Abstract
Background
Cilostazol overcomes high on-treatment platelet reactivity (HTPR) and reduces adverse cardiovascular (CV) outcomes after percutaneous coronary intervention (PCI). However, the role for triple antiplatelet therapy (TAPT) with cilostazol in addition to aspirin and clopidogrel after PCI is not well defined.
Methods
We conducted a MEDLINE/EMBASE/CENTRAL search for randomised trials, until May 2014, evaluating TAPT compared with dual antiplatelet therapy (DAPT) of aspirin and clopidogrel alone in patients undergoing PCI and reporting platelet reactivity and/or CV outcomes. The primary platelet reactivity outcome was differences in platelet reactivity unit (PRU) with secondary outcomes of %platelet inhibition and rate of HTPR. The primary CV outcome was major adverse cardiovascular events (MACE), with secondary outcomes of death, cardiovascular death, myocardial infarction, stent thrombosis (ST), target lesion revascularisation (TLR) and target vessel revascularisation (TVR) as well as safety outcomes of bleeding and drug discontinuations.
Results
In 17 trials that evaluated platelet reactivity outcomes, the mean PRU value was 47.73 units lower with TAPT versus DAPT (95% CI −61.41 to −34.04, p<0.0001; mean PRU 182.90 vs 232.65). TAPT also increased platelet inhibition by 12.71% (95% CI 10.76 to 14.67, p<0.0001), and led to a 60% reduction in the risk of HTPR (relative risk=0.40; 95% CI 0.30 to 0.53) compared with DAPT. Moreover, among the 34 trials that evaluated CV outcomes, TAPT reduced the risk of MACE (incident rate ratio (IRR)=0.68; 95% CI 0.60 to 0.78), TLR (IRR=0.57; 95% CI 0.44 to 0.73), TVR (IRR=0.69; 95% CI 0.59 to 0.81) and ST (IRR=0.63; 95% CI 0.40 to 0.98) with no difference for other outcomes including bleeding, even in trials using drug-eluting stents. Drug discontinuation due to adverse effects was, however, higher with TAPT vs DAPT (IRR=1.59; 95% CI 1.32 to 1.91).
Conclusions
In patients undergoing PCI, addition of cilostazol to DAPT results in decreased platelet reactivity and a significant reduction in CV outcomes including ST, even in the drug-eluting stent era.
Keywords: CORONARY ARTERY DISEASE
Key messages.
What is already known about this subject?
Cilostazol, a phosphodiesterase III inhibitor, exhibits antiplatelet effect and inhibits neointimal hyperplasia and smooth muscle proliferation. However, its role in addition to dual antiplatelet therapy (DAPT) of aspirin and clopidogrel in patients undergoing percutaneous coronary intervention (PCI) is not well defined.
What does this study add?
In patients undergoing PCI, addition of cilostazol to DAPT results in decreased platelet reactivity and a significant reduction in cardiovascular outcomes including stent thrombosis, even in the drug-eluting stent era.
How might this impact on clinical practice?
The current study provides evidence to support use of cilostazol as an attractive and strong competitor for newer antiplatelet regimens and should be evaluated in future trials in patients undergoing PCI.
Introduction
Dual antiplatelet therapy (DAPT) with aspirin and an ADP receptor inhibitor is the standard of care for patients undergoing percutaneous coronary intervention (PCI). However, there is significant interindividual variability in the extent of platelet inhibition achieved with clopidogrel.1–3 Several studies have shown a correlation between high levels of on-treatment platelet reactivity (HTPR) and adverse cardiovascular outcomes, such that patients with HTPR (also called clopidogrel resistance) have a threefold to fivefold increased risk for recurrent ischaemic events.4 5 Cilostazol, a phosphodiesterase III inhibitor, exhibits its antiplatelet effects via inhibition of the conversion of cyclic AMP (cAMP) to 5'-AMP causing a subsequent increase in cAMP within platelets, and has been shown to augment platelet inhibition when it is added to aspirin and clopidogrel as part of a triple therapy regimen.6 7 In addition, cilostazol inhibits neointimal hyperplasia and smooth muscle proliferation, and has the potential to reduce the risk of restenosis after coronary stent implantation.8–11 Despite these pharmacologic effects, clinical results from observational and small randomised trials have not shown a consistent clinical benefit.
Our objective was to evaluate whether triple antiplatelet therapy (TAPT) with cilostazol (in addition to aspirin and clopidogrel) decreases platelet reactivity and reduces adverse cardiovascular (CV) outcomes when compared with a dual antiplatelet (DAPT) regimen of aspirin and clopidogrel alone.
Methods
Eligibility criteria
We conducted a MEDLINE, EMBASE and CENTRAL search using the MeSH terms ‘cilostazol’ and ‘randomised clinical trial’. We limited our search to trials involving human subjects through May 2014. The search terms were broad with no language restrictions imposed. We checked the reference lists of review articles and prior meta-analyses to assess for additional eligible studies. Corresponding authors of studies were contacted for further information if relevant data were not reported. Trials in abstract format without a manuscript published were also included in the analysis.
To be included for analysis, eligible trials had to fulfil the following criteria: (1) randomised clinical trials of TAPT (aspirin, clopidogrel and cilostazol) in comparison to DAPT (aspirin and clopidogrel); (2) enrolment of patients undergoing PCI with drug-eluting or bare metal stents and (3) follow-up of at least 2 weeks for trials reporting platelet reactivity outcomes and at least 1 month for trials reporting cardiovascular outcomes.
Selection and quality assessment
Three authors (AS, BT and SB) independently reviewed trial eligibility and quality. Disagreements were resolved by consensus. Risk of bias was assessed using criteria recommended by the Cochrane Collaboration, specifically evaluating sequence generation of allocation; allocation concealment; blinding of participants, staff and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias.12 Trials with high or unclear risk of bias for the first three criteria were considered as high bias risk trials and the rest as low bias risk trials.
Data extraction and synthesis
The primary platelet reactivity outcome was differences in platelet reactivity unit (PRU) after treatment in TAPT versus DAPT groups. Secondary outcomes were percent platelet inhibition and rate of HTPR. We used a cut-off of PRU >235 as the threshold for identifying patients with HTPR who may be at high risk for ischaemic or thrombotic events following PCI, as has been recommended by a recent consensus document.13 Of note, definition of HTPR differed by study.
Our primary CV outcome was major adverse cardiovascular events (MACE), defined as death, myocardial infarction (MI) or target lesion revascularisation (TLR). We evaluated secondary CV outcomes of death, cardiovascular death, MI, stent thrombosis, TLR and target vessel revascularisation (TVR). Safety outcomes of major bleeding, minor bleeding, any (major or minor) bleeding and drug discontinuation due to adverse effects were also evaluated. The definitions of bleeding varied between the trials. Given the lack of consistent reporting of the Academic Research Consortium definitions of stent thrombosis from the studies, we used the individual trial protocol definitions of stent thrombosis.
Statistical analysis
We performed an intention to treat meta-analysis in line with recommendations from the Cochrane Collaboration and the PRISMA Statement14 15 and used standard software for statistical analysis (STATA V.9.0, STATA Corp, Texas, USA). Heterogeneity was assessed using the I2 statistic, defined as the proportion of total variation observed between the trials attributable to differences between trials rather than sampling error (chance), with values <25% considered as low and >75% as high.16 The pooled effect for each grouping of trials was derived from the point estimate for each separate trial weighted by the inverse of the variance (1/SE2). Continuous variable outcomes (PRU, per cent platelet inhibition) between the groups were compared with both a fixed effect model using the inverse variance method and a random effects model using the DerSimonian and Laird method. For cardiovascular outcomes, rates were expressed per patient-years to adjust for the varying duration of follow-up. Results were therefore reported as incident rate ratios (IRR) and 95% CIs with the use of both a fixed effect model using the method of Mantel and Haenszel and a random effects model using the method of DerSimonian and Laird, with the estimate of heterogeneity being taken from the Mantel-Haenszel model. Publication bias was estimated using the weighted regression tests of Begg and Egger.12
For platelet reactivity indices, analyses were stratified based on whether standard-dose (75 mg) or high-dose (150 mg) clopidogrel was used in the DAPT arm. In addition, further sensitivity analyses were performed based on the cohort enrolled: (1) acute coronary syndrome (ACS) versus not; and (2) enrolment of patients with HTPR at baseline versus not. For cardiovascular outcomes, analyses were stratified based on stent type—drug eluting stent (DES) versus Bare metal stent (BMS). A p value of <0.05 was considered significant.
Results
Study selection
We identified 41 trials that satisfied the inclusion criteria (figure 1). Seventeen trials reported platelet reactivity outcomes of which 10 comparator arms used high dose (150 mg) of clopidogrel. A total of 34 trials reported CV outcomes, the majority (25 trials) of which used DES.
Figure 1.
Study selection.
Baseline characteristics
The baseline characteristics, inclusion criteria and quality assessment are summarised in tables 1–4. In order to quantify platelet reactivity outcomes, we evaluated 17 trials with 20 comparator arms and 5056 patients. The median follow-up was 30 days and although the definition of HTPR was heterogeneous, all trials used the VerifyNow P2Y12 assay to measure platelet reactivity. The analysis of cardiovascular outcomes included 34 trials with 14 119 patients. The mean age of study participants was between 56.3 and 67.5 years, 37.9% of the patients had diabetes and the majority (77.6%) underwent PCI with DES.
Table 1.
