Abstract
Background:
Recently, our research group developed MA128, a novel herbal medicine, and demonstrated that MA128 is effective for the treatment of asthma and atopic dermatitis (AD). In particular, postinflammatory hyper-pigmentation in AD mice was improved with MA128 treatment. Thus, in this study, we determined the effect of MA128 on melanogenesis and its underlying mechanism in murine B16F10 melanoma cells.
Materials and Methods:
After treatment with MA128 at 100 and 250 μg/mL and/or alpha-melanocyte stimulating hormone (α-MSH) (1 μM), cellular melanin content and tyrosinase activity in B16F10 cells were measured. Using western blotting, expression levels of tyrosinase, tyrosinase-related protein-1 (TRP-1), TRP-2, microphthalmia-associated transcription factor (MITF), and activation of c-AMP-dependent protein kinase (PKA), c-AMP-related element binding protein (CREB) and mitogen-activated protein kinases (MAPKs) were examined.
Results:
MA128 significantly inhibited melanin synthesis and tyrosinase activity in a resting state as well as α-MSH-stimulating condition, and significantly decreased the expression of tyrosinase, TRP-1, TRP-2 and MITF. In addition, phosphorylation of PKA and CREB by α-MSH stimulation was efficiently blocked by MA128 pretreatment. Moreover, MA128 as an herbal mixture showed synergistic anti-melanogenic effects compared with each single constituent herb.
Conclusion:
MA128 showed anti-melanogenic activity through inhibition of tyrosinase activity mediated by p38 MAPK and PKA signaling pathways in B16F10 cells. These results suggest that MA128 may be useful as an herbal medicine for controlling hyper-pigmentation and as a skin-whitening agent.
Keywords: c-AMP-dependent protein kinase, MA128, melanogensis, microphthalmia-associated transcription factor, p38 mitogen-activated protein kinase, tyrosinase
INTRODUCTION
Melanin, the major pigment of skin and hair color in mammals, has beneficial functions in the photo-protection of human skin from harmful ultraviolet radiation and plays a critical evolutionary role in camouflage and animal mimicry.[1] However, abnormal hyper-pigmentation by overproduction of melanin, such as freckles, melasma, chloasma, senile lentigines and melanoderma incurred by inflammation (e.g. eczema, allergic contact dermatitis, and irritant contact dermatitis) can be an emotionally serious and distressing problem.[2] The tyrosinase gene family, including tyrosinase, tyrosinase-related protein-1 (TRP-1) and TRP-2 is essential for melanin synthesis. Microphthalmia-associated transcription factor (MITF) can regulate the genes encoding tyrosinase and TRP-1, thus substances capable of reducing MITF expression and activity could be potent melanogenesis suppressors.[3,4,5]
Identification of a tyrosinase inhibitor is important in the development of cosmetic products and medicinal drugs treating skin hyper-pigmentation.[5] Some tyrosinase inhibitors, such as arbutin, kojic acid, hydroquinone and stilbene, are used in the cosmetic industry due to their whitening effects. However, such synthetic agents often cause undesirable side-effects in the skin - such as contact dermatitis and erythema.[6] Therefore, alternative novel skin-whitening agents, including naturally occurring compounds with more potent efficacy but less adverse effect are needed for cosmetic and medicinal purposes.[7] Several herbal formulas such as Ssanghwa-tang, Qian-wang-hong-bai-san, and San-bai-tang have been shown to be effective tyrosinase inhibitors.[8,9,10] Properly formulated herbal cocktails may contain numerous phytochemicals and act in cooperation to amplify the efficacy of each single herb with no adverse effect.
Recently, our research group developed a novel herbal medicine, MA and its bioconversion product, MA128. MA was formulated with herbs that were traditionally used to treat allergic disease and inflammation. To achieve therapeutic advantages via increases in absorption and bioavailability of the active components in the body, we fermented MA with Lactobacillus rhamnosus and designated the product MA128. We found that MA and MA128 have anti-asthmatic effects by modulating OVA-specific Th1 (interferon-γ) and Th2 (interleukin-4) cytokines.[11] In addition, MA and MA128 were shown to be effective for the treatment of atopic dermatitis (AD) by reducing clinical features (e.g. severity score, and scratching) of AD and IgE production.[12] Interestingly, MA fermentation significantly enhanced the efficacy for asthma and AD by increasing its indicator molecules. In the AD BALB/c mice model, skin severity score calculated based on the following five major symptoms: Darkening/erythema, edema/population, excoriation, lichenification/prurigo and dryness, was significantly decreased in MA- and MA128-treated groups compared with the untreated group.
