Abstract
Background
Natriuretic peptides (NP) represent a critical pathway in heart failure (HF). However, there is wide individual variability in NP system activity, which could be partially genetic in origin. We explored genetic and non-genetic contributions to BNP inactivation.
Methods
Chronic HF patients (n=95) received recombinant human BNP (nesiritide) at standard doses and BNP levels were measured at baseline, 2 hours of infusion, and 30 minutes after discontinuation. Genomic DNA was genotyped for 91 single nucleotide polymorphisms (SNP) in two candidate genes. We tested the association of patient characteristics and genotype with 5 pharmacokinetic (PK) parameters: elimination rate constant, ΔBNP, BNP clearance, adjusted BNP clearance, and half-life. Linear regression with pleiotropic analysis was used to test genotype associations with PK.
Results
Participant mean age was 63 years, 44% were female, and 46% were African American. PK parameters varied widely, some >10-fold. HF type (preserved vs. reduced) was associated with PK (p<0.01), while renal function, demographics, and BMI and were not. Two SNPs in MME (rs989692, rs6798179) and two in NPR3 (rs6880564, rs2062708) also had associations with PK (p<0.05).
Conclusion
Pharmacokinetics of BNP varies greatly in HF patients, differs by HF type, and possibly MME or NPR3 genotype. Additional study is warranted.
Keywords: Natriuretic peptide, heart failure, drug metabolism, pharmacogenetics, genetic polymorphisms
INTRODUCTION
Heart failure (HF) continues to be an enormous public health problem despite the many advances in its pharmacotherapy over the past 25 years, with a prevalence of 5.7 million individuals affected and an incidence of over 500,000 new cases annually [1]. Thus better prognostics, improved targeting of current therapies, and novel therapies are still needed. The relevance of the natriuretic peptide (NP) system, particularly B-type NP (BNP), is well known in terms of HF pathophysiology [2,3], diagnosis [4], prognosis [5], and therapy [6,7]. Moreover, the response to extrinsic NP (e.g. nesiritide, carperitide) is also highly variable, with unclear therapeutic range and potential for adverse effects [8-10]. A portion of this variation in NP system functioning is likely to be genetic in origin,[11] evidenced by clear associations of genetic variants with cardiovascular disease states,[12,13] altered gene expression and protein abundance,[14,15] as well as native NP levels via either altered production or elimination [16-18]. NP system variability will continue to be a relevant clinical issue not only because our understanding of NP system biology is still incomplete, but also because BNP is being increasingly explored towards personalized care,[19] and because there are numerous investigational NP therapeutics currently under development. [20,21]
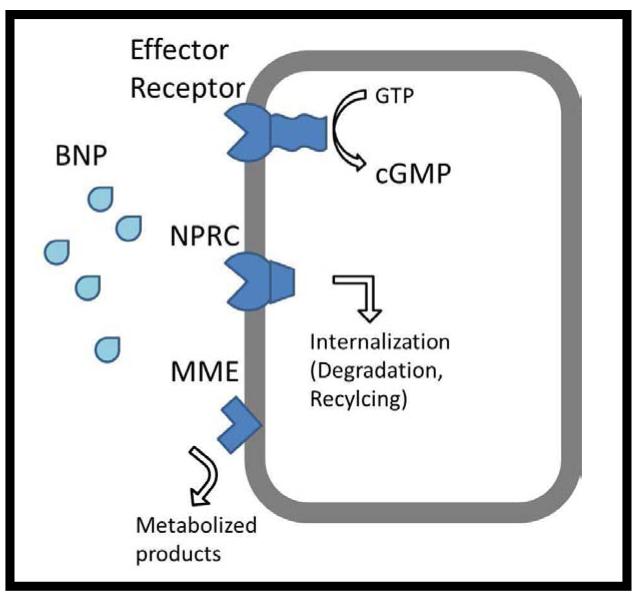
We sought to systematically assess the genetic and non-genetic associations of BNP clearance by infusing intravenous recombinant human BNP (nesiritide), quantifying key pharmacokinetic parameters, and focusing on common sequence variation in candidate genes relevant to BNP metabolism. The two primary molecular clearance mechanisms previously identified for NPs are Membrane Metallo-endopeptidase (aka NEP), encoded by the gene MME, and Natriuretic Peptide Receptor C [NPRc], encoded by the NPR3 gene (Figure 1). MME enzymatically degrades natriuretic (and other) peptides to predominantly inactive forms. NPRC is a non-catalytic NP receptor which shares homology with the other NP receptors in the transmembrane portion, but lacks the intracellular guanylate cyclase domain. It is thought that its primary role is in peptide internalization and clearance, though effector roles have recently been proposed.