Baseline characteristics of included trials for platelet reactivity outcomes
| Trial | Year | N | Comparison | SD or HD (DAPT group) | Mean age (years) | Follow-up (days) |
|---|---|---|---|---|---|---|
| ACCEL-AMI29 | 2009 | 90 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | Both | 62 | 30 |
| ACCEL-LOADING-ACS30 | 2012 | 218 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | SD | 63 | 30 |
| ACCEL-POLYMORPHISM31 | 2010 | 134 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | HD | 63 | 30 |
| ACCEL-PPI32 | 2012 | 90 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | HD | NR | 30 |
| ACCEL-RESISTANCE33 | 2009 | 60 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | HD | 63 | 30 |
| CILON-T34 | 2011 | 716 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | SD | 64 | 180 |
| Gao et al35 | 2013 | 428 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | SD | 56 | 365 |
| Guan et al36 | 2012 | 840 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | SD | 60 | 30 |
| HOST-ASSURE37 | 2013 | 1356 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | Both | 63 | 30 |
| Jeong et al38 | 2014 | 275 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | Both | NR | 30 |
| Jin et al39 | 2012 | 60 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | HD | 62 | 30 |
| Kim et al40 | 2011 | 126 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | HD | 62 | 30 |
| Kim et al41 | 2007 | 60 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | SD | 63 | 30 |
| Kum et al42 | 2009 | 66 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | SD | 62 | 14 |
| Lee et al43 | 2010 | 63 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | Both | NR | 14 |
| PIANO-2 CKD44 | 2011 | 74 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | Both | 53 | 14 |
| Shim et al45 | 2009 | 379 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | SD | 61 | 14 |
ACCEL-AMI, adjunctive Cilostazol versus High Maintenance Dose Clopidogrel in patients with AMI; ACCEL-LOADING-ACS, Multicentre Randomised Trial Evaluating Efficacy of Cilostazol on Platelet Aggregation, Inflammation and Myonecrosis in ACS Patients; ACCEL-POLYMORPHISM, Cytochrome 2C19 Polymorphism and Response to Adjunctive Cilostazol versus High Maintenance-Dose Clopidogrel in Patients Undergoing Percutaneous Coronary Intervention; ACCEL-PPI, Pharmacodynamics Effects of Adding Cilostazol versus Double-dose Clopidogrel in Patients with Acute Myocardial Infarction During Proton Pump Inhibitor Co-administration; ACCEL-RESISTANCE, Adjunctive Cilostazol Versus High Maintenance Dose Clopidogrel in Patients with Clopidogrel Resistance; CILON-T, Influence of Cilostazol-based Triple Antiplatelet Therapy on Ischaemic Complication After Drug-eluting Stent Implantation; HD, high-dose clopidogrel (150 mg); HOST-ASSURE, Harmonising Optimal Strategy for Treatment of Coronary Artery Stenosis—Safety and Effectiveness of Drug-Eluting Stents and Antiplatelet Regimen; NR, not reported; PIANO-2 CKD, Platelet Reactivity in Patients with Chronic Kidney Disease Receiving Adjunctive Cilostazol Compared with a High-Maintenance Dose of Clopidogrel; SD, Standard-dose clopidogrel (75 mg).
Table 2.
Inclusion criteria and study quality for platelet reactivity outcomes trials
| Trial | Cohort | Definition of HTPR | Platelet reactivity assay | Quality of study* |
|---|---|---|---|---|
| ACCEL-AMI29 | Patients with ACS undergoing PCI | 5 and 20 μM ADP-induced maximal platelet aggregation >50% | VerifyNow P2Y12; LTA | +++ |
| ACCEL-LOADING-ACS30 | Patients with non-ST-elevation MI undergoing PCI | NR | VerifyNow P2Y12 | ±±± |
| ACCEL-POLYMORPHISM31 | Patients with high post-treatment platelet reactivity or diabetes undergoing PCI | 5 μM ADP-induced maximal platelet aggregation >50% | VerifyNow P2Y12; LTA | +++ |
| ACCEL-PPI32 | Patients with acute MI undergoing PCI | 20 μM ADP-induced maximal platelet aggregation >59% | LTA | ±±± |
| ACCEL-RESISTANCE33 | Patients with high on-treatment platelet reactivity undergoing PCI | 5 μM ADP-induced maximal platelet aggregation >50% | VerifyNow P2Y12; LTA | ++± |
| CILON-T34 | Patients with angina undergoing PCI | NR | VerifyNow P2Y12 | ++± |
| Gao et al35 | Obese patients undergoing PCI | Post-treatment platelet aggregation absolute difference 10% or less | LTA | ±±± |
| Guan et al36 | Patients with ACS and high on-treatment platelet reactivity undergoing PCI | 20 μM ADP-induced maximal platelet aggregation >55% | LTA | ±±± |
| HOST-ASSURE37 | All-comer patients undergoing PCI | NR | VerifyNow P2Y12 | ±±+ |
| Jeong et al38 | Patients with ACS undergoing PCI | NR | LTA | ±±± |
| Jin et al39 | Patients undergoing PCI | % platelet inhibition <20 | VerifyNow P2Y12; LTA | ±++ |
| Kim et al40 | Patients with acute MI undergoing PCI | 20 μM ADP-induced maximal platelet aggregation >59% | VerifyNow P2Y12; LTA | ++± |
| Kim et al41 | Patients with ST-elevation MI undergoing PCI | % platelet inhibition <20 | VerifyNow P2Y12; LTA | ±±± |
| Kum et al42 | Patients undergoing PCI | NR | VerifyNow P2Y12 | ±±± |
| Lee et al43 | Patients with high on-treatment platelet reactivity undergoing PCI | % platelet inhibition <20 | VerifyNow P2Y12 | +±± |
| PIANO-2 CKD44 | Patients with renal disease on haemodialysis undergoing PCI | 5 μM ADP-induced maximal platelet aggregation >50% | VerifyNow P2Y12; LTA | +++ |
| Shim et al45 | Patients undergoing PCI with DES | % platelet inhibition <20 | VerifyNow P2Y12 | +±± |
*Represents risk of bias based on: sequence generation of allocation; allocation concealment and blinding. ‘+’ represents low bias risk, ‘−’ high bias risk and ‘±’ unclear bias risk.
ACCEL-AMI, adjunctive Cilostazol versus High Maintenance Dose Clopidogrel in patients with AMI; ACCEL-LOADING-ACS, Multicentre Randomised Trial Evaluating Efficacy of Cilostazol on Platelet Aggregation, Inflammation and Myonecrosis in ACS Patients; ACCEL-POLYMORPHISM, Cytochrome 2C19 Polymorphism and Response to Adjunctive Cilostazol versus High Maintenance-Dose Clopidogrel in Patients Undergoing Percutaneous Coronary Intervention; ACCEL-PPI, Pharmacodynamics Effects of Adding Cilostazol versus Double-dose Clopidogrel in Patients with Acute Myocardial Infarction During Proton Pump Inhibitor Co-administration; ACCEL-RESISTANCE, Adjunctive Cilostazol Versus High Maintenance Dose Clopidogrel in Patients with Clopidogrel Resistance; ACS, acute coronary syndrome; CILON-T, Influence of Cilostazol-based Triple Antiplatelet Therapy on Ischaemic Complication After Drug-eluting Stent Implantation; HD, high-dose clopidogrel (150 mg); HOST-ASSURE, Harmonising Optimal Strategy for Treatment of Coronary Artery Stenosis—Safety and Effectiveness of Drug-Eluting Stents and Antiplatelet Regimen; LTA, light transmittance aggregometry; MI, myocardial infarction; NR, not reported; PIANO-2 CKD, Platelet Reactivity in Patients with Chronic Kidney Disease Receiving Adjunctive Cilostazol Compared with a High-Maintenance Dose of Clopidogrel; SD, Standard-dose clopidogrel (75 mg).
Table 3.