In this study, we evaluated the effect of MA128 on melanin production in murine melanoma B16F10 cells and investigated the mechanism underlying its anti-melanogenic activity.
MATERIALS AND METHODS
Cell lines
The murine melanoma B16F10 cell line and immortalized human keratinocyte HaCaT cell line were purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained as a monolayer culture in Dulbecco's Modified Eagle Medium (DMEM; Lonza, Walkersville, MD) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS; GIBCO/Invitrogen, Carlsbad, CA), 100 units/mL penicillin, and 100 μg/mL streptomycin (Welgene, Korea) at 37°C in a humidified 5% CO2 incubator.
Antibodies and reagents
Synthetic melanin, mushroom tyrosinase, L-3,4-Dihydroxyphenylalanine (L-DOPA), alpha-melanocyte stimulating hormone (α-MSH), arbutin, and 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Anti-tyrosinase, anti-TRP-1, anti-TRP-2, anti-MITF, anti-c-AMP-dependent protein kinase (PKA), and anti-phospho-PKA (Thr198) antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Anti-c-AMP-related element binding protein (CREB), anti-phospho-CREB (Ser133), anti-p38, anti-phospho-p38 (Thr180/Tyr182), anti-extracellular signal-related kinase 1/2 (ERK), anti-phospho-ERK (Thr202/Tyr204), anti-c-Jun-N-terminal kinase (JNK), anti-phopsho-JNK (Thr183/Tyr185), and anti-tubulin antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). All of the other chemicals and solvents used were analytical grade.
Preparation and fermentation of novel herbal medicine
All herbs for preparing MA (Glycyrrhizae Radix, Polygoni Cuspidati Rhizoma, Sophorae Radix, Cnidii Rhizoma, Arctii Fructus, Ginseng Radix Alba, Scrophulariae Radix, Zizyphi Semen, Angelica Gigantis Radix, Saposhnikovia divaricata Schischkin, etc., Korean Patent Application no. 10-2010-0093901, PCT/KR2010/007523) were purchased from Korea Medicinal Herbs Association (Yeongcheon, Korea). Identification of all herbs was confirmed by Prof. KiHwan Bae of the College of Pharmacy, Chungnam National University (Daejeon, Korea), and all voucher specimens were deposited in the herbal band in Korea Institute of Oriental Medicine (KIOM, Korea). A total of 1840 g MA formula was soaked in 18.4 L distilled water, boiled for 3 h using an Herb Extractor (Cosmos-600 Extractor, Gyungseo Co., Inchon, Korea), and then filtered through standard testing sieves (150 μm, Retsch, Haan, Germany). Prior to fermentation, decoction MA was adjusted to pH 8.0 using 1 M NaOH and then sterilized by autoclave at 121°C for 5 min. Pure cultures of L. rhamnosus Korea Food Research Institute (KFRI 128) was obtained from the KFRI. L. rhamnosus was prepared by incubation in MRS medium (10 g/L peptone, 10 g/L beef extract, 5 g/L yeast extract, 20 g/L glucose, 1 ml/L Tween 80, 2 g/L K2HPO4, 5 g/L sodium acetate, 2 g/L triammonium citrate, 0.2 g/L MgSO4•7H2O, 0.2 g/L MnSO4•4H2O, pH 6.2-6.6) at 37°C for 24 h. The decoction MA (10 L) was added with 10 ml of L. rhamnosus (1 × 108 CFU/mL) and fermented at 37°C for 48 h. The fermented MA, designated as MA128, was then passed through a 60 μm nylon net filter (Millipore, MA, USA), freeze-dried, and stored in desiccators at 4°C until use.
Cell viability assay
After seeding at a density of 5 × 103 cells/well in 96-well culture plates, cells were allowed to adhere and then incubated with various concentrations of MA128 (50, 100, 250, 500, and 1000 μg/mL) or single herbal extract with individual concentration in MA128 250 μg/mL for the 48 h. After incubation with 10 μL of MTT solution (5 mg/mL in phosphate buffered saline [PBS]) for additional 4 h, the formazan precipitates were dissolved by dimethyl sulfoxide (DMSO) and then absorbance at 570 nm was measured with Infinite® M200 microplate reader (TECAN Group Ltd., Switzerland). Cell viability was presented as the percentage of viable cells compared with untreated, control cells.
