Abstract
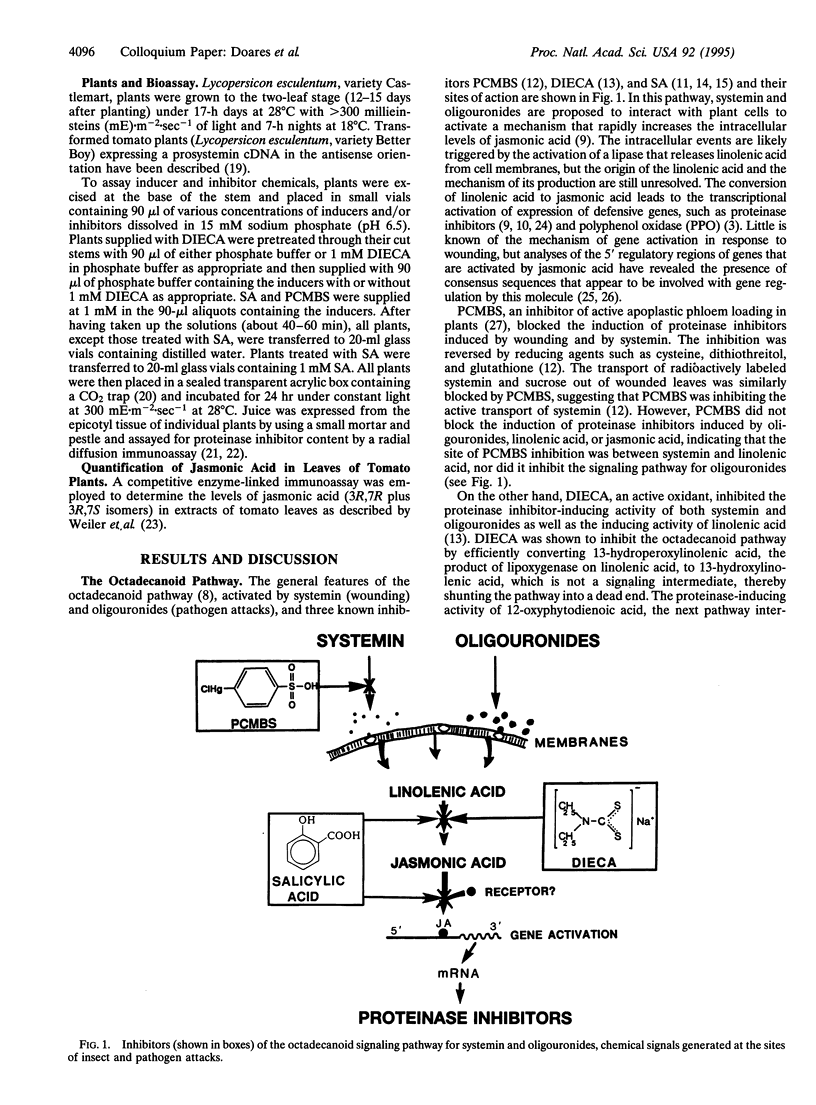
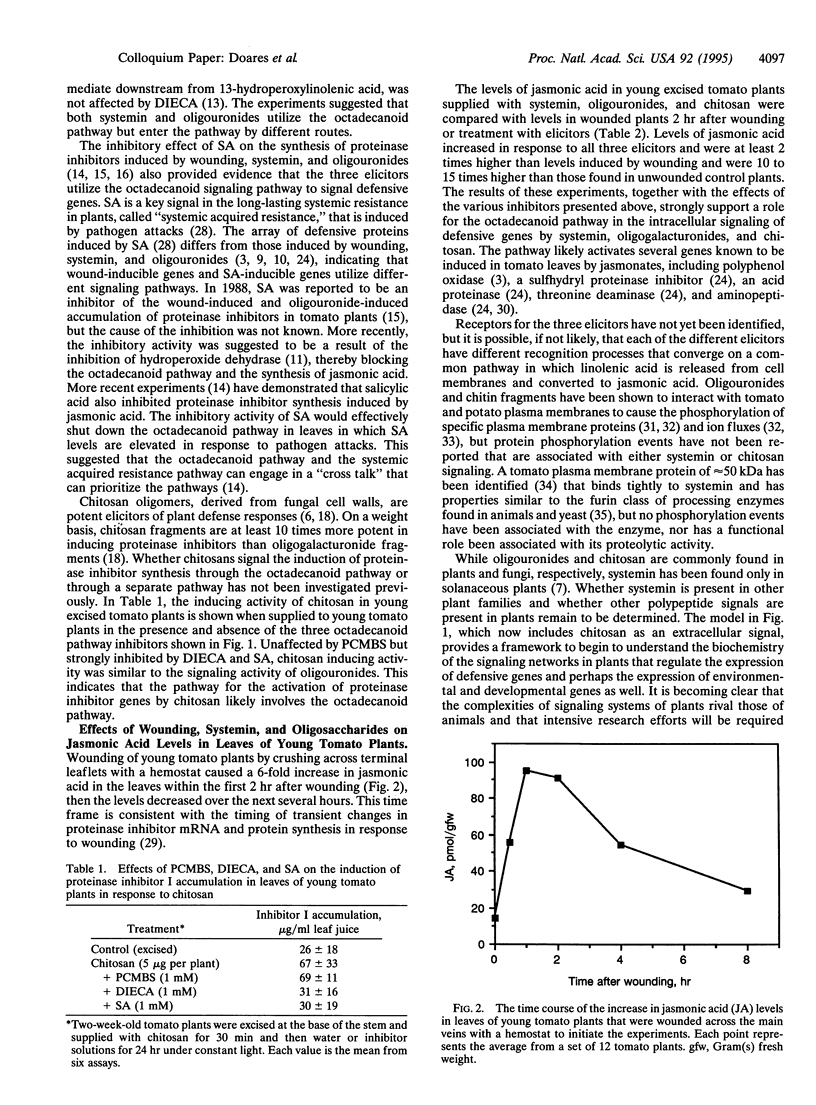
Jasmonic acid, synthesized from linolenic acid (the octadecanoid pathway), has been proposed to be part of a signal transduction pathway that mediates the induction of defensive genes in plants in response to oligouronide and polypeptide signals generated by insect and pathogen attacks. We report here that the induction of proteinase inhibitor accumulation in tomato leaves by plant-derived oligogalacturonides and fungal-derived chitosan oligosaccharides is severely reduced by two inhibitors (salicylic acid and diethyldi-thiocarbamic acid) of the octadecanoid pathway, supporting a role for the pathway in signaling by oligosaccharides. Jasmonic acid levels in leaves of tomato plants increased several fold within 2 hr after supplying the polypeptide systemin, oligogalacturonides, or chitosan to the plants through their cut stems, as expected if they utilize the octadecanoid pathway. The time course of jasmonic acid accumulation in tomato leaves in response to wounding was consistent with its proposed role in signaling proteinase inhibitor mRNA and protein synthesis. The cumulative evidence supports a model for the activation of defensive genes in plants in response to insect and pathogen attacks in which various elicitors generated at the attack sites activate the octadecanoid pathway via different recognition events to induce the expression of defensive genes in local and distal tissues of the plants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. D., Pearce G., Bryant J. E., Ryan C. A. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves. Identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984 Nov 10;259(21):13172–13177. [PubMed] [Google Scholar]
- Constabel C. P., Bergey D. R., Ryan C. A. Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):407–411. doi: 10.1073/pnas.92.