Baseline characteristics of included trials for cardiovascular outcomes
| Trial | Year | N | Comparison | Follow-up (months) | Mean age (years) | DM (%) | Stent type | DES (%) |
|---|---|---|---|---|---|---|---|---|
| ABCD46 | 2014 | 630 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 12 | 65 | 31 | BES | 100 |
| ACCEL-AMI29 | 2010 | 90 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 1 | 62 | 21 | PES>SES>ZES | 100 |
| ACCEL-LOADING-ACS30 | 2012 | 218 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 1 | 63 | 23 | DES, BMS | 95 |
| ACCEL-RESISTANCE33 | 2009 | 60 | Aspirin/clopidogrel/cilostazol vs aspirin/high-dose clopidogrel | 1 | 63 | 23 | DES | 100 |
| Ahn CM et al47 | 2011 | 130 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 24 | 64 | 22 | SES | 100 |
| Chen YD et al48 | 2006 | 120 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 9 | 58 | 30 | BMS | 0 |
| CIDES49 | 2008 | 280 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 6 | 62 | 100 | PES, SES | 100 |
| CILON-T34 | 2011 | 960 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 6 | 64 | 34 | PES, ZES | 100 |
| CLEAR50 | 2011 | 120 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 6 | 66 | 42 | SES>ZES>PES>EES | 100 |
| CREST51 | 2005 | 705 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 6 | 60 | 26 | BMS | 0 |
| DECLARE-DIABETES52 53 | 2008/2010 | 450 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 24 | 61 | 100 | PES, SES | 100 |
| DECLARE-LONG53 54 | 2007/2010 | 450 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 24 | 61 | 33 | PES, SES | 100 |
| DECLARE- LONG II55 | 2011 | 499 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 12 | 62 | 35 | ZES | 100 |
| Gao et al35 | 2013 | 428 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 12 | 56 | 18 | SES>PES | 100 |
| Guan et al36 | 2012 | 840 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 1 | 60 | NR | DES | 100 |
| Han et al56 | 2009 | 1212 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 12 | 60 | 22 | BMS, DES | 52 |
| Han et al57 | 2006 | 120 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 3 | 61 | 23 | BMS, DES | 43 |
| HOST-ASSURE37 | 2013 | 3755 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 1 | 63 | 32 | ZES-R>EES-PtCr | 100 |
| Hu et al58 | 2013 | 146 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 12 | 63 | NR | NR | NR |
| Jin et al39 | 2012 | 60 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 1 | 62 | 45 | DES | 100 |
| Kim et al59 | 2008 | 109 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 6 | 68 | 29 | PES>SES | 100 |
| Kim et al41 | 2007 | 60 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 1 | 63 | 29 | SES>PES>others | 100 |
| Kum et al42 | 2009 | 603 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 6 | 62 | 26 | DES | 100 |
| Lee et al60 | 2007 | 20 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 1 | 56 | 25 | NR | 100 |
| LONG- DES-II61 62 | 2007 | 500 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 9 | 61 | 33 | PES, SES | 100 |
| Lu et al63 | 2006 | 120 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 6–9 | 71 | NR | BMS | 0 |
| Lu et al64 | 2007 | 402 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 6 | 61 | 44 | BMS, DES | 85 |
| Min et al10 | 2007 | 59 | Aspirin/clopidogrel or ticlopidine/cilostazol vs aspirin/clopidogrel or ticlopidine | 6 | 62 | 26 | BMS | 0 |
| OPTIMUS-26 | 2008 | 50 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 1 | 64 | 100 | NR | 100 |
| Shen et al65 | 2010 | 160 | Aspirin/Clopidogrel/Cilostazol vs Aspirin/Clopidogrel | 12 | 69 | 100 | DES | 100 |
| Suh et al66 | 2009 | 143 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 25 | 62 | 100 | PES>SES | 100 |
| Wang et al67 | 2005 | 193 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 12 | 62 | 28 | BMS | 0 |
| Wang et al68 | 2010 | 164 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 12 | 68 | NR | BMS, DES | NR |
| Zang et al69 | 2008 | 263 | Aspirin/clopidogrel/cilostazol vs aspirin/clopidogrel | 12 | 59 | 100 | BMS, DES | 53 |
ABCD, Evaluating Additional Benefit of Cilostazol to Dual Antiplatelet Therapy in Patients with Long or Multivessel Coronary Artery Disease underwent Biolimus-Eluting Stent Implantation; ACS, acute coronary syndrome; AMI, acute myocardial infarction; BES, biolimus-eluting stent; BMS, bare metal stent; CIDES, comparison of cilostazol versus clopidogrel after drug-eluting stenting in diabetic patients; CILON-T, Influence of Cilostazol-based Triple Antiplatelet Therapy on Ischaemic Complication After Drug-eluting Stent Implantation; CLEAR, The Cilostazol Administration Before Percutaneous Coronary Intervention for Reduction of Periprocedural Myonecrosis Trial; CREST, Coronary Stent Restenosis in Patients Treated with Cilostazol; DECLARE-LONG II: Triple Antiplatelet Therapy With Dual Antiplatelet Therapy to Reduce Restenosis After Drug-Eluting Stent Implantation in Long Coronary Lesions; DECLARE-DIABETES, A Randomised Comparison of Triple Antiplatelet Therapy with Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation in Diabetic Patients; DECLARE-LONG, Drug-Eluting Stenting Followed by Cilostazol Treatment Reduces Late Restenosis in Patients with Long Coronary Lesions; DES, drug-eluting stent; DM, diabetes mellitus; EES, everolimus-eluting stent; EES-PtCr, everolimus-eluting platinum-chromium alloy stent; LONG-DES, Sirolimus-Eluting Stent Versus Paclitaxel-Eluting Stent for Patients With Long Coronary Artery Disease; OPTIMUS-2, Impact of Cilostazol on Platelet Function Profiles in Patients with Diabetes Mellitus and Coronary Artery Disease on Dual Antiplatelet Therapy; PES, Paclitaxel-eluting stent; SES, Sirolimus-eluting stent; ZES, Zotarolimus-eluting stent; ZES-R, Zotarolimus-eluting Resolute stent.
Table 4.
Inclusion criteria and study quality of included cardiovascular outcomes trials
| Trial | Cohort | Quality of study* |
|---|---|---|
| ABCD46 | Patients with long or multivessel disease undergoing PCI | ++± |
| ACCEL-AMI29 | Patients with ACS undergoing PCI | +++ |
| ACCEL-LOADING-ACS30 | Patients with non-ST-elevation MI undergoing PCI | ±±± |
| ACCEL-RESISTANCE33 | Patients with high on-treatment platelet reactivity undergoing PCI | ++± |
| Ahn et al47 | Patient with ACS undergoing PCI | ±±+ |
| Chen et al48 | Patients with ACS undergoing PCI | ±++ |
| CIDES49 | Patients with diabetes undergoing PCI | ±±± |
| CILON-T34 | Patients with angina undergoing PCI | ++± |
| CLEAR50 | Patients with stable angina undergoing PCI | ±±± |
| CREST51 | Patients with ACS/known stenosis undergoing PCI | +++ |
| DECLARE-DIABETES52 | Patients with ACS and diabetes undergoing PCI | +±± |
| DECLARE-LONG54 | Patients with ACS and stenosis of long (>25 mm) lesions undergoing PCI | +±± |
| DECLARE-LONG II55 | Patients with ACS/known stenosis of long (>25 mm) lesions undergoing PCI | +++ |
| Gao et al35 | Obese patients undergoing PCI | ±±± |
| Guan et al36 | Patients with ACS and high on-treatment platelet reactivity undergoing PCI | ±±± |
| Han et al56 | Patients with ACS undergoing PCI | ++± |
| Han et al57 | Patients with ACS undergoing PCI | ±±± |
| HOST-ASSURE37 | All-comer patients undergoing PCI | ±±+ |
| Hu et al58 | Patients with ACS undergoing PCI | ±±± |
| Jin et al39 | Patients undergoing PCI | ±++ |
| Kim et al59 | Patients with ACS/known stenosis undergoing PCI | ±±± |
| Kim et al41 | Patients with ST-elevation MI undergoing PCI | ±±± |
| Kum et al42 | Patients with ACS/known stenosis undergoing PCI | ±±± |
| Lee et al60 | Patients undergoing elective PCI | +±± |
| LONG-DES-II61 | Patients with stenosis of long lesions undergoing PCI | ++± |
| Lu et al70 | Patients undergoing PCI | ±±+ |
| Lu et al64 | Patients with ADP-induced platelet inhibition rates <30% undergoing PCI | +±± |
| Min et al10 | Patients with ACS/known stenosis undergoing elective PCI | ±+± |
| OPTIMUS-26 | Patients with diabetes undergone PCI | +++ |
| Shen et al65 | Patients with ACS undergoing PCI | ±±± |
| Suh et al66 | Patients with diabetes and chronic total occlusion undergoing PCI | ±±± |
| Wang et al67 | Patients with small vessel stenosis undergoing PCI | ±±± |
| Wang et al68 | Patients with non-ST-elevation MI undergoing PCI | ±±± |
| Zang et al69 | Patients with ACS undergoing PCI | ±±± |
*Represents risk of bias based on: sequence generation of allocation; allocation concealment and blinding. ‘+’ represents low bias risk, ‘−’ high bias risk and ‘±’ unclear bias risk.
ABCD, Evaluating Additional Benefit of Cilostazol to Dual Antiplatelet Therapy in Patients with Long or Multivessel Coronary Artery Disease underwent Biolimus-Eluting Stent Implantation; ACS, acute coronary syndrome; AMI, acute myocardial infarction; BES, biolimus-eluting stent; BMS, bare metal stent; CIDES, comparison of cilostazol versus clopidogrel after drug-eluting stenting in diabetic patients; CILON-T, Influence of Cilostazol-based Triple Antiplatelet Therapy on Ischaemic Complication After Drug-eluting Stent Implantation; CLEAR, The Cilostazol Administration Before Percutaneous Coronary Intervention for Reduction of Periprocedural Myonecrosis Trial; CREST, Coronary Stent Restenosis in Patients Treated with Cilostazol; DECLARE-LONG II: Triple Antiplatelet Therapy With Dual Antiplatelet Therapy to Reduce Restenosis After Drug-Eluting Stent Implantation in Long Coronary Lesions; DECLARE-DIABETES, A Randomised Comparison of Triple Antiplatelet Therapy with Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation in Diabetic Patients; DECLARE-LONG, Drug-Eluting Stenting Followed by Cilostazol Treatment Reduces Late Restenosis in Patients with Long Coronary Lesions; DES, drug-eluting stent; DM, diabetes mellitus; EES, everolimus-eluting stent; EES-PtCr, everolimus-eluting platinum-chromium alloy stent; LONG-DES, Sirolimus-Eluting Stent Versus Paclitaxel-Eluting Stent for Patients With Long Coronary Artery Disease; OPTIMUS-2, Impact of Cilostazol on Platelet Function Profiles in Patients with Diabetes Mellitus and Coronary Artery Disease on Dual Antiplatelet Therapy; PES, Paclitaxel-eluting stent; SES, Sirolimus-eluting stent; ZES, Zotarolimus-eluting stent; ZES-R, Zotarolimus-eluting Resolute stent.