Measurement of cellular melanin contents
Cellular melanin content was determined as described previously.[9] In brief, B16F10 cells were seeded at a density of 3 × 105 cells on the 100 mm culture dishes, treated with 100 and 250 μg/mL of MA128 for 12 h, and then stimulated with 1 μM of α-MSH for additional 36 h. Harvested cells (1 × 107 cells/sample) were lysed in 100 μL of 1 N NaOH/10% DMSO for 1 h at 80°C, and solubilized melanin was measured at 475 nm using Infinite® M200 microplate reader. Melanin content was calculated from a standard curve using synthetic melanin.
Measurement of cellular tyrosinase activity
B16F10 cells seeded in 6-well plates (1 × 105 cells/well) were treated with 100 and 250 μg/mL of MA128 for 12 h, and then further incubated with 1 μM of α-MSH for 36 h. To examine cellular tyrosinase activity, cells were lysed with 1% Triton X-100 in PBS by repeated freezing/thawing, and then centrifuged at 12000 rpm for 15 min at 4°C to obtain a supernatant as a source of tyrosinase. The reaction mixture containing the same amount of supernatant (or mushroom tyrosinase) compensated with 50 mM phosphate buffer (pH 6.8) up to 90 μL and 10 μL of 10 mM L-DOPA as a substrate for tyrosinase was incubated at 37°C in a 96-well plate, and then monitored the dopachrome formation from L-DOPA by measuring the absorbance at 475 nm using Infinite® M200 microplate reader. Relative tyrosinase activity was calculated from that of standard mushroom tyrosinase.
Mushroom tyrosinase inhibition assay
Colorimetric tyrosinase inhibition assay was performed as described previously with slight modification.[13] Each sample solution (80 μL) diluted in 50 mM potassium phosphate buffer (pH 6.5) were combined with 20 μL of tyrosinase (10 μg/mL in phosphate buffer, pH 6.5) in triplicate in 96-well microplates. After incubation for 5 min at room temperature, 100 μL of L-DOPA (10 mM in phosphate buffer, pH 6.5) were added into each well, and then incubated for 30 min at room temperature. Optical densities of the wells were determined at 475 nm using Infinite® M200 microplate reader.
Western blot analysis
Whole cell lysates were extracted in M-PER mammalian protein extraction reagent (Thermo Scientific, Rockford, IL) by centrifugation (12000 g × 15 min, 4°C), and the protein concentration was determined using bicinchoninic acid (BCA) Kit (Sigma). Total protein (10-20 μg) was separated by electrophoresis on sodium dodecyl sulfate-polyacrylamide gels, and transferred to Immobilon® -P PVDF transfer membrane (Millipore, Bedford, MA). After immunoblotting, proteins were visualized using a PowerOpti-ECL Western blotting detection reagent (Animal Gentetics, Inc., Korea) and an ImageQuant LAS 4000 mini (GE Healthcare, Piscataway, NJ). Band intensities were quantified using ImageJ software (National Institutes of Health, USA).
Statistical analysis
Data are presented as the mean ± standard deviation (SD) values of at least three independent experiments, unless otherwise specified. Statistical significance was analyzed by the two-tailed Student's t-test in Sigma Plot 8.0 software (SPSS Inc., Chicago, IL) and a P < 0.05 was considered as statistically significant.
RESULTS
MA128 at noncytotoxic concentrations suppressed melanin production and tyrosinase activity in B16F10 cells
First, MA128 cytotoxicity in B16F10 cells was determined by MTT assay. MA128 at concentrations up to 250 μg/mL did not cause any noticeable cytotoxicity during incubation for 48 h, but MA128 at higher doses slightly reduced the number of viable cells [Figure 1a]. Thus, in all subsequent experiments, MA128 was used at 100 and 250 μg/mL. We evaluated the cytotoxicity of MA128 when applied as a therapeutic agent to human skin by examining the effect of MA128 on human keratinocyte HaCaT cells. As shown in Figure 1a, MA128 did not cause cytotoxicity in HaCaT cells, suggesting that MA128 is safe for therapeutic use. During incubation, B16F10 cells produced a substantial amount of melanin, resulting in a black-pigmented cell pellet. Treatment with MA128 at 100 and 250 μg/mL for 48 h reduced melanin accumulation in cells [Figure 1b] and melanin content to 68.9 ± 6.4% and 21.2 ± 4.2% of that in untreated control cells, respectively [Figure 1c]. Next, we investigated the effect of MA128 on the cellular tyrosinase activity by measuring L-DOPA oxidation using cell lysates as a source of tyrosinase. MA128 treatment with 100 and 250 250 μg/mL for 48 h decreased tyrosinase activity by 26.2 ± 4.1% and 51.4 ± 4.3%, respectively, compared with untreated controls [Figure 1d]. These results provide a pharmacological basis for the use of MA128 in skin-whitening.
