresh medium containing one of the following treatments: 4 µg/mL of doxycycline (Sigma Co., St. Louis, MO, USA), 4 µg/mL of rifampin (Sigma), 0.5 µg/mL of azithromycin (Sigma), 1 µg/mL of chloroquine (Sigma), doxycycline plus chloroquine, rifampin plus chloroquine, or azithromycin plus chloroquine. The concentrations used were the expected serum concentrations that can be achieved after administration of conventional doses of the antibiotics, as employed in a previous study [19]. The medium was replaced with fresh medium containing the same antibiotic or its respective combination with chloroquine on day 4 post-inoculation with O. tsutsugamushi (piO). Uninfected ECV304 cells and cultures containing no antibiotic or chloroquine were used as controls. On day 8 piO, the ECV304 cells were washed 2 times with phosphate buffered saline (PBS), and IF staining for O. tsutsugamushi was performed. The above experiments were performed in triplicate in Boryong-infected cells and in duplicate in AFSC-4-infected cells.
2. Immunofluorescence staining
The ECV304 cells infected with each strain of O. tsutsugamushi were fixed with acetone and treated with FS15, a monoclonal antibody that reacts with the linear epitope on the 56-kDa major outer membrane protein of the bacterium, for 30 min at 37℃. Then, the cells were rinsed briefly with PBS Tween-20 (PBST), treated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (Jackson ImmunoResearch, West Grove, PA, USA) for 30 min in a moist chamber, and finally rinsed 3 times with PBST. To clearly define O. tsutsugamushi, the host cells were counter-stained with 0.003% Evans Blue in PBS. After the slides were rinsed, they were placed in a mounting medium (Vector Laboratories, Burlingame, CA, USA) and examined at 400× magnification under a fluorescence microscope (Zeiss, Germany) with a confocal laser scanning system (Bio-Rad, Hercules, CA, USA). IF-positive foci were enumerated using the ImageJ program [20].
3. Statistical analysis
Continuous variables were expressed as the median and interquartile range (IQR) and analyzed using SPSS version 19.0 (SPSS Inc, Chicago, IL, USA) for Windows. MedCalc Statistical Software version 12.7.3 (MedCalc Software bvba, Ostend, Belgium) was used to calculate the ratios and the 95% confidence intervals (CIs) between the 2 groups. Wilcoxon signed-rank tests were used for comparisons of 2-paired values, and Mann-Whitney U tests were used for comparisons of 2 independent values. All P values were two-tailed and statistical significance was defined as P < 0.05.
Results
At 4 hr piO, the median (IQR) of IF-positive foci was 311.0 (260.0-346.0), and the number in AFSC-4 strain-infected cells was 126.7% (95% CI: 122.6 to 131.0%) larger than that in Boryong strain-infected cells (346.00 [328.0-364.0] versus 273.0 [247.0-311]), although the difference was not statistically significant (Mann-Whitney U test, P = 0.08) (Table 1). On day 8 piO, the median number of IF-positive foci increased to 437.0 (380.5-502.0) (502.0 [483.0-521.0] for the AFSC-4 strain-infected cells and 407.0 [354.0-437.0] for the Boryong strain-infected cells). Therefore, there were 123.3% (95% CI: 120.0 to 126.8%) more IF-positive foci in AFSC-4-infected cells (Mann-Whitney U test, P = 0.08) in medium containing neither antibiotics nor chloroquine. Because the inoculated size was somewhat variable among experiments and the AFSC-4 strain was greater in number than the Boryong strain, the observed values were converted to percentages of inhibition (calculated as the observed values divided by the corresponding bacterial numbers in cultures containing no antibiotic on day 8 piO). The percentage of bacteria (with respect to cultures containing no antibiotic) was significantly reduced by 15.5% (95% CI: 13.2 to 18.1%) after the addition of chloroquine (Wilcoxon signed-rank test, P = 0.043)(Fig. 1).
Table 1.