Figure 1.
NPRC=Natriuretic Peptide Receptor C; MME= Membrane Metallo-endopeptidase; BNP= B-type natriuretic peptide
We prospectively enrolled patients with heart failure who were planned to receive nesiritide infusion in order to evaluate the overall variation in BNP kinetics in a typical HF population, examine the association with clinical and demographic characteristics, and test the association of genetic variation in the two candidate genes with pharmacokinetic parameters. We hypothesized that genetic variation in key genes may associate with differences in drug elimination. If true this could help improve patient selection and dosing of NP-based therapeutics, as well as possibly impact the interpretation of native BNP levels and prognostication.
METHODS
Patients and sample collection
The study was approved by the Henry Ford Hospital Institutional review board and all patients provided written informed consent. Patients with a history of heart failure who were receiving nesiritide either as part of clinical care (hospitalized HF patients) or only for the purposes of the study (ambulatory chronic HF patients) were included. Subjects with baseline systolic blood pressure less than 110 mmHg or with end stage renal disease were excluded. Patients received intravenous nesiritide at standard doses; 2mcg/kg bolus followed by 0.01mcg/kg/min continuous infusion. Patients were monitored for 1 hour prior to bolus, then received drug and were monitored for additional 2 hours. Blood samples were drawn at baseline and at 2 hours of infusion. Blood pressure was monitored every 15 minutes throughout. For patients not receiving nesiritide as part of standard care during an episode of hospitalized HF nesiritide was discontinued after the 2 hour blood draw, and another level was checked after 30 minutes to assess drug half-life. All investigational patient samples were centrifuged, aliquoted, and frozen within 30 minutes. These were stored at −70 C until batch testing could be performed at the Henry Ford Hospital clinical chemistry lab.
Genotyping
DNA samples were genotyped using a custom Illumina Goldengate® array which contained candidate-gene coverage relevant to HF including focused attention on the genes of interest to the natriuretic peptide pathway. Single nucleotide polymorphisms (SNP) were chosen for the array by attempting to include all coding variants, and also utilizing HAPMAP to select optimal non-coding (‘tag’) variants to capture blocks with minor allele frequency >0.1 prevalence in whites or African Americans within the gene regions of interest. After processing requirements for the Goldengate technology and quality control of genotyping, 91 SNPs in the two candidate genes of interest, membrane metallo-endopeptidase (MME, 41 SNPs) and natriuretic peptide receptor 3 (NPR3, 50 SNPs), were thus available for analysis. Genotyping calls were made using GenomeStudio software automatic algorithms (Illumina, San Diego, CA), and then individual SNPs were reviewed manually. Sample call rates were >90% and none of the SNPs analyzed deviated significantly from Hardy-Weinberg Equilibrium.
B-type Natriuretic Peptide Levels and Pharmacokinetic Parameters
B-type natriuretic peptide levels were tested in plasma samples using the Henry Ford Hospital Clinical Chemistry lab and personnel. The lab utilizes the Centaur (ADVIA, Bayer Diagnostics), which has high precision (coefficient of variation <5%) and is capable of directly measuring levels up to 5000 ng/L (higher levels measured with dilution). [22] The upper limit reported by our laboratory is 8000 ng/L after dilutions, which was assigned as the maximum possible BNP value. BNP levels were measured at two time-points in all patients, baseline (BNPB) and at 2 hours of infusion. Two hours of infusion was chosen because it was felt to be representative of steady state BNP level (BNPSS) since this is more than 6 times the previously reported average half-life of roughly 18 minutes.[23] A third time-point of 30 minutes after discontinuation of drug (BNPD) was also tested in patients where the nesiritide could be stopped (i.e. those subjects who were not prescribed nesiritide for clinical purposes in the hospital). We then derived and tested 4 pharmacokinetic endpoints, calculated as follows:
Change in BNP level (ΔBNP= BNPSS – BNPB)
Elimination rate constant (K= (1,000,000 × infusion rate)/(73 × ΔBNP))
BNP clearance rate (CL=1000 × infusion rate × weight/BNPSS)
BNP adjusted clearance rate (CL=1000 × infusion rate × weight/ΔBNP)
Half-life (HL= −30(Ln(0.5))/Ln(BNPSS/BNPD)).