Figure 1.
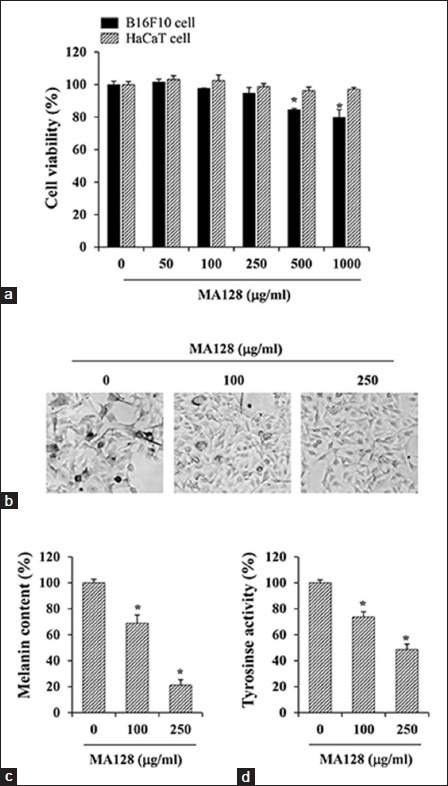
Effects of MA128 on the cell viability and melanogenic activity in B16F10 cells. (a) B16F10 cells and HaCaT cells seeded onto a 96-well culture plate were incubated with indicated concentrations of MA128 for 48 h, and then the cell viability was estimated by 3-4,5-Dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide assay. (b) B16F10 cells were incubated with or without 100 and 250 μg/ml MA128 for 48 h, and then observed for the accumulation of melanin under phase contrast microscope. Image magnification, ×200. (c) B16F10 cells (1 × 107) incubated for 48 h with or without 100 and 250 μg/ml MA128 were determined for the melanin content. (d) Cellular tyrosinase activity in B16F10 cells was examined by measuring the L-3,4-Dihydroxyphenylalanine oxidation. Each percentage value was calculated by comparing to that of untreated “control” cells. Data are expressed as the mean ± standard deviation of two independent experiments. *P < 0.05 versus untreated control
MA128 down-regulated the levels of melanogenic enzymes and inhibited the phosphorylation of p38 mitogen-activated protein kinase (MAPK) in B16F10 cells
To elucidate the anti-melanogenic mechanism of MA128, we examined the expression of major signaling molecules involved in melanin synthesis by western blotting in cells treated with 100 and 250 μg/mL MA128 for 48 h. MA128 treatment at 250 μg/mL significantly reduced tyrosinase, TRP-1, TRP-2 and MITF levels by 92.8 ± 0.9%, 62.3 ± 1.0%, 77.6 ± 0.8% and 58.6 ± 2.0%, respectively, compared with untreated control cells [Figure 2a]. To further investigate mitogen-activated protein kinase (MAPK) pathway involvement in the hypopigmentation of MA128, p38, ERK and JNK phosphorylation were examined by western blotting after treatment of cells with 100 and 250 μg/mL MA128 for 48 h [Figure 2b]. At 50, 100 and 250 250 μg/mL, MA128 decreased phosphorylation of p38 by 15.8 ± 5.2%, 42.1 ± 4.3% and 50.9 ± 6.7%, respectively, compared with MA128-untreated cells. However, MA128 treatment did not significantly influence the phosphorylation of ERK or JNK, suggesting that ERK and JNK are not involved in the anti-melanogenic activity of MA128. These observations suggest that inhibition of p38 MAPK phosphorylation together with reduced levels of MITF and melanogenic enzymes with MA128 treatment led to suppression of melanin synthesis in B16F10 cells.
Figure 2.