Number of immunofluorescence-positive foci 4 hr and 7 days after inoculation of Orientia tsutsugamushi, by the antibiotic and bacterial strain
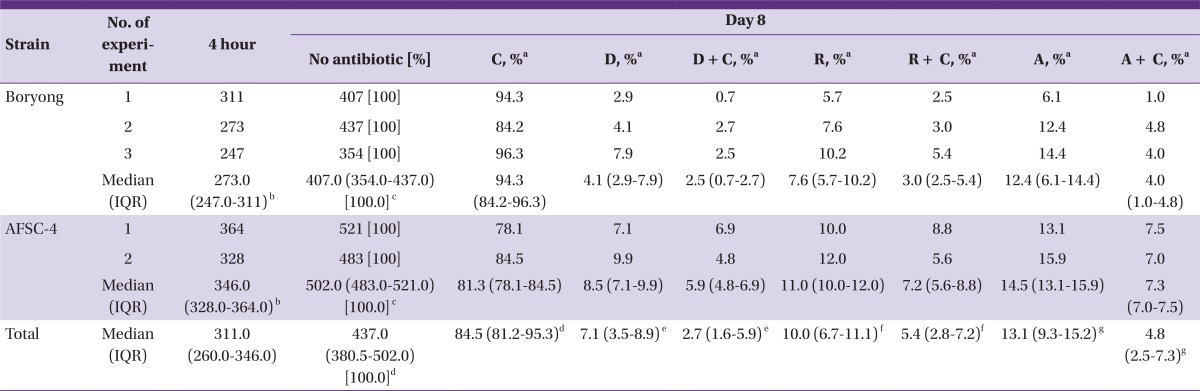
C, chloroquine; D, doxycycline; R, rifampin; A, azithromycin; IQR, interquartile range.
aPercentages of inhibition were calculated as the observed values divided by the corresponding bacterial numbers in cultures containing no antibiotic on day 8 post-inoculation with O. tsutsugamushi.
b,cP = 0.08 by Mann-Whitney U test.
d,e,f,gP = 0.043 by Wilcoxon signed-rank tests.
e,f,gThe difference in the overall antimicrobial effect between the antibiotics and their combinations with chloroquine was statistically significant (P = 0.001 by Wilcoxon signed-rank test).
Figure 1.
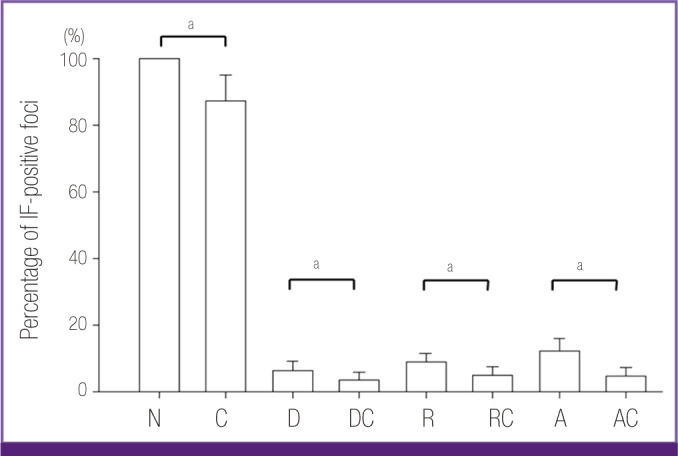
Inhibition of Orientia tsutsugamushi growth by antibiotics and their combinations with chloroquine. The Boryong or AFSC-4 strain of O. tsutsugamushi was inoculated into ECV304 cells and incubated in medium containing no antibiotic (N), chloroquine (C), doxycycline (D), doxycycline plus chloroquine (DC), rifampin (R), rifampin plus chloroquine (RC), azithromycin (A), or azithromycin plus chloroquine (AC) for 7 days. The number of immunofluorescence (IF)-positive foci was counted on day 8 post-inoculation. Chloroquine inhibits the growth of O. tsutsugamushi and augments the antimicrobial effect of the antibiotics against the bacterium.
aP=0.043 by Wilcoxon signed-rank tests.
When each of the antibiotics was added to the PET disc, the median percentages of bacteria were reduced by 92.9%, 90.0%, and 86.9% in doxycycline-, rifampin-, and azithromycin-treated cells, respectively. When the antibiotics were combined with chloroquine, the median percentages of IF-positive foci decreased further by 4.4%, 4.6%, and 8.3% in doxycycline- (resulting in a 97.3% reduction by doxycycline and chloroquine), rifampin- (94.6%), and azithromycin- (95.2%) treated cells, respectively. The difference for two pooled groups (all antibiotics individually and all combined treatments) was statistically significant (Wilcoxon signed-rank test, P = 0.001). Every pair-wise comparison between the antibiotic and its respective combination with chloroquine was also significantly different (Wilcoxon signed-rank tests, P-values = 0.043)(Fig. 1). The median percentage of bacterial cells after the treatment with antibiotics alone was 206.2% (95% CI: 167.6 to 251.1%) larger than after treatment with the antibiotic and chloroquine combination (9.9 [6.1-12.4] versus 4.8 [2.5-6.9]). The median number of bacteria in AFSC-4 strain-infected cells treated with the antibiotic and chloroquine combinations was nearly equal to that observed for the Boryong strain-infected cells treated with the antibiotics alone (7.0 [5.4-7.8] versus 7.6 [4.9-11.3]) (Mann-Whitney U test, P = 0.56).
