Abstract
Background:
Mimosa caesalpiniifolia Benth. (Leguminosae) is widely found in the Brazilian Northeast region and markedly contributes to production of pollen and honey, being considered an important honey plant in this region.
Objective:
To investigate the chemical composition of the ethanol extract of leaves from M. caesalpiniifolia by GC-MS after derivatization (silylation), as well as to evaluate the in vitro and in vivo toxicological effects and androgenic activity in rats.
Materials and Methods:
The ethanol extract of leaves from Mimosa caesalpiniifolia was submitted to derivatization by silylation and analyzed by gas chromatography-mass spectrometry (GC-MS) to identification of chemical constituents. In vitro toxicological evaluation was performed by MTT assay in murine macrophages and by Artemia salina lethality assay, and the in vivo acute oral toxicity and androgenic evaluation in rats.
Results:
Totally, 32 components were detected: Phytol-TMS (11.66%), lactic acid-2TMS (9.16%), α-tocopherol-TMS (7.34%) and β-sitosterol-TMS (6.80%) were the major constituents. At the concentrations analyzed, the ethanol extract showed low cytotoxicity against brine shrimp (Artemia salina) and murine macrophages. In addition, the extract did not exhibit any toxicological effect or androgenic activity in rats.
Conclusions:
The derivatization by silylation allowed a rapid identification of chemical compounds from the M. caesalpiniifolia leaves extract. Besides, this species presents a good safety profile as observed in toxicological studies, and possess a great potential in the production of herbal medicines or as for food consumption.
Keywords: Cytotoxicity, derivatization, GC-MS, Leguminosae, Mimosa caesalpiniifolia, toxicology
INTRODUCTION
The Mimosaceae family (or Leguminosae-Mimosoideae) comprises 4000 species distributed in 60 genera, occurring in tropical and subtropical regions, especially in arid regions.[1] The Mimosa Linnaeus is a genus of about 500 species of herbs and shrubs, distributed predominantly in Central and South Americas. Brazil is the main distribution center of the Mimosa genus, with approximately 340 species, and these 60% are endemic in different regions.[2,3] The chemical composition of the Mimosa genus includes primary and secondary metabolites, such as tryptophan-derivative alkaloids (single and β-carboline),[4] isoprenoids (diterpenes, triterpenes, carotenoids and steroids),[5,6] phenolic acids, lignans and flavonoids,[1,7] fatty acids, carbohydrates and amino acids.[8]
The Mimosa caesalpiniifolia Benth. is a native plant in Brazilian Caatinga and Cerrado vegetation, and is widely found in the Brazilian Northeast region. This species is popularly known as “unha-de-gato”, “sabiα”, “angiquinho-sabiα” and “sansão-do-campo”, and presents a high capacity for adaptation and regeneration of the soil, as well as is tolerant to acid soils.[9,10,11] It is used in traditional medical practices in treatment of inflammatory processes.[12] Besides, their dried or green leaves are often used for food consumption as fodder for sheep, goats and cattle, since they have high protein and minerals content.[13] Considering its chemical composition, many triterpenes and phenolic compounds were identified from various parts (leaves, fruits, flowers, twigs and stem barks) of M. caesalpiniifolia.[14]
Considering the pollen analysis of honey, propolis and pollen of Apis mellifera and native stingless bees of Brazilian Northeastern region, M. caesalpiniifolia markedly contributes to production of honey and pollen in this region.[9,15,16] Interestingly, a previously reported palynological analysis has characterized this species as the dominant pollen in the region of Monsenhor Gil City, Piauí State, Brazil.[17] Therefore, these evidences characterize this species as an important honey plant in this region.
This study aims to investigate the chemical composition of the ethanol extract from M. caesalpiniifolia leaves by GC-MS after derivatization (silylation reaction) as well as to evaluate the in vitro and in vivo toxicological effects and androgenic activity in rats.