Statistical Analysis
The cohort size was chosen based on previously published data regarding BNP level achieved during infusion and the standard error of measurement [24], with which we calculated that 95 subjects would provide 90% power to detect a 25% relative difference in ΔBNP between genotype groups for genotype frequencies as low as 10%. We investigated the association of clinical and demographic characteristics as well as the individual SNPs with the pharmacokinetic parameters above. Since the PK parameters had a skewed distribution we tested the association with clinical characteristics using the Wilcoxon test, for which the continuous variables (creatinine, age, BMI) were dichotomized at the median. Testing the association of genotype with PK was accomplished via linear regression and pleiotropic association analysis, using Fisher’s statistic with permutation to estimate p-values. This strategy was chosen so that we could combine all PK endpoints and thus examine the association of each SNP with its overall pharmacokinetic impact. P <0.05 was considered of interest for all tests. Multiple comparisons were assessed in the genetic analysis by the false discovery rate using the Benjamini and Hochberg approach.[25] All analyses were performed in SAS version 9.1.3 (SAS Institute, Cary, North Carolina) and R statistical software.
RESULTS
Study subjects and Pharmacokinetics
The primary study cohort included 95 subjects with a mean age of 63 years, 30% were female, and 50% non-white race (Table 1). All the subjects had established diagnosis of HF. Thirteen of the subjects were hospitalized for heart failure at the time of study entry and received nesiritide by clinical indication. The remaining 82 subjects were volunteer patients with a history of chronic HF that received nesiritide for study purposes only, in a controlled ambulatory setting. All patients received nesiritide at standard doses for at least two hours and had BNP levels collected at baseline and at two hours of treatment. For subjects receiving nesiritide for study purposes only, treatment was discontinued after the on-treatment sample was collected, and another sample was drawn after 30 minutes in order to calculate half-life.
Table 1.
Baseline Characteristics
| Characteristic | N= 95 |
|---|---|
| Age, years | 62 (±15.6) |
| Female gender | 29 (31%) |
| Non-white race | 47 (49%) |
| Ejection Fraction ≥ 50% | 25 (26%) |
| Mean Ejection Fraction (among those <50%) |
25% (±11) |
| Creatinine | 1.38 (±0.54) |
| Weight, kg | 96.6 (±24.5) |
| Body mass index | 32.6 (±7.9) |
| Inpatient status | 25% |
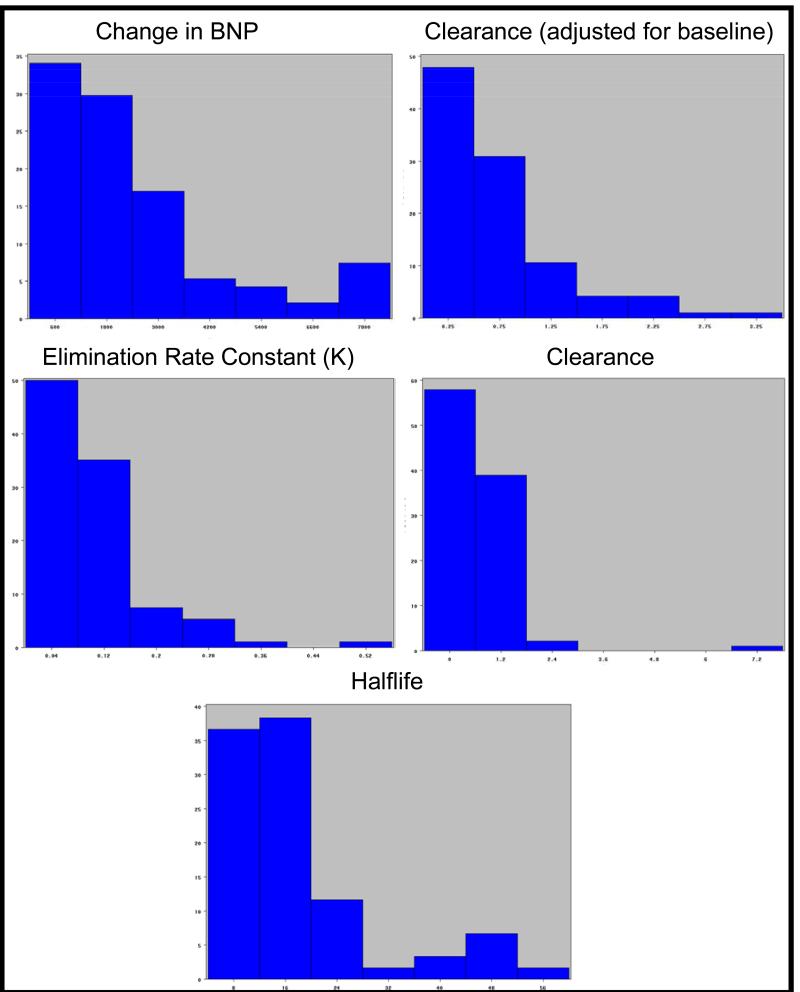