Discussion
In the present study, chloroquine was observed to have a mild inhibitory effect and increased the effect of antibiotics against O. tsutsugamushi. The growth of O. tsutsugamushi was inhibited by chloroquine itself by 15.5%, consistent with previous results for Coxiella burnetii (6.1%) and less extreme than the inhibition observed for Listeria monocytogenes and Legionella pneumophila (45-80%) [19, 21, 22]. These organisms differ in their endocytic pathways: O. tsutsugamushi, Rickettsia species, and L. monocytogenes escape from phagosomes before fusion with the lysosomes and multiply in the cytosol; L. pneumophila inhibits phagolysosomal fusion and multiplies in the phagosomes; lastly, C. burnetii survives and multiplies in the phagolysosomes [13]. When phagosomes fuse with lysosomes, the pH of these vesicles becomes more acidic, from 6.5 in phagosomes to 4.5 in phagolysosomes [19]. Because chloroquine alkalizes these acidic vesicles and augments the antibacterial effects of the antibiotic, it is expected that chloroquine more potently suppresses C. burnetii than O. tsutsugamushi; however, the inhibition of C. burnetii growth by chloroquine was not as great when compared with O. tsutsugamushi. It is not certain whether this finding is due to differences in experimental designs or due to characteristics of the organisms.
Despite its rather low intrinsic antibacterial effect, chloroquine increased the antibacterial effect of various antibiotics against O. tsutsugamushi, as was previously shown in other organisms including C. burnetii [19, 21, 22]. The increases in the antimicrobial effect against O. tsutsugamushi after the addition of chloroquine were larger than the sum of the independent antibiotic activities, indicating that the combinations were synergistic. This synergistic effect suggests several therapeutic implications; for instance, the combination of an antibiotic and chloroquine is one option for the treatment of scrub typhus when it responds poorly to doxycycline therapy. The AFSC-4 strain in cultures containing neither antibiotic nor chloroquine showed 126.7% and 123.3% larger bacterial populations than the Boryong strain at 4 hr and 7 days piO, respectively. Similar among-strain differences were also shown in other studies where the AFSC-4, C1, and C27 strains, which are reported to be insensitive to doxycycline, exhibited a 10-18% higher inoculum number than the Karp strain, an antibiotic-susceptible strain [6, 18]. These differences in the inoculum numbers may be due to higher in vitro infectivity of these strains, as shown in the AFSC-4 strain [23]. Thus, if this high in vitro infectivity is associated with delayed defervescence in the treatment of scrub typhus, a 206.2% difference between the bacterial numbers in cultures containing the antibiotic combined with chloroquine and those with the antibiotic alone indicates that this combination could be used to treat scrub typhus caused by doxycycline-insensitive strains. This assumption is supported by data indicating that the bacterial numbers in AFSC-4 strain-infected cells, treated with combinations of an antibiotic and chloroquine, were nearly equal to those in the Boryong strain-infected cells treated with the antibiotics alone. Another potential application of the antibiotic and chloroquine combination treatments may be the rapid resolution of scrub typhus. Because the amount of O. tsutsugamushi organisms in severe scrub typhus is larger than in mild scrub typhus [24], a rapid reduction in the amount of bacteria in critically ill patients may result in more favorable clinical outcomes. Lastly, these combinations may be useful in the management of chronic O. tsutsugamushi infections, if these conditions are identified in the near future. Q fever endocarditis is an example of a chronic intracellular bacterial infection that is managed with doxycycline plus chloroquine [11]. Therefore, by combining antibiotics with chloroquine, higher efficacies may be achieved against O. tsutsugamushi infections. Alternatively, the same efficacy may be achieved with reduced doses of antibiotics and thereby this regimen may reduce the incidence of adverse effects during long-term treatment.
Although there is a previous report that a traveler taking chloroquine as a malaria prophylaxis contracted scrub typhus and improved within 48 hours after taking doxycycline [25], the theoretical benefits of the antibiotic combined with chloroquine must be investigated in clinical trials. This case also suggests that previous clinical trials conducted in malaria endemic areas might include patients with scrub typhus who were taking chloroquine as a malaria chemoprophylaxis. Chloroquine at a dose of 300 mg per week, a typical regimen for the prophylaxis of malaria, results in a plasma concentration of 1.6 µg/mL or more [26]. Therefore, these studies may estimate better responses to an anti-rickettsial agent. For example, Sheehy et al. [27] evaluated the clinical efficacies of chloramphenicol and tetracycline in United States servicemen with scrub typhus who had been deployed during the Vietnam War and were likely administered chloroquine prophylaxis; therefore, this study may overestimate the antibiotic efficacies and underestimate relapse rates.