MATERIALS AND METHODS
Plant material
The leaves of M. caesalpiniifolia were collected in December, 2010, from native forest of Federal University of Piauí (UFPI), Teresina, Brazil (5° 03’ 25.24” S 42° 47’ 42.48” W; elevation of 71 m). The voucher specimen was identified and deposited at Graziela Barroso Herbarium/UFPI under registration number TEPB 26,824.
Extraction
M. caesalpiniifolia leaves were pulverized in a knife mill providing 1500 g of powder, which was macerated with ethanol (4.5 l) and submitted to ultrasonic agitation for 30 minutes daily. The organic phase (supernatant) was filtered every 72 hours. This procedure was performed in triplicate. The collected supernatants were concentrated on a rotary evaporator at reduced pressure to yield 243.1 g (16.2%, w/w) of ethanol extract of M. caesalpiniifolia leaves (Mc-EtOH).
Derivatization by silylation
In a round bottom flask of 5 ml, 3 mg of the Mc-EtOH and 100 μl of the silylating reagent were added, a mixture of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) and trimethylchlorosilane (TMCS) (99:1, v/v). The system was maintained under stirring in inert atmosphere of N2 and heated in an oil bath to 85°C for 1 hour. The reaction was followed by thin layer chromatography (TLC).
Gas Chromatography - Mass Spectrometry analysis
Gas chromatography (GC) analyses were performed on a SHIMADZU GC-17A coupled to a mass spectrometer QP5050A-GCMS equipped with a DB-5 capillary column HT (95% methylpolysiloxane and 5% phenyl, 30 m × 0.25 mm of internal diameter and 0.1 μm film). The GC operating conditions were: injetor temperature: 260°C and the interface temperature: 300°C. The column temperatures was: initial temperature 60°C with heating rate of 6°C.min-1 to 260°C and then to 300°C at a heating rate of 12°C.min-1. The compounds were identified by comparison with the mass spectra of the computational library Wiley 229 and literature data. Helium was used as carrier gas at a constant flow of 1 ml.min-1. The acquisition of the mass spectra was done in Scan mode with the acquisition time of 52.21 min and cutting of the solvent within 2 minutes, mass range between 40 − 650 Daltons, using the electron ionization method (70 eV), 1.5 KV voltage, analyzer quadrupole and ion source to 200°C.
In vitro toxicological evaluation
Cytotoxic activity against brine shrimp (Artemia salina)
The toxicity test brine shrimp (Artemia salina.) was developed according to the methodology of Meyer et al.[18] with modifications. Eggs of Artemia sp. were hatched in a mini-aquarium containing a glass divider, which allowed the migration of the larvae between the two environments: One light and one dark. The mini-aquarium was filled with a saline solution to 16.5 g.l-1, prepared with sea salt and mineral water. Then, the eggs were incubated in the dark and the larvae were attracted by a light source. In test of lethality were used larvae after 24 hours of hatching. The sample was prepared by dissolving 100 mg of Mc-EtOH in 10 ml solution of 1.0% Tween 40, yielding a stock solution of 10 mg.ml-1( 10,000 μg.ml-1). Aliquots of 1.7, 1.4, 1.3 and 0.8 ml were transferred to vials and volume was completed to 2 ml of saline solution, and an aliquot of 0.5 ml of each vial, including the stock solution was transferred to test tubes. Then, 1 ml of saline solution and 10 larvae were added to the tubes and adjusted the volume to 5 ml.
The final concentrations of Mc-EtOH in the sample tubes were 1000, 850, 700, 650 and 400 μg.ml-1, respectively. The control was carried out with salt water (control I) and solution of Tween 40 1.0% (v/v) (control II), under the same conditions of analysis. The test was performed in triplicate. The value of median lethal dose (LD50) was determined by counting the dead brine shrimps after an incubation period of 24 hours. The data were processed in the computer program SPSS15.0 and analyzed by the probit method.[19] Mc-EtOH was considered biologically active when LD50 ≤ 1000 μg.ml-1.