The pharmacokinetic parameters of interest varied widely across the study cohort. Figure 2 displays histograms of the distribution of each PK parameter; the x axis shows seven equal sized bins dividing the range of PK values (units vary by measure) and the y axis shows the percentage of the cohort within each bin. The range of ΔBNP (14 to 7900 pg/ml), Adjusted BNP Clearance (0.1 ml/min to 67 ml/min), BNP Clearance (0.027 ml/min to 7.25 ml/min), K (0.002 to 9.7) and Half-life (6.6 to 56.4) were roughly 10-fold or greater. The PK parameters stratified by patient characteristics are shown in Table 2. None of the PK parameters differed significantly across age, race, gender, creatinine, or body mass index. However there was a difference in 4 of the 5 pharmacokinetic measures by HF type (preserved vs. reduced ejection fraction); these included ΔBNP, Adjusted Clearance, Clearance, and K (all p<0.01). There was one subject that was an extreme outlier who was excluded from genetic analyses.
Figure 2.
Distribution of each PK parameter (ΔBNP in ng/L; K, unitless; BNP clearance in mL/min; BNP adjusted clearance rate in mL/min; Half-life in minutes). The x axis shows seven equal sized bins dividing the range of PK values. The y axis shows the percentage of the cohort within each bin.
Table 2.
Pharmacokinetic Parameters Compared to Baseline Characteristics in Primary Cohort
| Characteristic | ΔBNP, pg/ml |
p | Clearance (adjusted), ml/min |
p | Clearance, ml/min |
p | Halflife, minutes |
p | K | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall (Q1, Median, Q3) |
1034 1655 3120 |
NA | 0.29 0.58 1.43 |
NA | 0.23 0.51 0.76 |
NA | 11.2 14.4 21.2 |
NA | 0.0 0.081 0.13 |
NA |
| Female | 2533 | 0.22 | 0.36 | 0.18 | 0.305 | 0.29 | 16.2 | 0.57 | 0.054 | 0.28 |
| Male | 1411 | 0.63 | 0.54 | 13.6 | .0.094 | |||||
| Non-white | 1889 | 0.14 | 0.52 | 0.53 | 0.41 | 0.80 | 13.3 | 0.49 | 0.073 | 0.21 |
| white | 1458 | 0.58 | 0.45 | 14.9 | 0.088 | |||||
| EF < 50% | 1967 | 0.001 | 0.44 | 0.002 | 0.3 | <0.001 | 12.9 | 0.064 | 0.067 | <0.001 |
| EF ≥ 50% | 1102 | 0.84 | 0.72 | 16.8 | 0.12 | |||||
| Age*, Low | 1907 | 0.45 | 0.56 | 0.98 | 0.46 | 0.94 | 12.3 | 0.42 | 0.072 | 0.47 |
| High | 1507 | 0.58 | 0.39 | 15.8 | 0.086 | |||||
| BMI*, Low | 1643 | 0.65 | 0.49 | 0.08 | 0.37 | 0.08 | 14.9 | 0.67 | 0.083 | 0.79 |
| High | 1656 | 0.62 | 0.52 | 12.5 | 0.073 | |||||
| Creatinine*, Low |
1327 | 0.19 | 0.65 | 0.19 | 0.56 | 0.063 | 14.4 | 0.55 | 0.10 | 0.13 |
| High | 1870 | 0.48 | 0.35 | 14.4 | 0.073 |
Divided at the median
Genetic Associations of Pharmacokinetic Parameters
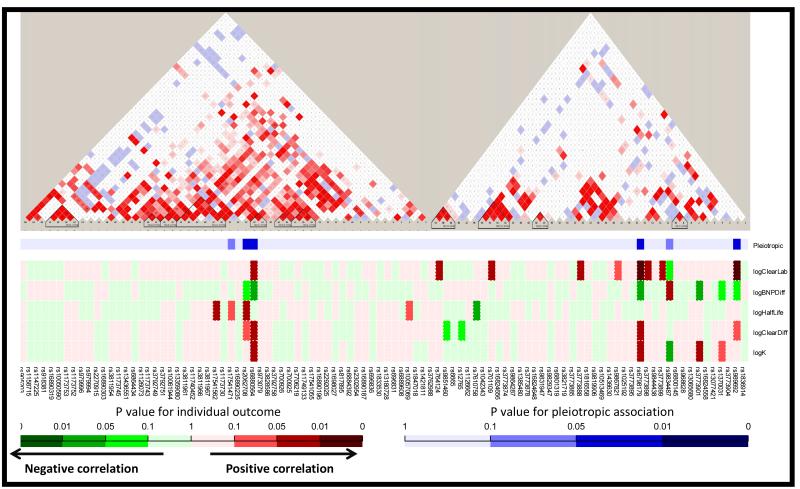