Primary platelet reactivity outcomes
Primary outcome: differences in PRU
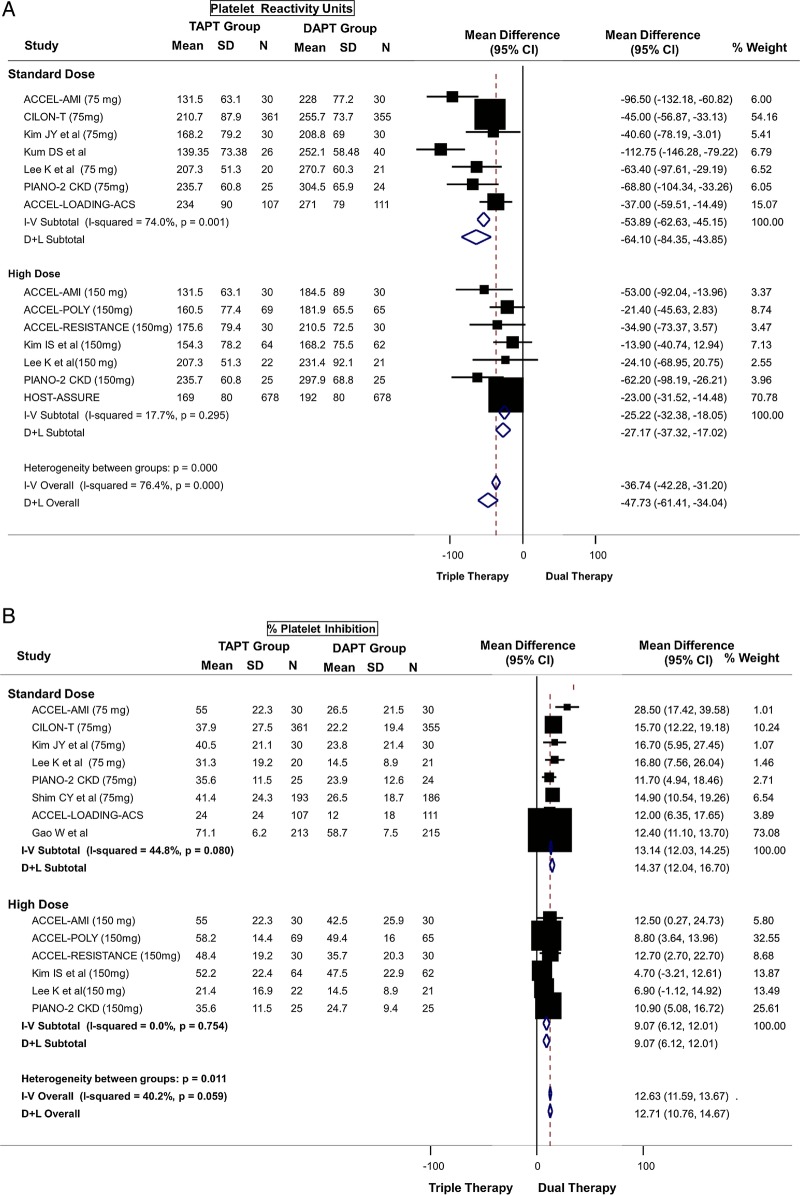
TAPT resulted in a mean PRU reduction of 47.73 (95% CI −61.41 to −34.04, p<0.0001; mean PRU 182.90 vs 232.65) compared with DAPT (figure 2A). There was a larger mean difference between the TAPT and DAPT groups when the analysis was restricted to a DAPT group using standard-dose clopidogrel (mean PRU 189.54 vs 255.83) where the PRU value was lower by a mean of 64.10 (95% CI −84.35 to −43.85). Moreover, TAPT was associated with a lower PRU value even when compared with DAPT using high-dose clopidogrel (mean difference of 27.17) (mean PRU 176.27 vs 209.48) (figure 2A). The results were similar when stratified by ACS status (see web appendix figure A1) or by baseline clopidogrel resistance status (see web appendix figure A2). There was moderate-to-high heterogeneity for the above analysis. However, the heterogeneity was reduced in subgroup analysis restricted to comparison with high-dose clopidogrel (figure 2A), in trials enrolling patients with baseline clopidogrel resistance (see web appendix figure A2 and in trials enrolling patients without ACS (see web appendix figure A1).
Figure 2.
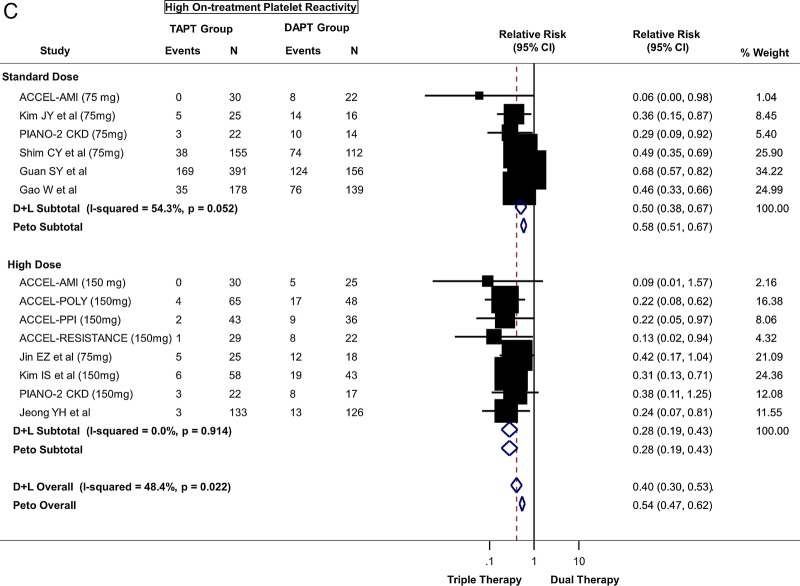
(A) Primary platelet reactivity outcome: difference in platelet reactivity units (PRU) after treatment between triple antiplatelet therapy (TAPT) versus dual antiplatelet therapy (DAPT). (B) Secondary platelet reactivity outcome: difference in percent platelet inhibition after treatment between TAPT versus DAPT. (C) Secondary platelet reactivity outcome: risk of high on-treatment platelet reactivity (HTPR) after treatment between TAPT versus DAPT.
Figure 2.
Continued
In addition, the mean PRU values on treatment in the TAPT group in each of the trials were below a PRU of 235, which has been cited in the literature as the suggested threshold for defining HTPR.13
Secondary outcomes: percent platelet inhibition and high on-treatment platelet reactivity
TAPT was associated with a 12.71% greater platelet inhibition compared to DAPT for the overall cohort (95% CI 10.76 to 14.67, p<0.0001) (figure 2B). TAPT was also associated with a greater platelet inhibition in comparison with DAPT using standard-dose clopidogrel (14.37% mean greater platelet inhibition) and remained significant even when compared with DAPT using high-dose clopidogrel (9.07% mean greater platelet inhibition) (figure 2B). There was moderate heterogeneity for the above analysis. The results were similar when stratified by ACS status (see web appendix figure A3) or by baseline clopidogrel resistance status (see web appendix figure A4).
In addition, TAPT was associated with a 60% reduction in the risk of HTPR when compared with DAPT (figure 2C) (relative risk=0.40; 95% CI 0.30 to 0.53, p<0.0001). When stratified by clopidogrel dose, TAPT was associated with a 50% reduction in risk of HTPR compared to standard-dose DAPT and a 72% reduction compared to high-dose DAPT (figure 2C). Heterogeneity was moderate with no evidence for significant publication bias. The results were similar when stratified by ACS status (see web appendix figure A5) or by baseline clopidogrel resistance status (see web appendix figure A6).