Cytotoxic activity against murine macrophages (MTT Assay)
To evaluate the possible cytotoxicity in vitro induced by Mc-EtOH on mammalian cells, the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was performed. Macrophages obtained from peritoneal cavity of Swiss mice were used. Macrophages were removed by administering 8.0 ml of sterile phosphate buffered saline (PBS) pH 7.4, at 4°C to the abdominal cavity. Macrophages were then added to sterile cell culture plates, at a concentration of 1 × 105 cells per well in RPMI 1640 medium (Sigma, St Louis, USA). Mc-EtOH at concentrations of 100, 50, 25, 12.5, 6.25 and 3.12 mg ml-1 were evaluated in this test.[20]
In vivo toxicological and androgenic evaluation
Animals
Male adult Wistar rats weighing between 180-220 g were used. The animals were kept under 12 hour light and 12 hours of darkness in cages suitable for rats, with a maximum of five animals per cage in a room with air conditioning and free access to water and food.
The studies were conducted in accordance with the requirements of the Ethics Committee on Animal Experimentation of Federal University of Piauí (no. 001/11). The animals were manipulated only when necessary and they were not exposed to any kind of pain and stress caused by noise.
Experimental protocol
Orchiectomy
Each rat was anesthetized by intraperitoneal administration of a combination of ketamine (50 mg.kg-1; CEVA Animal Health, Paulinia, SP, Brazil) and xylazine (11.5 mg.kg-1; CEVA Animal Health, Paulinia, SP, Brazil). Before surgical procedure, plantar reflex were evaluated to check the status of anesthesia. The incisions in the scrotum were performed to expose the testicles, and then, each spermatic cord was plugged and the corresponding testicle was removed. In order to prevent infection and pain in the postoperative period, the animals received intramuscularly an association containing: 300,000 IU Procaine benzylpenicillin, 300,000 IU potassium benzylpenicillin, 600,000 IU benzathine penicillin, 500 mg streptomycin base and 45 mg sodium diclofenac.
The animals were maintained standing for 30 days for complete recovery of the surgical process, with free access to food and water, and observed daily to monitor the healing of animals and their health status.
Daily treatment with Mc-EtOH
Four groups of eight animals each, randomly divided, as follows: G1 (control), G2 (Mc-EtOH 250 mg.kg-1 bw), G3 (Mc-EtOH 500 mg.kg-1 bw) and G4 (Mc-EtOH 750 mg.kg-1 bw). The control group was orally treated with saline solution. All groups were daily treated for 32 days.
Evaluation of the biochemical parameters
After 32 days of treatment, animals were anesthetized with a combination of ketamine and xylazine according to the procedures previously described, and blood samples were collected by cardiac puncture in vials without anticoagulant and added clot activator (BD-Z serum Vacuette clot activator, BD-Surgical Industry Ltd., Juiz de Fora, MG, Brazil). The collected blood samples were centrifuged at 3500 rpm for 5 minutes to separate the serum samples. Biochemical measurements were performed using reagent kits (Labtest, Belo Horizonte, MG, Brazil) for the following serum parameters: Alkaline phosphatase (ALP), aspartate aminotransferase (AST), urea and creatinine.
Histopathological analysis
For evaluation of internal organs, the rats were euthanized by sobredosis of sodium thiopental. Then, prostate, pituitary and adrenal glands, heart, liver and kidneys were dissected, removed and the relative weighed. The tissue sections of excised organs were fixed in formalin buffer (formaldehyde solution 10%) and after 24 hours they were dehydrated with these series of increasing alcohol (70-100%), diafanized in xylene and finally was impregnated and embedded in paraffin according to routine protocol of histological methods.[21] The tissue fragments were sectioned in a thickness of 3.0 μm, subsequently stained with hematoxylin-eosin and then examined by light microscopy.
Statistical Analysis
The results were analyzed by analysis of variance (ANOVA) followed by Student-Newman Keuls × s test and expressed as mean ± SEM (standard error of the mean). The analysis of significance was considered for values P < 0.05. All analyses were performed using SigmaStat® software, version 3.5.