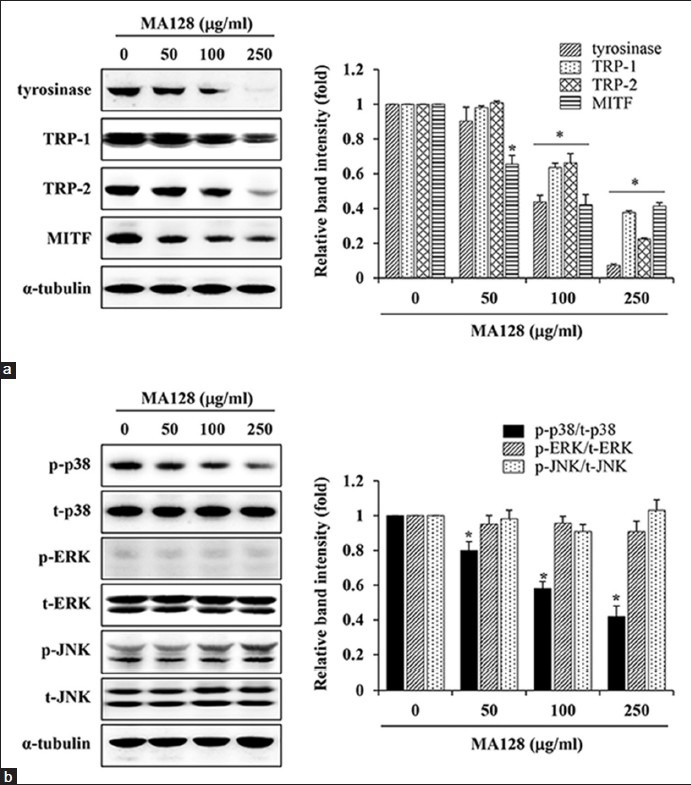
Effects of MA128 on the expression levels of melanogenic proteins in B16F10 cells. (a) B16F10 cells were treated with 50, 100, and 250 μg/mL MA128 for 48 h. Harvested cells were lysed and then examined for the expression levels of tyrosinase, tyrosinase-related protein-1 (TRP-1), TRP-2, and microphthalmia-associated transcription factor by western blotting. After normalization to α-tubulin, relative ratios were quantitated with ImageJ. (b) The levels of p-mitogen-activated protein kinases, including p-p38, p-extracellular signal-related kinase 1/2, and p-c-Jun-N-terminal kinase were also examined by western blotting. Relative ratios of phosphorylated forms to total levels were determined after normalization to α-tubulin expression. Data are expressed as mean ± standard deviation of two independent experiments. *P < 0.05 versus untreated control
MA128 blocked alpha-melanocyte stimulating hormone -induced elevation of melanin synthesis and tyrosinase activity in B16F10 cells
c-AMP-inducing agents, such as α-MSH and forskolin, activate PKA and CREB transcription factor, increase MITF expression, a master regulator of melanogenesis, and consequently induce melanin synthesis.[3,14] As shown in Figure 3a, treatment with α-MSH caused considerable melanin production, resulting in black pigmentation in cells, while pretreatment with MA128 dramatically blocked α-MSH-induced melanin production in a dose-dependent manner. The α-MSH treatment significantly increased melanin content and tyrosinase activity by 265.8 ± 9.8% and 312.5 ± 21.5%, respectively, compared with untreated control cells. These increases were dose-dependently inhibited by MA128 pretreatment; levels in cells treated with 250 μg/mL MA128 were similar to untreated control cells, suggesting that MA128 almost completely blocked α-MSH-mediated melanogenesis [Figure 3b and c]. Arbutin used as a positive control also inhibited α-MSH-induced elevation of melanin synthesis and tyrosinase activity to about 50% of untreated control cells.
Figure 3.