Cardiovascular outcomes
Primary outcome
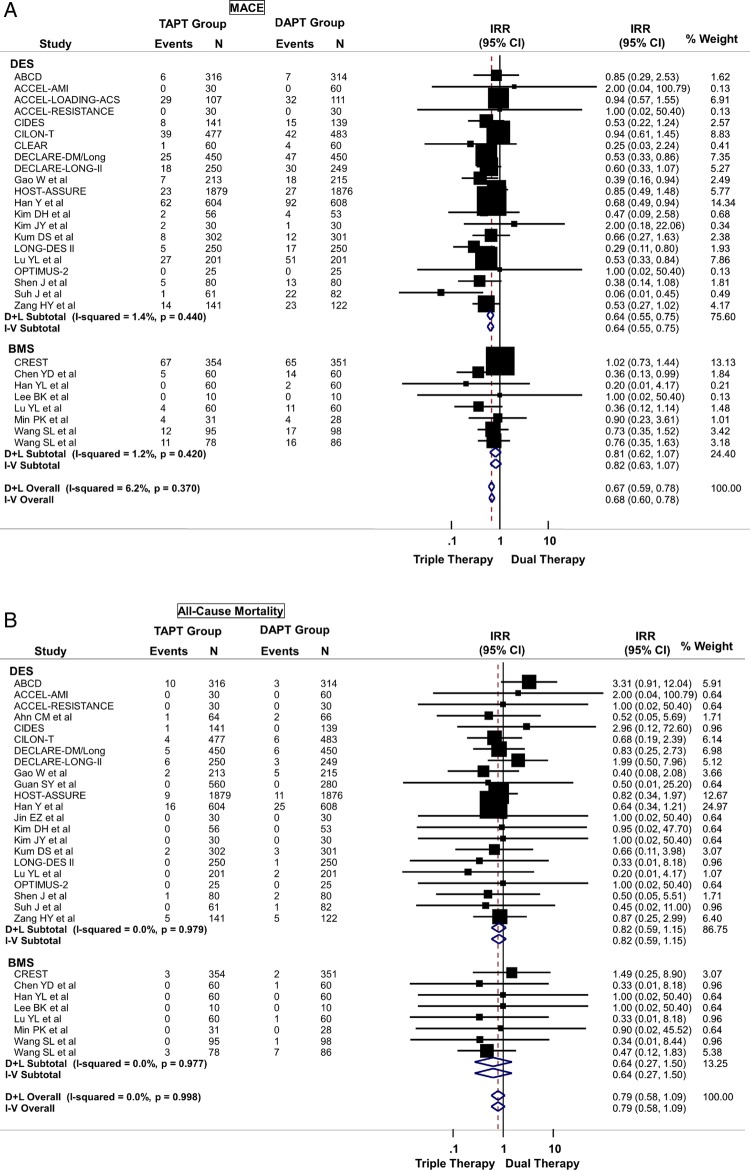
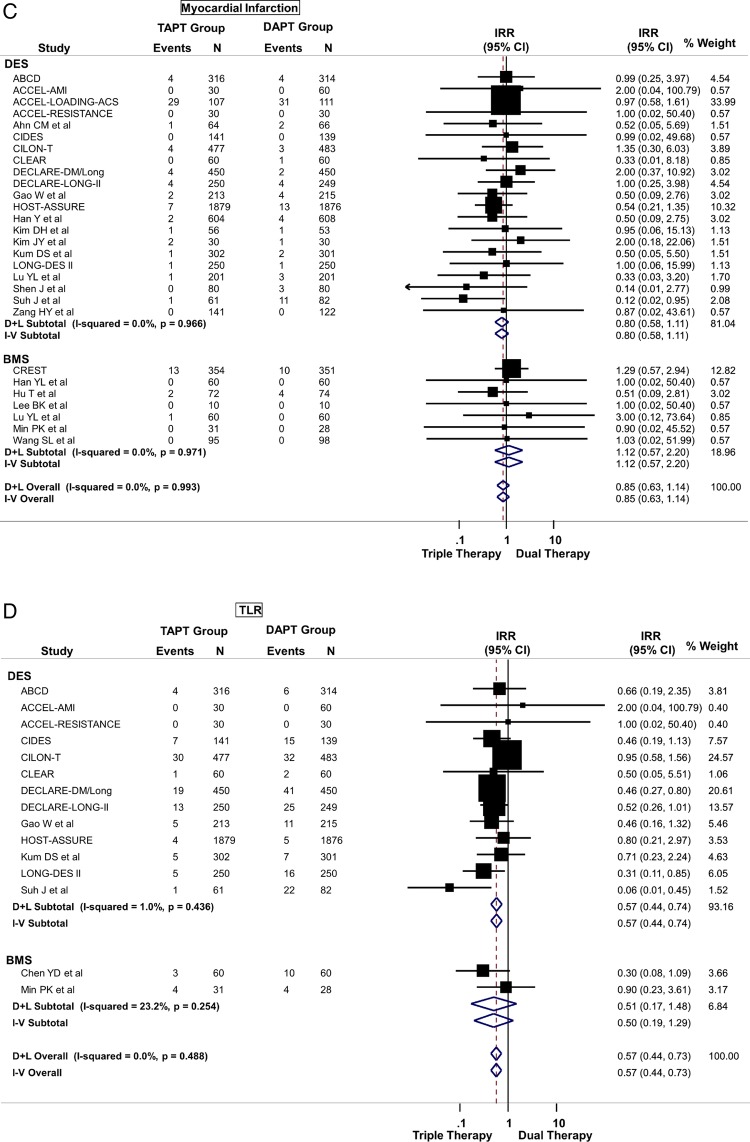
TAPT was associated with a 32% reduction in the risk of MACE (IRR=0.68; 95% CI 0.60 to 0.78) when compared with DAPT for the overall cohort (figure 3A). This effect was observed regardless of stent type (Pinteraction >0.05) such that even in patients undergoing PCI with DES, TAPT resulted in a 36% reduction in MACE (IRR=0.64; 95% CI 0.55 to 0.75) when compared with DAPT alone (figure 3A). There was low heterogeneity in the analysis and no evidence for significant publication bias.
Figure 3.
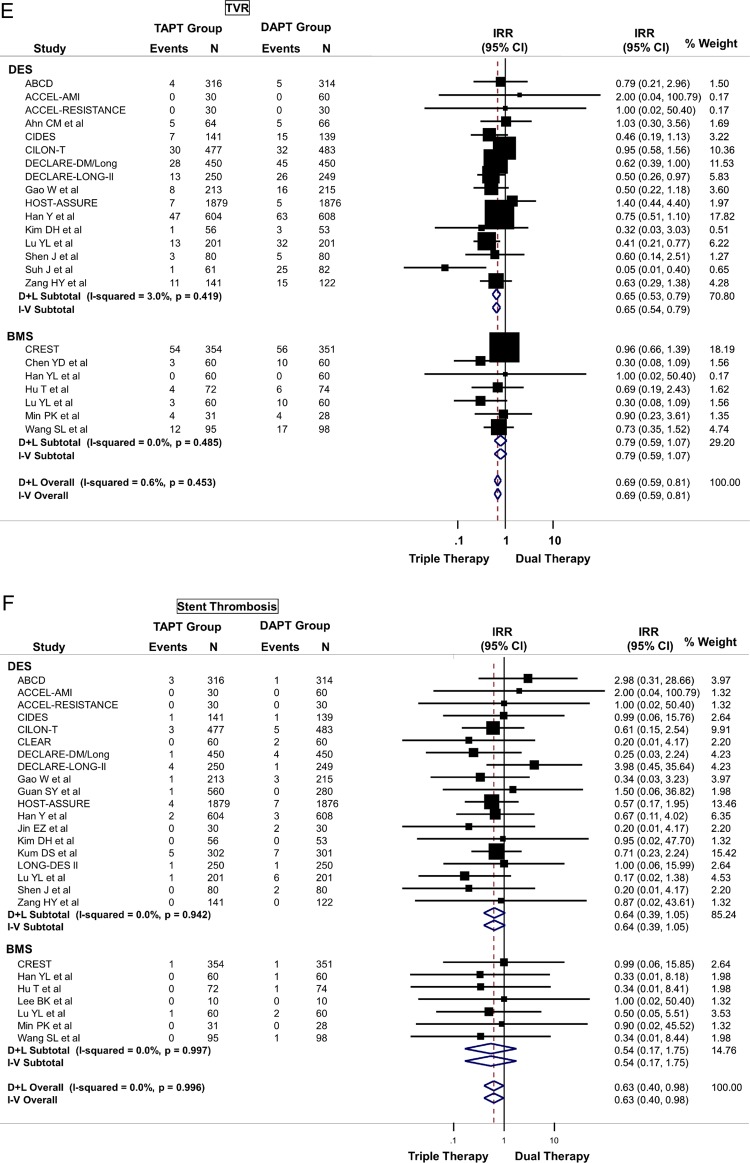
(A) Primary cardiovascular outcome: risk of major adverse cardiovascular effects (MACE) between triple antiplatelet therapy (TAPT) versus dual antiplatelet therapy (DAPT). (B) Secondary cardiovascular outcome: risk of all-cause mortality between TAPT versus DAPT. (C) Secondary cardiovascular outcome: risk of myocardial infarction between TAPT versus DAPT. (D) Secondary cardiovascular outcome: risk of target lesion revascularisation (TLR) between TAPT versus DAPT. (E) Secondary cardiovascular outcome: risk of target vessel revascularisation (TVR) between TAPT versus DAPT. (F) Secondary cardiovascular outcome: risk of stent thrombosis between TAPT versus DAPT.
Figure 3.
Continued
Figure 3.
Continued
Secondary outcomes
TAPT was associated with similar IRR for death (IRR=0.79; 95% CI 0.58 to 1.09) (figure 3B), cardiovascular death (IRR=0.74; 95% CI 0.42 to 1.30) and MI (IRR=0.85; 95% CI 0.63 to 1.14) (figure 3C) for the overall cohort. The IRR was independent of stent type as TAPT showed benefit regardless whether BMS and DES was used (stent type, Pinteraction >0.05). In the overall cohort, TAPT was associated with a 43% reduction in the risk of TLR (IRR=0.57; 95% CI 0.44 to 0.73) (figure 3D) and a 31% reduction in the risk of TVR (IRR=0.69; 95% CI 0.59 to 0.81) (figure 3E) compared with DAPT. TAPT efficacy for reducing TLR and TVR was present even when the analyses were restricted to studies using DES. In DES-treated patients, TAPT resulted in a 43% reduction in TLR (IRR=0.57; 95% CI 0.44 to 0.74) and a 35% reduction in TVR (IRR=0.65; 95% CI 0.54 to 0.79) with TAPT compared with DAPT.
TAPT was associated with significantly lower stent thrombosis rate when compared with DAPT (IRR=0.63; 95% CI 0.40 to 0.98) (figure 3F). There was no heterogeneity (0%) in all of the above analyses and no evidence for significant publication bias.