RESULTS AND DISCUSSION
The ethanol extract from leaves of M. caesalpiniifolia yielded 16.2%, and its chemical derivatization followed by GC-MS analysis showed the presence of a large class number of constituents. This method is useful due to possibility to identify compounds of a sample without the need of prior purification. In this reaction, the substitution of active hydrogens from OH, SH or NH groups occurs by trimethylsilyl bonds (SiMe3), decreasing the polarity of molecules, making it more volatile and enabling the analysis by GC-MS. Figure 1 shows the total ion chromatogram (TIC) of Mc-EtOH, where 32 substances were detected and the mass spectra compared with the literature. The identified compounds are shown in Table 1.
Figure 1.
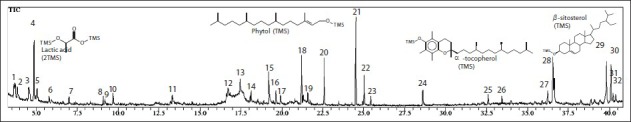
Total ion chromatogram of ethanol extract from leaves of Mimosa caesalpiniifolia (Mc-EtOH) submitted to derivatization (silylation) and analyzed by GC-MS
Table 1.
Compounds identified from Mc-EtOH by GC-MS after derivatization
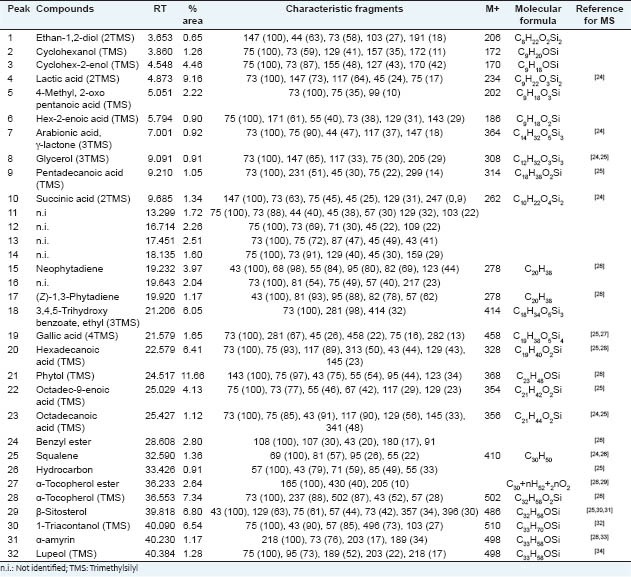
The presence of hydrocarbons, acids, alcohols, isoprenoids and phenolic compounds was observed. The constituents of high relative abundance were phytol-TMS (11.66%), lactic acid-2TMS (9.16%), α-tocopherol-TMS (7.34%) and β-sitosterol-TMS (6.80%). In previous study, β-sitosterol was also identified in the hexane fraction of the leaves, fruits, branches and barks of this species.[14] Gallic acid, also identified, was previously isolated from the aerial parts of another species of the same genus, M. hamata,[22] as well as lupeol was identified in the leaves of M. artemisiana,[23] in the aerial parts of M. hostiles[6] and flowers of M. caesalpiniifolia.[14]
The in vitro cytotoxicity assays are also useful in the study of toxicity of natural products.[35] The MTT method is spectrophotometric analysis, which uses (3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyl tetrazolium bromide), known as MTT, a yellow collor and water-soluble compound. The MTT enters the cells through the plasma membrane and, in contact with superoxide produced by the mitochondrial activity, is oxidized to MTT-formazan, a salt purplish color and insoluble in water. Then, the oxidation of MTT is proportional to the mitochondrial activity and therefore to cell viability.[36]
Another method to evaluate cytotoxicity comprises the brine shrimp (Artemia salina) bioassay, a microcrustacean found in seawater. This bioactivity test may be indicative of antitumor and/or insecticide activity. The toxicity of extracts, fractions and chemical constituents of plants is often evaluated against brine shrimp because it is a simple, rapid and low cost test. The substances tested having lethal dose 50% of the specimens (LD50) of less than 1000 mg.ml-1, are considered active (toxic).[1] This bioassay provides an advantage in evaluation of bioactivity of botanical products with medicinal applications in traditional medical practices, confirming their therapeutic potentials, as well as evaluating their cytotoxic profiles, representing a support for bioguided obtention of plant-derived compounds and further toxicological studies in animal models.[37]
The in vitro toxicity of Mc-EtOH was previously analyzed in order to cause no further damage in evaluating the possible in vivo toxicity. The Mc-EtOH showed LC50 of 1765 mg.l-1 against Artemia salina, and 706.5 mg.l-1, with confidence interval between 412.3 and 1210 mg.l-1 against murine macrophages in the MTT assay. Then, these results indicated absence of toxicity for Mc-EtOH, and reinforcing the food properties due to safe intake of the aerial parts from M. caesalpiniifolia by ruminants.[13]
Even Mc-EtOH did not shown toxicity against Artemia salina and murine macrophages, in vivo toxicological evaluation was carried out in rats. Additionally, the toxicity against the male reproductive system, represented by the androgenic activity, was investigated. Body weight, internal organs with highest blood flow and metabolic activity were evaluated.