We examined the association of all five pharmacokinetic parameters of interest simultaneously using a pleiotropic statistical analysis. Ninety one SNPs in the two genes of interest were tested. The linkage maps of NPR3 and MME and primary association testing results are depicted in Figure 3. Within MME two SNPs were significant for association with PK parameters by pleiotropic testing (rs989692, p=0.034; rs6798179 p=0.014). Within NPR3 an additional two SNPs were significant for association with PK parameters by pleiotropic testing (rs6880564, p=0.046; rs2062708, p=0.049). Given the number of SNPs examined and modest cohort size, the associations did not survive adjustment for multiple comparisons. However, among these, rs6798179 showed the strongest and most consistent relationship, with associations to individual parameters as follows; K (p=0.024), change in BNP (p=0.019), raw clearance (p=0.0068), adjusted clearance (p=0.014), but no association to half-life (p=0.88). For the NPR3 SNPs, rs6880564 was most consistent with individual parameter associations; K (p=0.047), change in BNP (p=0.0497), raw clearance (p=0.019), adjusted clearance (p=0.037), and no association to half-life (p=0.90). In terms of magnitude of effect, on average the genotype at rs6798179 was associated with roughly 50% increase in K and Clearance, and a nearly 40% smaller change in BNP due to infusion.
Figure 3.
The linkage maps of NPR3 and MME by each SNP (red and white blocked triangle) and heat map of association tests with overall PK (from pleotropic testing, blue) and individual PK parameters (red-green). Dark shades indicate stronger association.
DISCUSSION
This is the first pharmacogenetic observation of nesiritide, and the largest study of its PK characteristics in a real-world HF cohort to our knowledge. Our data yields several key observations; that BNP elimination varies dramatically across typical HF patients, that HF type (preserved vs. reduced EF) impacts this substantially, and finally our data suggest that genetic variation in molecular clearance mechanisms may affect BNP metabolism. Specifically, genetic variation at rs6798179 within the MME gene was associated with a 40-50% change in BNP pharmacokinetics. While our study is relatively small and should be viewed as hypothesis generating, it supports the need for additional investigation and has potential implications in terms of HF pathophysiology and treatment. The variants identified as potentially impacting clearance are relatively common, occurring in roughly 20% of healthy populations of European ancestry, 10% of those of African ancestry, and up to 50% in Asian populations. In addition the magnitude of pharmacokinetic effect that we observed could be expected to be clinically significant, relating a 1.5 fold difference in steady state BNP levels and 50% difference in clearance rates.