Safety outcomes
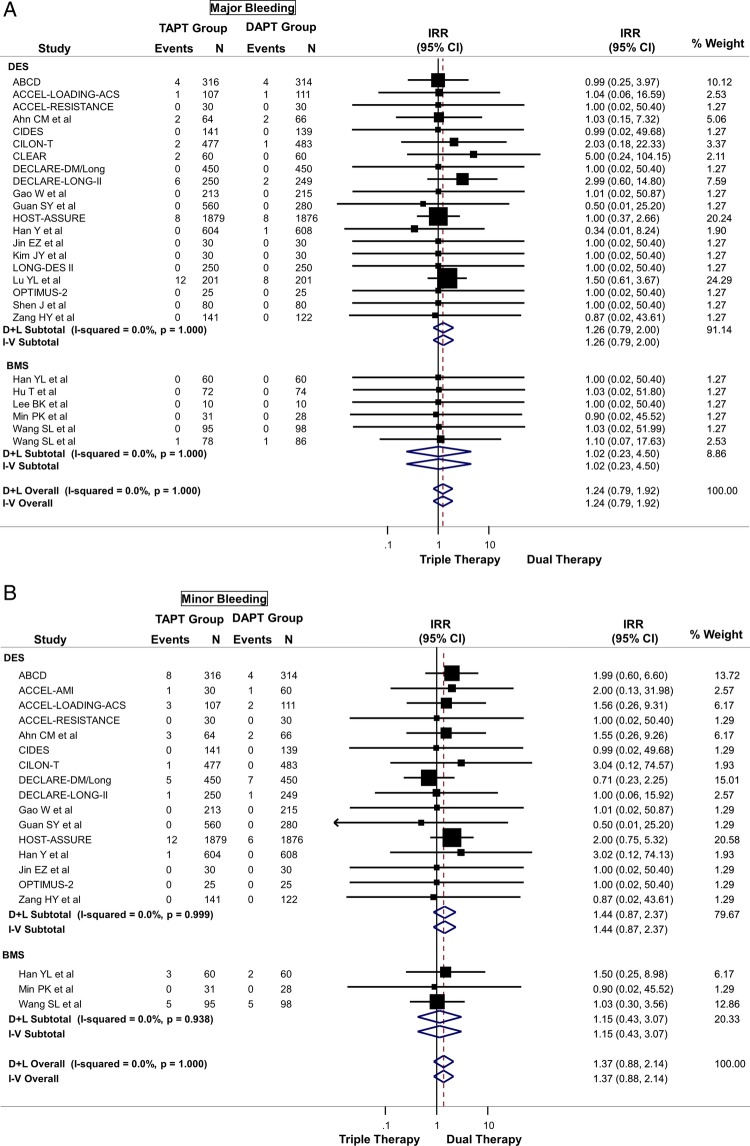
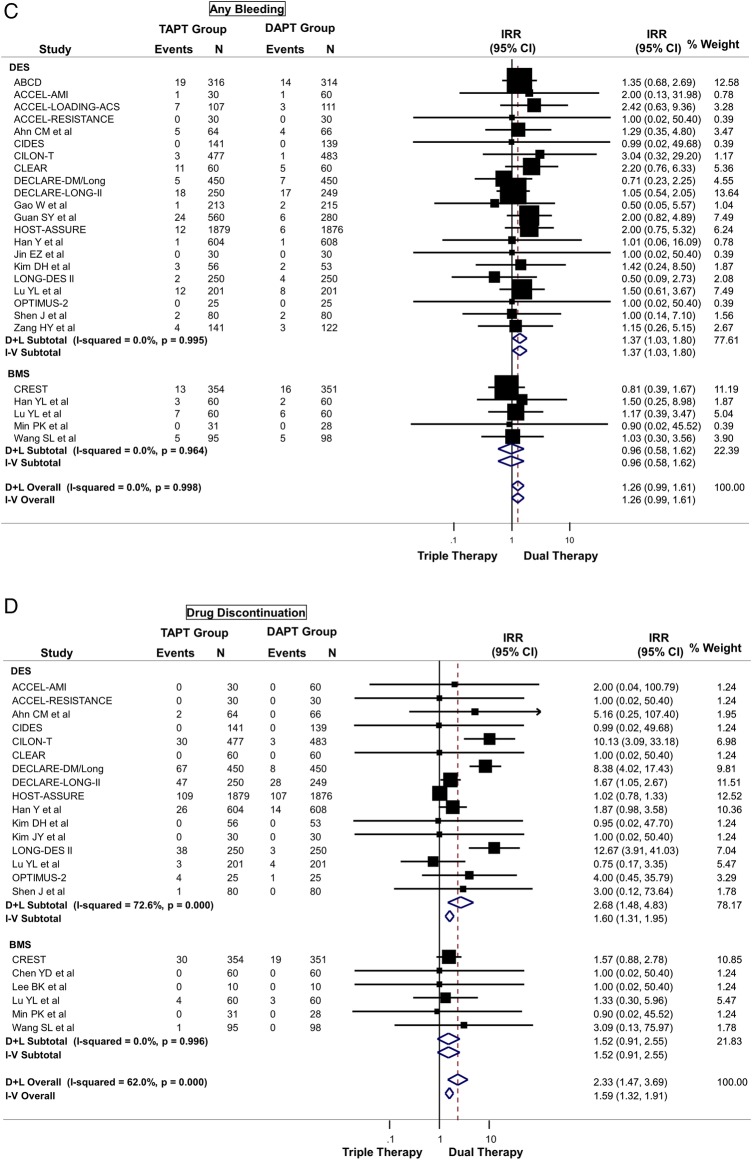
TAPT was associated with a numerically increased risk of major (IRR=1.24; 95% CI 0.79 to 1.92) (figure 4A), minor (IRR=1.37; 95% CI 0.88 to 2.14) (figure 4B), or any bleeding (IRR=1.26; 95% CI 0.99 to 1.61) (figure 4C) compared with DAPT, although these were not statistically significant. TAPT was also associated with a 59% increase in drug discontinuation due to adverse events (IRR=1.59; 95% CI 1.32 to 1.91) (figure 4D) when compared with DAPT. The most commonly listed causes for drug discontinuation were headache, skin rash and palpitations/tachycardia. There was no-to-modest (for drug discontinuation outcomes) heterogeneity in all of the above analyses and no evidence for significant publication bias.
Figure 4.
(A) Safety outcome: risk of major bleeding between triple antiplatelet therapy (TAPT) versus dual antiplatelet therapy (DAPT). (B) Safety outcome: risk of minor bleeding between TAPT versus DAPT. (C) Safety outcome: risk of any bleeding between TAPT versus DAPT. (D) Safety outcome: risk of drug discontinuation due to adverse effects between TAPT versus DAPT.
Figure 4.
Continued
Discussion
In patients undergoing PCI, TAPT using cilostazol results in significant decrease in platelet reactivity and reduced risk of HTPR. TAPT resulted in significantly lower mean PRU, greater platelet inhibition and reduced risk of HTPR in the setting of DAPT with both standard-dose and high-dose clopidogrel. In addition, TAPT was associated with a significant reduction in CV events, including reduction in MACE, driven largely by significant reductions in TLR and TVR. Most importantly, there was a significant lower stent thrombosis with TAPT versus DAPT. Moreover, the reduction of restenosis with TAPT remained even when the analysis was restricted to trials using DES. In addition, there was numerically higher bleeding with TAPT versus DAPT, although this did not reach statistical significant. However, there was a significant increase in the risk of drug discontinuation due to adverse effects when compared with DAPT.
Platelet reactivity and outcomes
Prior studies have shown a relationship between on-treatment platelet reactivity and adverse CV events in patients undergoing PCI. In an analysis of individual patient data from six studies with 3059 patients, for every 10 U increase in PRU there was a 4% increase in primary endpoint rate of death, MI or stent thrombosis (HR 1.04; 95% CI 1.03 to 1.06; p<0.0001).17 A recent consensus statement recommended a cut-off of PRU >235 U as the threshold for identifying patients with HTPR who may be at high risk for ischaemic or thrombotic events following PCI.13 Patients with HTPR have been shown to have an increased risk of death (110% increase), MI (104% increase) and stent thrombosis (211% increase).17 18
Although platelet reactivity is a surrogate marker, given the wide interindividual variability in clopidogrel-induced platelet inhibition,1–3 various strategies have been tested to improve platelet inhibition. These strategies have utilised higher loading and maintenance doses of clopidogrel, or next-generation P2Y12 inhibitors such as prasugrel and ticagrelor, which are more potent that clopidogrel and have a more uniform antiplatelet effect. Doubling of the clopidogrel dose (150 mg) has been shown to significantly reduce PRU in patients with HTPR.19–21 Similarly, data from the next-generation P2Y12 inhibitors such as prasugrel and ticagrelor have shown improved platelet reactivity indices when compared with clopidogrel.22 Although the newer agents prasugrel and ticagrelor reduce MACE in randomised trials, these agents increase bleeding in patients with PCI and cost significantly more than generic clopidogrel.23 24
Cilostazol, a phosphodiesterase III inhibitor, exhibits antiplatelet effects by increasing cAMP within platelets, and is available as a generic drug. Our results show a significant benefit of TAPT with cilostazol in improving platelet reactivity indices in patients undergoing PCI, with lower PRU, greater platelet inhibition and a significant reduction in the risk of HTPR regardless of comparison with either standard-dose or high-dose clopidogrel. In addition, these results were seen even in comparison with DAPT using high-dose clopidogrel. Given that generic clopidogrel is now available, many clinicians opt to prescribe high-dose clopidogrel to address HTPR in patients who cannot afford newer antiplatelet agents. The results of the present study show that TAPT with cilostazol is superior even to DAPT with high-dose clopidogrel. Despite these promising results, a number of limitations must be acknowledged. Although platelet reactivity is a risk factor/surrogate marker for adverse CV events, clinical studies have not yet demonstrated that a pharmacological treatment strategy based on platelet reactivity improves outcomes.20 25 In the ARCTIC trial of 2440 patients randomised to platelet-function monitoring and drug adjustment group versus conventional strategy of no monitoring and drug adjustment, there were no differences in composite of death, MI, stent thrombosis, stroke, or urgent revascularisation at 1 year between the two groups, calling into question the utility of adjusting therapies based on platelet function monitoring.25
However, because cilostazol inhibits both platelet activation and smooth muscle proliferation, it has the potential to target two dreaded complications of PCI—stent thrombosis and restenosis. TAPT may reduce MACE by two or more cellular mechanisms.8–11 Our study shows significant reduction in both stent thrombosis and restenosis using TAPT with cilostazol, even in patients treated with DES. This is a potential advantage for this agent, as no antiplatelet agent, including prasugrel or ticagrelor, has been shown to have any antirestenosis property.