Evaluation of androgenic activity is related to the reproductive system. The term androgenic comes from the greek, where andro means man and gennan produce. Therefore, the definition of a biological androgen is any substance capable of producing specifically the growth of male reproductive system.[38] According Golan et al.,[39] androgens, such as dehydroepiandrosterone (DHEA), androstenedione, testosterone and dihydrotestosterone (DHT), possess masculinizing properties. Among these, testosterone, a circulating androgen, and DHT, an intracellular androgen, are the classic androgens. Androgens are important for the development of a male phenotype during male development and sexual maturation.
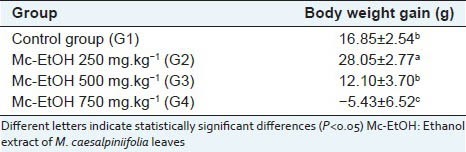
Table 2 shows the body weight gain of castrated rats submitted to oral administration of Mc-EtOH during 32 days. The treatment with Mc-EtOH 250 mg.kg-1 (G2) induced a high body weight gain compared with control group (G1), probably related to a high nutritional value or a stimulus to food consumption. At dose of 500 mg.kg-1 (G3) body weight gain does not significantly varied compared with control group. Otherwise, oral treatment with 750 mg.kg-1 (G4) promoted a significant body weight loss during treatment. These results suggest that Mc-EtOH may provide nutritional value and then body weight gain, but the opposite effect in increasing doses, probably due to a toxic response.
Table 2.
Body weight gain of rats after treament with Mc-EtOH
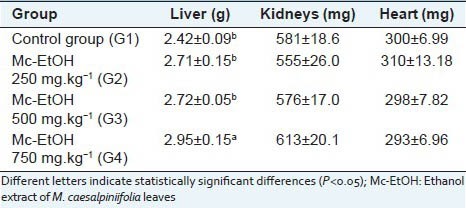
The evaluation of biochemical parameters did not induced any significant change in serum levels of alkaline phosphatase (ALP), AST, urea and creatinine [Table 3], which indicates that Mc-EtOH did not promote liver and kidney damages. Accordingly, there was no observed tissue damages in the organs analyzed (heart, liver and kidneys) at any tested doses in macroscopical observation as well as in the histolopathological analysis. At the dose of 750 mg.kg-1, a significant augment in liver weight (hepatomegaly) was observed, contrasted with any observed biochemical and histological changes related to this organ [Table 4]. On the other hand, there was not significant change in kidney and heart weights in any tested doses. These results indicate the absence of toxicity for Mc-EtOH according to the measured parameters, which are consistent with the biochemical results, where any damage in these organs was observed.
Table 3.
Serum biochemical parameters of rats after treament with Mc-EtOH
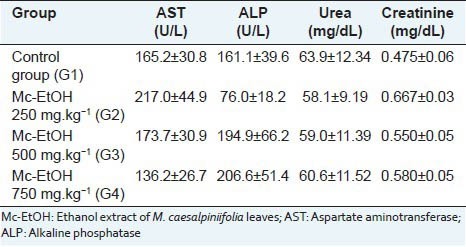
Table 4.