Some differences were observed between the 3 antibiotics with respect to their efficacy in combination with chloroquine. In particular, when azithromycin was combined with chloroquine, O. tsutsugamushi growth was more strongly inhibited than when chloroquine was combined with doxycycline or rifampin, which could be attributed to increased antimicrobial activity of azithromycin in the alkaline pH of the growth medium.
Because there were discordant values between the bacteria at 4 hr and those on day 8 piO in cultures lacking antibiotics, some variation may be due to counting error, particular when the bacteria are high in number (>300 per field). In this instance, IF-positive dots were confluent, so it may have been difficult to accurately enumerate IF positivity; however, when the number was small, the counting was rather accurate. Thus, the effects of various antibiotics must account for variations in the denominator (i.e., the bacterial numbers in cultures containing no antibiotic on day 8 piO), but the comparison of the antimicrobial effect, i.e., the numerator, between the antibiotics and their combinations with chloroquine is reliable.
Footnotes
No conflicts of interest.
References
- 1.Hayashi H, Watanabe M. On the possibility of appearance of rickettsiae in the circulating blood after the recovery of rickettsiosis. Kitasato Arch Exp Med. 1948;21:135–141. [Google Scholar]
- 2.Smadel JE, Ley HL, Jr, Diercks RH, Cameron JA. Persistence of Rickettsia tsutsugamushi in tissues of patients recovered from scrub typhus. Am J Hyg. 1952;56:294–302. doi: 10.1093/oxfordjournals.aje.a119553. [DOI] [PubMed] [Google Scholar]
- 3.Chung MH, Lee JS, Baek JH, Kim M, Kang JS. Persistence of Orientia tsutsugamushi in humans. J Korean Med Sci. 2012;27:231–235. doi: 10.3346/jkms.2012.27.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yotsukura M, Aoki N, Fukuzumi N, Ishikawa K. Review of a case of tsutsugamushi disease showing myocarditis and confirmation of Rickettsia by endomyocardial biopsy. Jpn Circ J. 1991;55:149–153. doi: 10.1253/jcj.55.149. [DOI] [PubMed] [Google Scholar]
- 5.Im JH, Baek JH, Lee JS, Chung MH, Lee SM, Kang JS. A case series of possibly recrudescent Orientia tsutsugamushi infection presenting as pneumonia. Jpn J Infect Dis. 2014;67:122–126. doi: 10.7883/yoken.67.122. [DOI] [PubMed] [Google Scholar]
- 6.Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, Jongsakul K, Teja-Isavadharm P, Bhodhidatta D, Corcoran KD, Dasch GA, Strickman D. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet. 1996;348:86–89. doi: 10.1016/s0140-6736(96)02501-9. [DOI] [PubMed] [Google Scholar]
- 7.Phimda K, Hoontrakul S, Suttinont C, Chareonwat S, Losuwanaluk K, Chueasuwanchai S, Chierakul W, Suwancharoen D, Silpasakorn S, Saisongkorh W, Peacock SJ, Day NP, Suputtamongkol Y. Doxycycline versus azithromycin for treatment of leptospirosis and scrub typhus. Antimicrob Agents Chemother. 2007;51:3259–3263. doi: 10.1128/AAC.00508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, Strickman D. Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: a randomised trial. Lancet. 2000;356:1057–1061. doi: 10.1016/S0140-6736(00)02728-8. [DOI] [PubMed] [Google Scholar]
- 9.Im JH, Baek JH, Lee JS, Chung MH, Lee SM, Kang JS. In vitro bacteriostatic effects of rifampin on Orientia tsutsugamushi. J Korean Med Sci. 2014;29:183–189. doi: 10.3346/jkms.2014.29.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee OH, Baek JH, Lee JS, Chung MH, Lee SM, Kang JS. In vitro antagonism between cefotaxime and anti-rickettsial antibiotics against Orientia tsutsugamushi. Infect Chemother. 2014;46:189–193. doi: 10.3947/ic.2014.46.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raoult D, Houpikian P, Tissot Dupont H, Riss JM, Arditi-Djiane J, Brouqui P. Treatment of Q fever endocarditis: comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch Intern Med. 1999;159:167–173. doi: 10.1001/archinte.159.2.167. [DOI] [PubMed] [Google Scholar]
- 12.Feurle GE, Moos V, Schneider T, Fenollar F, Raoult D. The combination of chloroquine and minocycline, a therapeutic option in cerebrospinal infection of Whipple's disease refractory to treatment with ceftriaxone, meropenem and co-trimoxazole. J Antimicrob Chemother. 2012;67:1295–1296. doi: 10.1093/jac/dks008. [DOI] [PubMed] [Google Scholar]