As indicated above, it’s important to note that our data demonstrate that in a real-world HF population the increment in BNP achieved, the BNP clearance rate, and the elimination rate constant all vary substantially, and likely to a clinically relevant degree. Moreover this variability was not explained by factors typically considered such as renal function, body mass, or race. Interestingly, HF subtype (preserved EF vs. reduced EF) was most associated with BNP clearance, with HFrEF showing about double the BNP bump (and a lower clearance rate) compared to HFpEF. This finding is consistent with the more attenuated BNP elevations seen in HFpEF, and could in theory contribute to potential differences of nesiritide effect of in this group. Subgroup analysis of the ASCEND HF study based on EF did not show any trend towards a difference in the primary outcome. However this was defined somewhat differently (dichotomized at 40%) and there was not data presented regarding blood pressure lowering. While preserved EF patients were included in both ASCEND and VMAC, our search of the literature did not identify published data addressing efficacy of nesiritide by EF subgroups; this may be an opportunity for further investigation utilizing existing studies.
The high degree of variability in drug elimination that we found (which seems to have both genetic and non-genetic contributors), could very reasonably be expected to result in sub or super-therapeutic BNP levels during nesiritide infusion, despite uniform dosing, and thus underlie part of the variable impact demonstrated in clinical trials. The largest study of nesiritide, the ASCEND HF trial, showed little or no benefit in terms of dyspnea improvement or clinical outcomes, but it also showed modest or no risk in terms of hypotension and renal insufficiency.[8] This is in contrast to previous studies that sometimes utilized higher doses,[26,7] were associated with more robust symptomatic and hemodynamic changes,[27] but also were associated with higher risks of hypotension and renal insufficiency.[9,10] Our data demonstrate substantial underlying variability in drug clearance and BNP level increment on therapy, and suggest that a portion of this variability is genetic in origin. Thus improved targeting or dosing of drug via genetics or using therapeutic drug monitoring could be theorized to improve the risk:benefit ratio of nesiritide treatment and thus potentially salvage clinical utility for this vasoactive peptide. This remains an important health issue despite the existence of a large negative clinical trial because there are still no effective therapies for acutely decompensated heart failure to date, and this remains an extremely common and high risk reason to be hospitalized. Furthermore, there are additional NP agents in use and under investigation so that genetic factors impacting their elimination or effect may be relevant for future drug development.
Moreover, the genetic-based differences in clearance would be expected to also affect the native NP pathway functioning, potentially making the naturally occurring peptides more or less abundant relative to the level of expression/release. Whether and how this may impact disease development, progression, or diagnosis/prognostic BNP test performance is not yet known. Additional studies are needed to examine the prevalence of the genotypes in HF cohorts and compare this to healthy populations, to test whether the alleles of interest correlate with disease incidence, progression among patients with established HF, or BNP test performance. Similarly the altered clearance in HFpEF (relative to HFrEF) may help explain the differences in BNP levels noted in the two conditions. On the other hand, the relevance of our findings to NTproBNP testing is less clear. Certainly the genetic factors are more established as being relevant to BNP itself, and thus would not obviously impact NTproBNP levels. In regards to whether the relatively enhanced BNP clearance in HFpEF could have any effect on NTproBNP, the mechanism of this effect is unknown and our data cannot shed any light. Additional study with NTproBNP testing would be needed to clarify this further.
The biologic mechanism of a potential pharmacogenetic effect for this intronic variant is unknown. It’s possible that the relationship described in this study could be mediated via linkage disequilibrium with one or more functional variants, indeed rs6798179 appears in the linkage map to be part of a block of LD at that region. Additional investigation will be required to further test this. In terms of mechanism, we have previously evaluated RNA and protein expression of MME in relation to genotype but did not detect and alteration by rs6798179 genotype,[14] although that study was small (n= 103).
Limitations
Our study has several limitations that should be noted. Given our limited sample size, we did not adjust for multiple comparisons and therefore these findings should be interpreted as preliminary; they could have occurred by chance and replication and external validation is needed. On the other hand, the modest sample size could have also limited our power to detect true differences in pharmacokinetics by genotype, thus we may have missed potentially important associations. In addition, this study was focused on elimination of BNP, particularly exogenously administered BNP, so that we do not have information on genetic variants that might affect native production.
Conclusion
In a real-world cohort of HF patients, BNP PK parameters during nesiritide infusion varied greater than 10 fold. Variation in BNP elimination was associated with HF subtype (preserved vs. reduced EF) and possibly genetic variation in MME and NPR3, with rs6798179 in MME showing the most consistent and strong association. Additional study is warranted to validate and extend these observations, towards enhanced understanding of the NP system and increased utility (via improved targeting or dosing) of exogenously administered NP.