Mean weight of liver, kidneys and heart of rats after treatment with Mc-EtOH
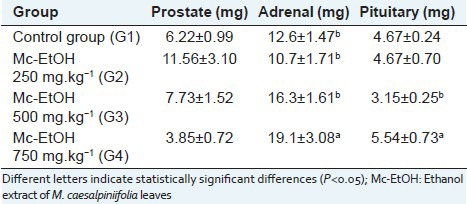
There was no significant differences between weights of prostate of rats treated with Mc-EtOH [Table 5], indicating the absence of androgenic activity. Otherwise, a significant increase of pituitary and adrenal glands weights were observed after treatment at dose of 750 mg.kg-1, as well as a reduction in the body weight. In summary, the adrenal hypertrophy may be indicative of stress, which can lead to tissue catabolism, and lipid and weight loss, leading to a reduction of body weight.
Table 5.
Mean weights of accessory glands (prostate and seminal vesicles) and endocrine (adrenal and pituitary) of rats after treatment with Mc-EtOH
In conclusion, the silylation derivatization was a useful method in the identification of compounds by GC-MS of ethanol extract from leaves of M. caesalpiniifolia. Besides, this extract did not induce in vitro cytotoxicity. However, in vivo toxicological evaluation induced a body weight loss was observed at the highest tested doses, probably as a slight signal of toxicity. Also, the extract has not androgenic activity in any doses. Therefore, this species presents a good safety profile at lower doses, and possess a great potential in the production of nutraceuticals and herbal phytotherapics.
ACKNOWLEDGMENTS
This work was supported by UFPI (Federal University of Piauí, Brazil), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil), FAPEPI (Fundação de Amparo à Pesquisa do Estado do Piauí, Brazil) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Nunes XP, Mesquita RF, Silva DA, Lira DP, Costa VC, Silva MV, et al. Chemical constituents, evaluation of antioxidant and cytotoxic activities of Mimosa paraibana Barneby (Mimosaceae) [in portuguese] Rev Bras Farmacogn. 2008;18:718–23. [Google Scholar]
- 2.Grether R. Nomenclatural changes in the genus mimosa (Fabaceae, Mimosoideae) in Southern Mexico and Central America. Novon. 2000;10:29–37. [Google Scholar]
- 3.Silva AS, Secco RS. A new species of Mimosaceae from Brazilian Amazon, Mimosa dasilvae [in portuguese] Acta Amaz. 2000;30:449–52. [Google Scholar]
- 4.Moraes EH, Alvarenga MA, Ferreira ZM, Akisue G. The nitrogenous bases of Mimosa scabrella Benth [in portuguese] Quím Nova. 1990;13:308–9. [Google Scholar]
- 5.Kudritskaya SE, Fishman GM, Zagorodskaya LM, Chikovani DM. Carotenoids from leaves of Mimosa biuncifera. Chem Nat Compd. 1988;24:258. [Google Scholar]
- 6.Ohsaki A, Yokoyama R, Miyatake H, Fukuyama Y. Two diterpene rhamnosides, Mimosasides B and C, from Mimosa hostilis. Chem Pharm Bull (Tokyo) 2006;54:1728–9. doi: 10.1248/cpb.54.1728. [DOI] [PubMed] [Google Scholar]
- 7.Souza RS, Albuquerque UP, Monteiro JM, Amorim EL. Jurema-Preta (Mimosa tenuiflora [Willd.] Poir.): A review of its traditional use, phytochemistry and pharmacology. Braz Arch Biol Technol. 2008;51:937–47. [Google Scholar]
- 8.Anton R, Jiang Y, Weniger B, Beck JP, Rivier L. Pharmacognosy of Mimosa tenuiflora (Willd) Poiret J Ethnopharmacol. 1993;38:153–7. doi: 10.1016/0378-8741(93)90010-3. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho PE. Sabiá (Mimosa caesalpiniifolia) Circular Ténica da Embrapa. 2007;135:1–7. [Google Scholar]