Therefore, a strategy of using TAPT with cilostazol has several advantages: (1) it improves the surrogate outcome of platelet reactivity relative to DAPT, including high-dose clopidogrel; (2) the antismooth muscle proliferative properties of cilostazol may make it an excellent agent to prevent restenosis resulting in reduced TVR even in patients treated with a DES; (3) the improvement in platelet reactivity indices translate into significant reduction in stent thrombosis and (4) the medication is available generically and is therefore less expensive than newer antiplatelet therapy. Thus, when used following PCI, TAPT with cilostazol has the potential to be a cost-effective therapy to improve clinical outcomes by reducing thrombotic events and restenosis. The results of this study therefore call for a randomised trial comparing a strategy of TAPT with DAPT using newer antiplatelet agents.
Our results differ from the studies of Jang et al26 and Sakurai et al27 in that these studies did not evaluate platelet reactivity outcomes and had far fewer trials than the current analysis. In our analysis, TAPT was associated with significant increase in drug discontinuation. The most commonly listed causes for drug discontinuation were headache, skin rash and palpitations/tachycardia. Sakurai et al27 similarly found a significant increase in rash and gastrointestinal side effects with TAPT.
Study limitations
As in other meta-analyses without individual patient data, we were unable to adjust for dosages of medication used or with compliance with assigned therapies. Given heterogeneity in the study protocols, clinically relevant differences could have been missed and are best assessed in a meta-analysis of individual patient data. Stroke would have been interesting to examine, as there is some evidence that cilostazol reduces stroke.28 All of the trials did not report all of the outcomes. The subgroup analyses might suffer from multiple testing. In addition, the results need to be confirmed in an ethnically diverse population, as most of the trials were done in Asian populations. However, the CREST and the OPTIMUS-2 trials, performed mainly in a non-Asian population, showed similar efficacy of cilostazol when compared with controls. The individual trials did not provide sufficient data to stratify analyses by early versus newer generation DES.
Conclusions
In patients undergoing PCI, TAPT with cilostazol is associated with significantly improved platelet reactivity indices, even when compared with DAPT with high-dose clopidogrel, and is associated with significant reduction in CV events, including reduction in BMS and DES restenosis and stent thrombosis. The dual properties of antiplatelet and antiproliferative action, the availability as a generic medication combined with the above data makes TAPT with aspirin, clopidogrel and cilostazol an attractive and strong competitor for newer antiplatelet regimens and should be evaluated in future trials.
Supplementary Material
Footnotes
Contributors: SB was involved in study concept and design, analysis and interpretation of the data, statistical analysis, and study supervision and also takes responsibility for the integrity of the data and the accuracy of the data analysis. SB and AS were involved in acquisition of the data and drafting of the manuscript. SB, AS, JJD, KC, FF and DLB were involved in critical revision of the manuscript for important intellectual content.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: DLB: Advisory Board: Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Get With The Guidelines Steering Committee; Data Monitoring Committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), Journal of the American College of Cardiology (Section Editor, Pharmacology); Research Grants: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Roche, Sanofi Aventis, The Medicines Company; Unfunded Research: FlowCo, PLx Pharma, Takeda.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Gurbel PA, Bliden KP, Hiatt BL, et al. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003;107:2908–13 [DOI] [PubMed] [Google Scholar]
- 2.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol 2007;49:1505–16 [DOI] [PubMed] [Google Scholar]
- 3.Serebruany VL, Steinhubl SR, Berger PB, et al. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005;45:246–51 [DOI] [PubMed] [Google Scholar]
- 4.Tousoulis D, Briasoulis A, Dhamrait S, et al. Effective platelet inhibition by aspirin and clopidogrel: where are we now? Heart 2009;95:850–8 [DOI] [PubMed] [Google Scholar]
- 5.Aradi D, Komosci A, Vorobcsuk A, et al. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: Systematic review and meta-analysis. Am Heart J 2010;160:543–51 [DOI] [PubMed] [Google Scholar]
- 6.Angiolillo DJ, Capranzano P, Goto S, et al. A randomized study assessing the impact of cilostazol on platelet function profiles in patients with diabetes mellitus and coronary artery disease on dual antiplatelet therapy: results of the OPTIMUS-2 study. Eur Heart J 2008;29:2202–11 [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Shakur Y, Yoshitake M, et al. Cilostazol (pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc Drug Rev 2001;19:369–86 [DOI] [PubMed] [Google Scholar]
- 8.Fujinaga K, Onoda K, Yamamoto K, et al. Locally applied cilostazol suppresses neointimal hyperplasia by inhibiting tenascin-C synthesis and smooth muscle cell proliferation in free artery grafts. J Thorac Cardiovasc Surg 2004;128:357–63 [DOI] [PubMed] [Google Scholar]
- 9.Tsai C-S, Lin F, Chen Y, et al. Cilostazol attenuates MCP-1 and MMP-9 expression in vivo in LPS-administrated balloon-injured rabbit aorta and in vitro in LPS-treated monocytic THP-1 cells. J Cell Biochem 2007;103:54–66 [DOI] [PubMed] [Google Scholar]
- 10.Min P-K, Jung J, Ko Y, et al. Effect of cilostazol on in-stent neointimal hyperplasia after coronary artery stenting. Circ J 2007;71:1685–90 [DOI] [PubMed] [Google Scholar]
- 11.Lee S-W, Park S, Yun S, et al. Triple antiplatelet therapy reduces ischemic events after drug-eluting stent implantation: Drug-eluting stenting followed by cilostazol treatment reduces adverse serious cardiac events (DECREASE registry). Am Heart J 2010:284–91 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Green S. Assessing risk of bias in included studies. Cochrane handbook for systematic reviews of interventions version. 500 edn Oxford: The Cochrane Collaboration, 2008 [Google Scholar]
- 13.Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010;56:919–33 [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 1999;354:1896–900 [DOI] [PubMed] [Google Scholar]
- 15.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration, 2008. http://www.cochrane-handbook.org (accessed 12 Dec 2013). [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brar SS, ten Berg J, Marcucci R, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. J Am Coll Cardiol 2011;58:1945–54 [DOI] [PubMed] [Google Scholar]
- 18.Gurbel PA, Tantry US. Acceptance of high platelet reactivity as a risk factor: now, what do we do about it? JACC Cardiovasc Interv 2010;3:1008–10 [DOI] [PubMed] [Google Scholar]
- 19.Barker CM, Murray SS, Teirstein PS, et al. Pilot study of the antiplatelet effect of increased clopidogrel maintenance dosing and its relationship to CYP2C19 genotype in patients with high on-treatment reactivity. JACC Cardiovasc Interv 2010;3:1001–7 [DOI] [PubMed] [Google Scholar]
- 20.Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011;305:1097–105 [DOI] [PubMed] [Google Scholar]
- 21.Price MJ, Angiolillo DJ, Teirstein PS, et al. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial. Circulation 2011;124:1132–7 [DOI] [PubMed] [Google Scholar]
- 22.Michelson AD, Frelinger AL, III, Braunwald E, et al. Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial. Eur Heart J 2009;30:1753–63 [DOI] [PubMed] [Google Scholar]
- 23.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–15 [DOI] [PubMed] [Google Scholar]
- 24.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57 [DOI] [PubMed] [Google Scholar]
- 25.Collet JP, Cuisset T, Range G, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 2012;367:2100–9 [DOI] [PubMed] [Google Scholar]
- 26.Jang JS, Jin HY, Seo JS, et al. A meta-analysis of randomized controlled trials appraising the efficacy and safety of cilostazol after coronary artery stent implantation. Cardiology 2012;122:133–43 [DOI] [PubMed] [Google Scholar]
- 27.Sakurai R, Koo BK, Kaneda H, et al. Cilostazol added to aspirin and clopidogrel reduces revascularization without increases in major adverse events in patients with drug-eluting stents: a meta-analysis of randomized controlled trials. Int J Cardiol 2013;167:2250–8 [DOI] [PubMed] [Google Scholar]
- 28.Kumbhani DJ, Bhatt DL. Secondary prevention of stroke: can we do better than aspirin? Lancet Neurol 2010;9:942–3 [DOI] [PubMed] [Google Scholar]
- 29.Jeong Y-H, Hwang J, Kim I, et al. Adding cilostazol to dual antiplatelet therapy achieves greater platelet inhibition than high maintenance dose clopidogrel in patients with acute myocardial infarction: results of the adjunctive cilostazol versus high maintenance dose clopidogrel in patients with AMI (ACCEL-AMI) study. Circ Cardiovasc Interv 2010;3:17–26 [DOI] [PubMed] [Google Scholar]
- 30.Jeong Y-H. ACCEL-LOADING-ACS—Multicenter randomized trial evaluating efficacy of cilostazol on platelet aggregation, Inflammation, and myonecrosis in ACS patients. TCT-AP 2012
- 31.Hwang SJ, Jeong YH, Kim IS, et al. Cytochrome 2C19 polymorphism and response to adjunctive cilostazol versus high maintenance-dose clopidogrel in patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv 2010;3:450–9 [DOI] [PubMed] [Google Scholar]
- 32.Jeong YH, Hwang S, Park T, et al. Pharmacodynamic effects of adding cilostazol versus double-dose clopidogrel in patients with acute myocardial infarction during proton pump inhibitor co-administration (ACCEL-PPI). J Am Coll Cardiol 2012;59:A127 [Google Scholar]
- 33.Jeong Y-H, Lee SW, Choi BR, et al. Randomized comparison of adjunctive cilostazol versus high maintenance dose clopidogrel in patients with high post-treatment platelet reactivity: results of the ACCEL-RESISTANCE (Adjunctive cilostazol versus high maintenance dose clopidogrel in patients with clopidogrel resistance) randomized study. J Am Coll Cardiol 2009;53:1101–9 [DOI] [PubMed] [Google Scholar]
- 34.Suh J-W, Lee S, Park K, et al. Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: results of the CILON-T (influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. J Am Coll Cardiol 2011;57:280–9 [DOI] [PubMed] [Google Scholar]
- 35.Gao W, Zhang Q, Ge H, et al. Efficacy and safety of triple antiplatelet therapy in obese patients undergoing stent implantation. Angiology 2013;64:554–8 [DOI] [PubMed] [Google Scholar]
- 36.Guan SY, Han YL, Li Y, et al. [Effects of intensive antiplatelet therapy for patients with high on-treatment platelet reactivity after coronary stent implantation]. Zhonghua Xin Xue Guan Bing Za Zhi 2012;40:25–9 [PubMed] [Google Scholar]
- 37.Park KW, Kang SH, Park JJ, et al. Adjunctive cilostazol versus double-dose clopidogrel after drug-eluting stent implantation: the HOST-ASSURE randomized trial (Harmonizing optimal strategy for treatment of coronary artery stenosis-safety & effectiveness of drug-eluting stents & anti-platelet regimen). JACC Cardiovasc Interv 2013;6:932–42 [DOI] [PubMed] [Google Scholar]
- 38. Jeong YH, Park Y, Koh JS, et al. Reappraisal of Pharmacodynamic Effect of Adjunctive Cilostazol and High-dose Clopidogrel in East Asian ACS Patients. TCT-AP. 2014.