Study of 95 patients with heart failure receiving human recombinant BNP (nesiritide) infusion and measuring BNP elimination.
BNP clearance varied greatly across the cohort.
Heart failure type (i.e. preserved vs. reduced ejection fraction) correlated with BNP clearance rates.
Genetic variation in MME and NPR3 may affect BNP clearance rates; this needs replication.
FUNDING SOURCES
This research was supported in part by the National Heart, Lung, and Blood Institute (Lanfear K23HL085124, R01HL103871; Williams R01HL079055), the National Institute of Allergy and Infectious Diseases (Williams R01AI079139, R01AI061774) and the National Institute of Diabetes and Digestive and Kidney Diseases (Williams R01DK064695).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have no disclosures.
CITATIONS
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the american heart association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. doi:CIR.0b013e31823ac046 [pii] 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maeda K, Tsutamoto T, Wada A, Hisanaga T, Kinoshita M. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135(5 Pt 1):825–832. doi: 10.1016/s0002-8703(98)70041-9. [DOI] [PubMed] [Google Scholar]
- 3.Ellmers LJ, Scott NJ, Piuhola J, Maeda N, Smithies O, Frampton CM, Richards AM, Cameron VA. Npr1-regulated gene pathways contributing to cardiac hypertrophy and fibrosis. J Mol Endocrinol. 2007;38(1-2):245–257. doi: 10.1677/jme.1.02138. doi:10.1677/jme.1.02138. [DOI] [PubMed] [Google Scholar]
- 4.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–167. doi: 10.1056/NEJMoa020233. doi:10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 5.Pascual-Figal DA, Domingo M, Casas T, Gich I, Ordonez-Llanos J, Martinez P, Cinca J, Valdes M, Januzzi JL, Bayes-Genis A. Usefulness of clinical and NT-proBNP monitoring for prognostic guidance in destabilized heart failure outpatients. Eur Heart J. 2008;29(8):1011–1018. doi: 10.1093/eurheartj/ehn023. doi:10.1093/eurheartj/ehn023. [DOI] [PubMed] [Google Scholar]
- 6.VMAC Investigators (Vasodilation in the Management of Acute CHF) Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 7.Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang CS, Neibaur M, Haught WH, LeJemtel TH. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343(4):246–253. doi: 10.1056/NEJM200007273430403. doi:10.1056/nejm200007273430403. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr., Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. doi: 10.1056/NEJMoa1100171. doi:10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 9.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. Jama. 2005;293(15):1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 10.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111(12):1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 11.Lanfear DE. Genetic variation in the natriuretic peptide system and heart failure. Heart Fail Rev. 2010;15(3):219–228. doi: 10.1007/s10741-008-9113-y. doi:10.1007/s10741-008-9113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannone V, Huntley BK, Olson TM, Heublein DM, Scott CG, Bailey KR, Redfield MM, Rodeheffer RJ, Burnett JC., Jr. Atrial Natriuretic Peptide Genetic Variant rs5065 and Risk for Cardiovascular Disease in the General Community: A 9-Year Follow-Up Study. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.01344. doi:HYPERTENSIONAHA.113.01344 [pii] 10.1161/HYPERTENSIONAHA.113.