- 10.Risbaki J, Lima PC, Oliveira VR, Drumond MA. Sabiá (Mimosa caesalpiniaefolia): A tree of multiple uses in Brazil [in portuguese] Comunicado Ténico da Embrapa. 2003;104:1–3. [Google Scholar]
- 11.Agra MF, Silva KN, Basílio IJ, Freitas PF, Barbosa-Filho JM. Survey of Medicinal plants used in the region Northeast of Brazil. Rev Bras Farmacogn. 2008;18:472–508. [Google Scholar]
- 12.Albuquerque UP, Muniz de Medeiros P, de Almeida AL, Monteiro JM, Machado de Freitas Lins Neto E, Gomes de Melo J, et al. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: A quantitative approach. J Ethnopharmacol. 2007;114:325–54. doi: 10.1016/j.jep.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Vieira EL, Carvalho FF, Batista AM, Ferreira RL, Santos MV, Lira MA, et al. Chemical composition of forage and selectivity by bovines of “sabiá” (Mimosa caesalpiniaefolia Benth.) in the rainy and dry seasons [in portuguese] Rev Bras Zootec. 2005;34:1505–11. [Google Scholar]
- 14.Araújo BQ. Teresina, Brazil: Federal University of Piauí; 2010. Chemical and biological studies of Mimosa caesalpiniaefolia Benth. (Leguminosae-Mimosoideae) [in Portuguese]. Master's Degree Dissertation, Graduation Program in Chemistry. [Google Scholar]
- 15.Sodré Gda S, Marchini LC, Carvalho CA, Moreti AC. Pollen analysis in honey samples from the two main producing regions in the Brazilian northeast. An Acad Bras Ciênc. 2007;79:381–8. doi: 10.1590/s0001-37652007000300003. [DOI] [PubMed] [Google Scholar]
- 16.Silva RA, Evangelista-Rodrigues A, Aquino IS, Felix LP, Mata MF, Peronico AS. Characterization of the honey bee flora in the semi-arid of Paraíba, Brazil. Arch Zootec. 2008;57:427–38. [Google Scholar]
- 17.Lima-Neto JS. Teresina, Brazil: Federal University of Piauí; 2009. Pollen of stingless bees (Scaptotrigona sp.) of Monsenhor Gil-PI: pollen analysis, chemical constituents and kinetic study against DPPH [in portuguese]. Master's Degree Dissertation, Graduation Program in Chemistry. [Google Scholar]
- 18.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–4. [PubMed] [Google Scholar]
- 19.Bliss CI. The calculator of the dosage-mortality curve. Ann Appl Biol. 1935;22:134–67. [Google Scholar]
- 20.Rodrigues KA, Amorim LV, de Oliveira JM, Dias CN, Moraes DF, Andrade EH, et al. Eugenia uniflora L. essential oil as a potential anti-leishmania agent: Effects on Leishmania amazonensis and possible mechanisms of action. Evid Based Complement Alternat Med 2013. 2013:279726. doi: 10.1155/2013/279726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacha WJ, Wood LM. 1st ed. Philadelphia: Lea & Febiger; 1990. Color Atlas of Veterinary Histology. [Google Scholar]
- 22.Hussain N, Modan MH, Shabbir SG, Zaidi SA. Antimicrobial principles in Mimosa hamata. J Nat Prod. 1979;42:525–7. doi: 10.1021/np50005a014. [DOI] [PubMed] [Google Scholar]
- 23.Nascimento IA, Braz-Filho R, Carvalho MG, Mathias L, Fonseca FA. Flavonolignoids and other compounds isolated from Mimosa artemisiana Heringer e Paula [in portuguese] Quí Nova. 2012;35:2159–64. [Google Scholar]
- 24.Isidorov VA, Isidorova AG, Sczczepaniak L, Czyzewska U. Gas chromatographic-mass spectrometric investigation of the chemical composition of beebread. Food Chem. 2009;115:1056–63. [Google Scholar]