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey D. A., Klessig D. F. Salicylic acid, active oxygen species and systemic acquired resistance in plants. Trends Cell Biol. 1994 Sep;4(9):334–338. doi: 10.1016/0962-8924(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Farmer E. E., Moloshok T. D., Saxton M. J., Ryan C. A. Oligosaccharide signaling in plants. Specificity of oligouronide-enhanced plasma membrane protein phosphorylation. J Biol Chem. 1991 Feb 15;266(5):3140–3145. [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Octadecanoid Precursors of Jasmonic Acid Activate the Synthesis of Wound-Inducible Proteinase Inhibitors. Plant Cell. 1992 Feb;4(2):129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Hadwiger L. A., Beckman J. M. Chitosan as a Component of Pea-Fusarium solani Interactions. Plant Physiol. 1980 Aug;66(2):205–211. doi: 10.1104/pp.66.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildmann T., Ebneth M., Peña-Cortés H., Sánchez-Serrano J. J., Willmitzer L., Prat S. General roles of abscisic and jasmonic acids in gene activation as a result of mechanical wounding. Plant Cell. 1992 Sep;4(9):1157–1170. doi: 10.1105/tpc.4.9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan A., Thornburg R. W. Auxin Levels Regulate the Expression of a Wound-Inducible Proteinase Inhibitor II-Chloramphenicol Acetyl Transferase Gene Fusion in Vitro and in Vivo. Plant Physiol. 1989 Sep;91(1):73–78. doi: 10.1104/pp.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. R., Choi J. L., Costa M. A., An G. Identification of G-Box Sequence as an Essential Element for Methyl Jasmonate Response of Potato Proteinase Inhibitor II Promoter. Plant Physiol. 1992 Jun;99(2):627–631. doi: 10.1104/pp.99.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., DeWald D. B., Mullet J. E. Identification of a methyl jasmonate-responsive domain in the soybean vspB promoter. Plant Cell. 1993 Mar;5(3):241–251. doi: 10.1105/tpc.5.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurl B., Pearce G., Orozco-Cardenas M., Ryan C. A. Structure, expression, and antisense inhibition of the systemin precursor gene. Science. 1992 Mar 20;255(5051):1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- Mueller M. J., Brodschelm W., Spannagl E., Zenk M. H. Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7490–7494. doi: 10.1073/pnas.90.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez-Vasquez J., Orozco-Cardenas M. L., Ryan C. A. A Sulfhydryl Reagent Modulates Systemic Signaling for Wound-Induced and Systemin-Induced Proteinase Inhibitor Synthesis. Plant Physiol. 