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannone V, Cefalu AB, Noto D, Scott CG, Bailey KR, Cavera G, Pagano M, Sapienza M, Averna MR, Burnett JC., Jr. The Atrial Natriuretic Peptide Genetic Variant rs5068 Is Associated With a Favorable Cardiometabolic Phenotype in a Mediterranean Population. Diabetes Care. 2013;36(9):2850–2856. doi: 10.2337/dc12-2337. doi:dc12-2337 [pii] 10.2337/dc12-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanfear DE, Sunkara B, Li J, Rastogi S, Gupta RC, Padhukasahasram B, Williams LK, Sabbah HN. Association of Genetic Variation with Gene Expression and Protein Abundance within the Natriuretic Peptide Pathway. J Cardiovasc Transl Res. 2013;6(5):826–833. doi: 10.1007/s12265-013-9491-y. doi:10.1007/s12265-013-9491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira NL, Lin D, Pelleymounter L, Moon I, Stilling G, Eckloff BW, Wieben ED, Redfield MM, Burnett JC, Jr., Yee VC, Weinshilboum RM. Natriuretic peptide receptor-3 gene (NPR3): nonsynonymous polymorphism results in significant reduction in protein expression because of accelerated degradation. Circ Cardiovasc Genet. 2013;6(2):201–210. doi: 10.1161/CIRCGENETICS.112.964742. doi:CIRCGENETICS.112.964742 [pii] 10.1161/CIRCGENETICS.112.964742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanfear DE, Stolker JM, Marsh S, Rich MW, McLeod HL. Genetic variation in the B-type natiuretic peptide pathway affects BNP levels. Cardiovasc Drugs Ther. 2007;21(1):55–62. doi: 10.1007/s10557-007-6007-5. doi:10.1007/s10557-007-6007-5. [DOI] [PubMed] [Google Scholar]
- 17.Lanfear DE, Stolker J, Marsh S, Rich MW, McLeod HL. Natriuretic Peptide Receptor 3 (NPR3) Genotype Modulates the Relationship between B-Type Natriuretic Peptide (BNP) and Left Ventricular End-Diastolic Pressure. Therapy. 2006;3(6):765–771. [Google Scholar]
- 18.Costello-Boerrigter LC, Boerrigter G, Ameenuddin S, Mahoney DW, Slusser JP, Heublein DM, Redfield MM, Rodeheffer RJ, Olson TM, Burnett JC., Jr. The Effect of the Brain-Type Natriuretic Peptide Single-Nucleotide Polymorphism rs198389 on Test Characteristics of Common Assays. Mayo Clin Proc. 2011;86(3):210–218. doi: 10.4065/mcp.2010.0708. doi:86/3/210 [pii] 10.4065/mcp.2010.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felker GM, Hasselblad V, Hernandez AF, O’Connor CM. Biomarker-guided therapy in chronic heart failure: a meta-analysis of randomized controlled trials. Am Heart J. 2009;158(3):422–430. doi: 10.1016/j.ahj.2009.06.018. doi:10.1016/j.ahj.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Luss H, Mitrovic V, Seferovic PM, Simeunovic D, Ristic AD, Moiseyev VS, Forssmann WG, Hamdy AM, Meyer M. Renal effects of ularitide in patients with decompensated heart failure. Am Heart J. 2008;155(6):1012 e1011–1018. doi: 10.1016/j.ahj.2008.02.011. doi:10.1016/j.ahj.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 21.McKie PM, Sangaralingham SJ, Burnett JC., Jr. CD-NP: an innovative designer natriuretic peptide activator of particulate guanylyl cyclase receptors for cardiorenal disease. Curr Heart Fail Rep. 2010;7(3):93–99. doi: 10.1007/s11897-010-0016-6. doi:10.1007/s11897-010-0016-6. [DOI] [PubMed] [Google Scholar]
- 22.Wu AH, Packer M, Smith A, Bijou R, Fink D, Mair J, Wallentin L, Johnston N, Feldcamp CS, Haverstick DM, Ahnadi CE, Grant A, Despres N, Bluestein B, Ghani F. Analytical and clinical evaluation of the Bayer ADVIA Centaur automated B-type natriuretic peptide assay in patients with heart failure: a multisite study. Clin Chem. 2004;50(5):867–873. doi: 10.1373/clinchem.2003.026138. [DOI] [PubMed] [Google Scholar]
- 23.FDA Nesiritide Prescribing Information
- 24.Marcus LS, Hart D, Packer M, Yushak M, Medina N, Danziger RS, Heitjan DF, Katz SD. Hemodynamic and renal excretory effects of human brain natriuretic peptide infusion in patients with congestive heart failure. A double-blind, placebo-controlled, randomized crossover trial. Circulation. 1996;94(12):3184–3189. doi: 10.1161/01.cir.94.12.3184. [DOI] [PubMed] [Google Scholar]
- 25.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behavioural brain research. 2001;125(1-2):279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 26.Silver MA, Horton DP, Ghali JK, Elkayam U. Effect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failure. J Am Coll Cardiol. 2002;39(5):798–803. doi: 10.1016/s0735-1097(01)01818-6. [DOI] [PubMed] [Google Scholar]
- 27.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. Jama. 2002;287(12):1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]