- 25.FO Silvério. Belo Horizonte, Brazil: Federal University of Minas Gerais; 2008. Characterization of extract of eucalyptus wood and pitch deposits involved in the manufacture of cellulose and paper [in portuguese]. Doctor's Degree Thesis, Graduation Program in Chemistry. [Google Scholar]
- 26.Nguyen-Tu TT, Egasse C, Zeller B, Dereme S. Chemotaxonomical investigations of fossil and extant beeches. I. Leaf lipids from the extant Fagus sylvatica L. C R Palevol. 2007;6:451–61. [Google Scholar]
- 27.Proestos C, Sereli D, Komaitis M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006;95:44–52. [Google Scholar]
- 28.Kuroda K. Pyrolysis-trimethylsilylation analysis of lignin: Preferential formation of cinnamyl alcohol derivatives. J Anal Appl Pyrolysis. 2000;56:79–87. [Google Scholar]
- 29.Pereira AS, Siqueira DS, Elias VO, Simoneit BR, Cabral JA, Aquino Neto FR. Three series of high molecular weight alkanoates found in Amazonian plants. Phytochemistry. 2002;61:711–9. doi: 10.1016/s0031-9422(02)00348-5. [DOI] [PubMed] [Google Scholar]
- 30.Pelillo M, Iafelice G, Marconi E, Fiorenza Caboni M. Identification of plant sterols in hexaploid and tetraploid wheats using gas chromatography with mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:2245–52. doi: 10.1002/rcm.1156. [DOI] [PubMed] [Google Scholar]
- 31.Nierop KG, Naafs DF, Van Bergen PF. Origin, occurrence and fate of extractable lipids in Dutch coastal dune soils along a pH gradient. Org Geochem. 2005;36:555–66. [Google Scholar]
- 32.Haim D, Berríos M, Valenzuela A, Videla LA. Trace quantification of 1-octacosanol and 1-triacontanol and their main metabolites in plasma by liquid-liquid extraction coupled with gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:4145–58. doi: 10.1016/j.jchromb.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 33.Mathe C, Culioli G, Archier P, Vieillescazes C. Characterization of archaeological frankincense by gas chromatography-mass spectrometry. J Chromatogr A. 2004;1023:277–85. doi: 10.1016/j.chroma.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Silva DM. Maceió, Brazil: Federal University of Alagoas; 2006. Chemical study and biological activities of Mansoa hirsuta (Bignoniaceae) [in portuguese]. Master's Degree Dissertation, Graduation Program in Chemistry and Biotechnology. [Google Scholar]
- 35.Harput US, Nagatsu A, Saracoglu I. Antioxidant and cytotoxic effects of Moltkia aurea Boiss. Rec Nat Prod. 2012;6:62–6. [Google Scholar]
- 36.van de Loosdrecht AA, Nennie E, Ossenkoppele GJ, Beelen RH, Langenhuijsen MM. Cell mediated cytotoxicity against V937 cells by human monocytes and macrophages in a modified colorimetric MTT assay. A methodological study. J Immunol Methods. 1991;141:15–22. doi: 10.1016/0022-1759(91)90205-t. [DOI] [PubMed] [Google Scholar]
- 37.Arcanjo DD, Albuquerque AC, Melo-Neto B, Santana LC, Medeiros MG, Citó AM. Bioactivity evaluation against Artemia salina Leach of medicinal plants used in Brazilian Northeastern folk medicine. Braz J Biol. 2012;72:505–9. doi: 10.1590/s1519-69842012000300013. [DOI] [PubMed] [Google Scholar]
- 38.Handa JR, Price RH. Androgen action. In: Fink G, editor. Encyclopedia of Stress. 1st ed. Vol. 1. New York: Academic Press; 2000. [Google Scholar]
- 39.Golan DE, Tashjian AH, Jr, Armstrong EJ, Armstrong AW. 2nd ed. Rio de Janeiro: Guanabara Koogan; 2009. Principles of Pharmacology: The pathophysiologic basis of drug therapy [in portuguese] [Google Scholar]