1994 Jun;105(2):725–730. doi: 10.1104/pp.105.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautot V., Holzer F. M., Reisch B., Walling L. L. Leucine aminopeptidase: an inducible component of the defense response in Lycopersicon esculentum (tomato). Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9906–9910. doi: 10.1073/pnas.90.21.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautot V., Holzer F. M., Walling L. L. Differential expression of tomato proteinase inhibitor I and II genes during bacterial pathogen invasion and wounding. Mol Plant Microbe Interact. 1991 May-Jun;4(3):284–292. doi: 10.1094/mpmi-4-284. [DOI] [PubMed] [Google Scholar]
- Pearce G., Strydom D., Johnson S., Ryan C. A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991 Aug 23;253(5022):895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Pēna-Cortés H., Sánchez-Serrano J. J., Mertens R., Willmitzer L., Prat S. Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Quantitative determination of soluble cellular proteins by radial diffusion in agar gels containing antibodies. Anal Biochem. 1967 Jun;19(3):434–440. doi: 10.1016/0003-2697(67)90233-3. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. The regulation by carbon dioxide of protein synthesis in tomato leaves. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1004–1008. doi: 10.1016/s0006-291x(77)80077-6. [DOI] [PubMed] [Google Scholar]
- Schaller A., Ryan C. A. Identification of a 50-kDa systemin-binding protein in tomato plasma membranes having Kex2p-like properties. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11802–11806. doi: 10.1073/pnas.91.25.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautman R., Cowan K. M., Wagner G. G. Data processing for radial immunodiffusion. Immunochemistry. 1971 Oct;8(10):901–916. doi: 10.1016/0019-2791(71)90429-0. [DOI] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Proteinase inhibitor synthesis in tomato leaves : induction by chitosan oligomers and chemically modified chitosan and chitin. Plant Physiol. 1984 Nov;76(3):787–790. doi: 10.1104/pp.76.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]