- 39.Jin EZ, Yu L, Li X. Loading effect of 200 mg cilostazol on platelet inhibition in patients undergoing percutaneous coronary intervention. Int Heart J 2012;53:1–4 [DOI] [PubMed] [Google Scholar]
- 40.Kim IS, Jeong YH, Park Y, et al. Platelet inhibition by adjunctive cilostazol versus high maintenance-dose clopidogrel in patients with acute myocardial infarction according to cytochrome P450 2C19 genotype. JACC Cardiovasc Interv 2011;4:381–91 [DOI] [PubMed] [Google Scholar]
- 41.Kim J-Y, Lee K, Shin M, et al. Cilostazol could ameliorate platelet responsiveness to clopidogrel in patients undergoing primary percutaneous coronary intervention. Circ J 2007;71:1867–72 [DOI] [PubMed] [Google Scholar]
- 42.Kum DS, Kim M, Paik J, et al. Clinical effects of additional cilostazol administration after drug-eluting stent insertion Korean Circ J 2009;39:21–5 [Google Scholar]
- 43.Lee K, Kim J, Yoo B, et al. Cilostazol augments the inhibition of platelet aggregation in clopidogrel low-responders. J Thromb Hemost 2010;8:2577–9 [DOI] [PubMed] [Google Scholar]
- 44.Woo JS, Kim W, Lee S, et al. Platelet reactivity in patients with chronic kidney disease receiving adjunctive cilostazol compared with a high-maintenance dose of clopidogrel: Results of the effect of platelet inhibition according to clopidogrel dose in patients with chronic kidney disease(PIANO-2 CKD) randomized study. Am Heart J 2011;162:1018–25 [DOI] [PubMed] [Google Scholar]
- 45.Shim CY, Yoon S, Park S, et al. The clopidogrel resistance can be attenuated with triple antiplatelet therapy in patients undergoing drug-eluting stent implantation. Int J Cardiol 2009;134:351. [DOI] [PubMed] [Google Scholar]
- 46.Youn YJ, Lee JW, Ahn SG, et al. Multicenter randomized trial of 3-month cilostazol use in addition to dual antiplatelet therapy after biolimus-eluting stent implantation for long or multivessel coronary artery disease. Am Heart J 2014;167:241–8 e1 [DOI] [PubMed] [Google Scholar]
- 47.Ahn C, Hong S, Park J, et al. Cilostazol reduces the progression of carotid intima-media thickness without increasing the risk of bleeding in patients with acute coronary syndrome during a 2-year follow-up. Heart Vessels 2011;26:502–10 [DOI] [PubMed] [Google Scholar]
- 48.Chen Y-d, Lu Y, Jin Z, et al. A prospective randomized antiplatelet trial of cilostazol versus clopidogrel in patients with bare metal stent. Chin Med J 2006;119:360–6 [PubMed] [Google Scholar]
- 49.Ahn Y, Jeong M, Jeong J, et al. Randomized comparison of cilostazol vs clopidogrel after drug-eluting stenting in diabetic patients (CIDES Trial). Circ J 2008;72:35–9 [DOI] [PubMed] [Google Scholar]
- 50.Kim BK, Oh SJ, Yoon SJ, et al. A randomized study assessing the effects of pretreatment with cilostazol on periprocedural myonecrosis after percutaneous coronary intervention. Yonsei Med J 2011;52:717–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Douglas JS, Jr, Holmes DR, Jr, Kereiakes DJ, et al. Coronary stent restenosis in patients treated with cilostazol. Circulation 2005;112:2826–32 [DOI] [PubMed] [Google Scholar]
- 52.Lee SW, Park SW, Kim YH, et al. Drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus the DECLARE-DIABETES trial (A randomized comparison of triple antiplatelet therapy with dual antiplatelet therapy after drug-eluting stent implantation in diabetic patients). J Am Coll Cardiol 2008;51:1181–7 [DOI] [PubMed] [Google Scholar]
- 53.Lee SW, Chun KJ, Park SW, et al. Comparison of triple antiplatelet therapy and dual antiplatelet therapy in patients at high risk of restenosis after drug–eluting stent implantation (from the Declare-Diabetes and Long Trials). Am J Cardiol 2010;105:168–73 [DOI] [PubMed] [Google Scholar]
- 54.Lee SW, Park SW, Kim YH, et al. Comparison of triple versus dual antiplatelet therapy after drug-eluting stent implantation (from the DECLARE-Long trial). Am J Cardiol 2007;100:1103–8 [DOI] [PubMed] [Google Scholar]
- 55.Lee SW, Park SW, Kim YH, et al. A randomized, double-blind, multicenter comparison study of triple antiplatelet therapy with dual antiplatelet therapy to reduce restenosis after drug-eluting stent implantation in long coronary lesions: results from the DECLARE-LONG II (Drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with long coronary lesions) trial. J Am Coll Cardiol 2011;57:1264–70 [DOI] [PubMed] [Google Scholar]
- 56.Han Y, Li Y, Wang S, et al. Cilostazol in addition to aspirin and clopidogrel improves long-term outcomes after percutaneous coronary intervention in patients with acute coronary syndromes: a randomized, controlled study. Am Heart J 2009;157:733–9 [DOI] [PubMed] [Google Scholar]
- 57.Han Y, Su Q, Li Y, et al. The effects of post coronary stenting triple antiplatelet therapies on platelet functions. Chin J Intern Med 2006;45:635–8 [PubMed] [Google Scholar]
- 58.Hu T, Ma H, Li H, et al. Efficacy of cilostazol in patients with acute coronary syndrome after percutaneous coronary intervention. Am J Ther 2013;20:151–3 [DOI] [PubMed] [Google Scholar]
- 59.Kim DH, Kim J, Moon S, et al. Effects of long-term triple anti-platelet therapy with low dose cilostazol after drug-eluting stent implantation. Korean J Med 2008;74:368–75 [Google Scholar]
- 60.Lee B-K, Lee SW, Park SW, et al. Effects of triple antiplatelet therapy (aspirin, clopidogrel, and cilostazol) on platelet aggregation and P-selectin expression in patients undergoing coronary artery stent implantation. Am J Cardiol 2007;100:610–14 [DOI] [PubMed] [Google Scholar]
- 61.Kim YH, Park SW, Lee SW, et al. Sirolimus-eluting stent versus paclitaxel-eluting stent for patients with long coronary artery disease. Circulation 2006;114:2148–53 [DOI] [PubMed] [Google Scholar]
- 62.Kelbaek H, Klovgaard L, Helqvist S, et al. Long-term outcome in patients treated with sirolimus-eluting stents in complex coronary artery lesions: 3-year results of the SCANDSTENT (Stenting coronary arteries in non-stress/benestent disease) trial. J Am Coll Cardiol 2008;51:2011–16 [DOI] [PubMed] [Google Scholar]
- 63.Baumgart D, Klauss V, Baer F, et al. One-year results of the SCORPIUS study: a German multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patients. J Am Coll Cardiol 2007;50:1627–34 [DOI] [PubMed] [Google Scholar]
- 64.Lu Y-L, Chen Y, Lu S. Effects of antiplatelet therapy in patients with high platelet aggregability after percutaneous coronary intervention. Chin J Cardiovasc Dis 2007;35:793–6 [PubMed] [Google Scholar]
- 65.Menichelli M, Parma A, Pucci E, et al. Randomized trial of sirolimus-eluting stent versus bare-metal stent in acute myocardial infarction (SESAMI). J Am Coll Cardiol 2007;49:1924–30 [DOI] [PubMed] [Google Scholar]
- 66. Suh J, Kim WJ, Seo HS, et al. Clinical outcome of patients with diabetes mellitus and chronic total occlusion after drug-eluting stenting followed by cilostazol treatment. Eurointervention. 2009;5(Supp E) [Google Scholar]
- 67.Ardissino D, Cavallini C, Bramucci E, et al. Sirolimus-eluting vs uncoated stents for prevention of restenosis in small coronary arteries: a randomized trial. JAMA 2004;292:2727–34 [DOI] [PubMed] [Google Scholar]
- 68.Menozzi A, Solinas E, Ortolani P, et al. Twenty-four months clinical outcomes of sirolimus-eluting stents for the treatment of small coronary arteries: the long-term SES-SMART clinical study. Eur Heart J 2009;30:2095–101 [DOI] [PubMed] [Google Scholar]
- 69.Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315–23 [DOI] [PubMed] [Google Scholar]
- 70.Lu YL, Chen YZ, Lv SZ, et al. Effect of cilostazol on restenosis in elderly patients with coronary bare-metal stents implantation. Chin J Geriatr 2006;25:537–78 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.