- 13.Rolain JM, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30:297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu H, Lee JH, Han SH, Kim SY, Cho NH, Kim IS, Choi MS. Exploitation of the endocytic pathway by Orientia tsutsugamushi in nonprofessional phagocytes. Infect Immun. 2006;74:4246–4253. doi: 10.1128/IAI.01620-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown J, Reading SJ, Jones S, Fitchett CJ, Howl J, Martin A, Longland CL, Michelangeli F, Dubrova YE, Brown CA. Critical evaluation of ECV304 as a human endothelial cell model defined by genetic analysis and functional responses: a comparison with the human bladder cancer derived epithelial cell line T24/83. Lab Invest. 2000;80:37–45. doi: 10.1038/labinvest.3780006. [DOI] [PubMed] [Google Scholar]
- 16.Kim MJ, Kim MK, Kang JS. Improved antibiotic susceptibility test of Orientia tsutsugamushi by flow cytometry using monoclonal antibody. J Korean Med Sci. 2007;22:1–6. doi: 10.3346/jkms.2007.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim ES, Kim MK, Lee HM, Chung MH, Lee JS, Park JE, Kang JS. In vitro antibiotic susceptibility of Orientia tsutsugamushi strain Boryong measured by flow cytometry. Infect Chemother. 2008;40:212–217. [Google Scholar]
- 18.Strickman D, Sheer T, Salata K, Hershey J, Dasch G, Kelly D, Kuschner R. In vitro effectiveness of azithromycin against doxycycline-resistant and -susceptible strains of Rickettsia tsutsugamushi, etiologic agent of scrub typhus. Antimicrob Agents Chemother. 1995;39:2406–2410. doi: 10.1128/aac.39.11.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurin M, Benoliel AM, Bongrand P, Raoult D. Phagolysosomal alkalinization and the bactericidal effect of antibiotics: the Coxiella burnetii paradigm. J Infect Dis. 1992;166:1097–1102. doi: 10.1093/infdis/166.5.1097. [DOI] [PubMed] [Google Scholar]
- 20.Siritantikorn S, Jintaworn S, Noisakran S, Suputtamongkol Y, Paris DH, Blacksell SD. Application of ImageJ program to the enumeration of Orientia tsutsugamushi organisms cultured in vitro. Trans R Soc Trop Med Hyg. 2012;106:632–635. doi: 10.1016/j.trstmh.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marathe SA, Sen M, Dasgupta I, Chakravortty D. Differential modulation of intracellular survival of cytosolic and vacuolar pathogens by curcumin. Antimicrob Agents Chemother. 2012;56:5555–5567. doi: 10.1128/AAC.00496-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrd TF, Horwitz MA. Chloroquine inhibits the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. A potential new mechanism for the therapeutic effect of chloroquine against intracellular pathogens. J Clin Invest. 1991;88:351–357. doi: 10.1172/JCI115301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MS, Baek JH, Lee JS, Chung MH, Lee SM, Kang JS. High in vitro infectivity of a doxycycline-insensitive strain of Orientia tsutsugamushi. Infect Chemother. 2013;45:431–434. doi: 10.3947/ic.2013.45.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonthayanon P, Chierakul W, Wuthiekanun V, Phimda K, Pukrittayakamee S, Day NP, Peacock SJ. Association of high Orientia tsutsugamushi DNA loads with disease of greater severity in adults with scrub typhus. J Clin Microbiol. 2009;47:430–434. doi: 10.1128/JCM.01927-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupon M, Rogues AM, Malou M, d'Ivernois C, Lacut JY. Scrub typhus: an imported Rickettsial disease. Infection. 1992;20:153–154. doi: 10.1007/BF01704608. [DOI] [PubMed] [Google Scholar]
- 26.Wetsteyn JC, De Vries PJ, Oosterhuis B, Van Boxtel CJ. The pharmacokinetics of three multiple dose regimens of chloroquine: implications for malaria chemoprophylaxis. Br J Clin Pharmacol. 1995;39:696–699. doi: 10.1111/j.1365-2125.1995.tb05731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheehy TW, Hazlett D, Turk RE. Scrub typhus. A comparison of chloramphenicol and tetracycline in its treatment. Arch Intern Med. 1973;132:77–80. doi: 10.1001/archinte.132.1.77. [DOI] [PubMed] [Google Scholar]