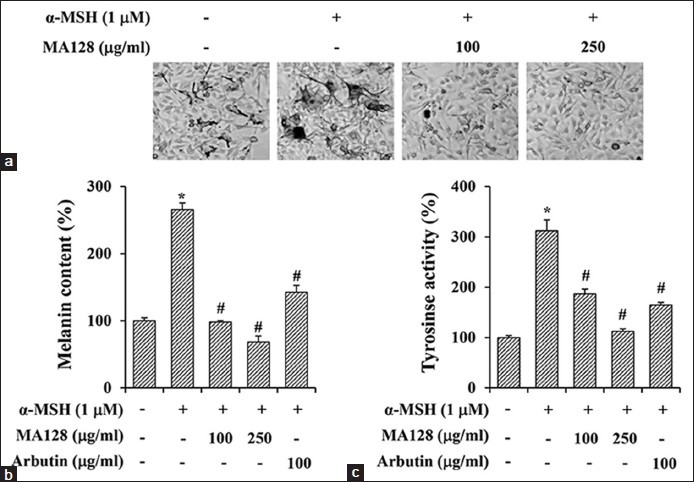
Effects of MA128 on the alpha-melanocyte stimulating hormone (α-MSH)-induced melanogenic activity in B16F10 cells. B16F10 cells were preincubated with or without 100 and 250 μg/ml MA128 for 12 h, and then simulated with 1 μM α-MSH for additional 36 h. (a) Accumulation of melanin in cells were observed under phase contrast microscope. Image magnification, ×200. Melanin content (b) and tyrosinase activity (c) were also determined. Arbutin (100 μg/ml) was used as positive control. Data are expressed as mean ± standard deviation of three independent experiments. *P < 0.05 versus untreated control, #P < 0.05 versus α-MSH stimulation
MA128 suppressed the alpha-melanocyte stimulating hormone -induced increase in the expression of melanogenic enzymes and phosphorylation of c-AMP-dependent protein kinase and c-AMP-related element binding protein in B16F10 cells
In cells stimulated with α-MSH, expression levels of tyrosinase, TRP-1, TRP-2 and MITF were considerably increased. However, pretreatment with 250 μg/mL MA128 significantly suppressed the α-MSH-induced increase in tyrosinase, TRP-1, TRP-2 and MITF by 93.5 ± 1.1%, 97.6 ± 0.7%, 40.5 ± 6.2% and 84.6 ± 1.0%, respectively, compared with expression in cells treated with α-MSH alone [Figure 4a]. To investigate the effect of MA128 on the PKA pathway, the effect of MA128 on α-MSH-induced phosphorylation of PKA and CREB was evaluated by western blotting. Upon stimulation with α-MSH for 30 min, the p-PKA and p-CREB levels were significantly increased by 5.5- and 3.2-fold, respectively, compared with the levels in untreated control cells. In contrast, pretreatment with MA128 (250 μg/mL) significantly decreased α-MSH-induced increase in the levels of p-PKA and p-CREB by 85.2 ± 0.4% and 85.1 ± 0.3%, respectively, compared with levels in cells treated with α-MSH alone [Figure 4b]. α-MSH stimulation and MA128 treatment did not change total expression of PKA and CREB. These results collectively indicate that MA128 regulated signal transduction upstream of c-AMP-induced melanogenesis and inhibited melanin synthesis via down-regulation of melanogenic enzymes.
Figure 4.
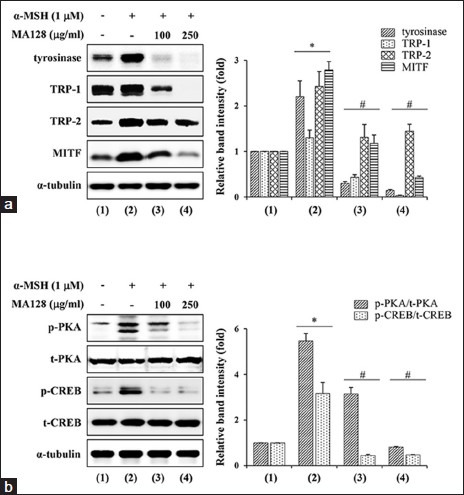
Effects of MA128 on the levels of melanogenic proteins, p-c-AMP-dependent protein kinase (PKA), and p-c-AMP-related element binding protein (CREB) in alpha-melanocyte stimulating hormone (α-MSH)-stimulated B16F10 cells. (a) B16F10 cells preincubated with or without 100 and 250 μg/mL MA128 for 12 h were simulated with 1 μM α-MSH for additional 36 h. After harvest of cells, lysates were subjected to western blotting for the detection of tyrosinase, tyrosinase-related protein-1 (TRP-1), TRP-2, and microphthalmia-associated transcription factor levels. (b) B16F10 cells were preincubated with or without 100 and 250 μg/mL MA128 for 12 h, and then further stimulated with 1 μM α-MSH for 30 min. Changes in the level of p-PKA and p-CREB were examined by western blotting followed by quantitation with ImageJ. Relative ratios were determined after normalization to α-tubulin expression. Data are expressed as mean ± standard deviation of two independent experiments. *P < 0.05 versus untreated control, #P < 0.05 versus α-MSH stimulation
MA128 showed synergistic anti-melanogenic activity
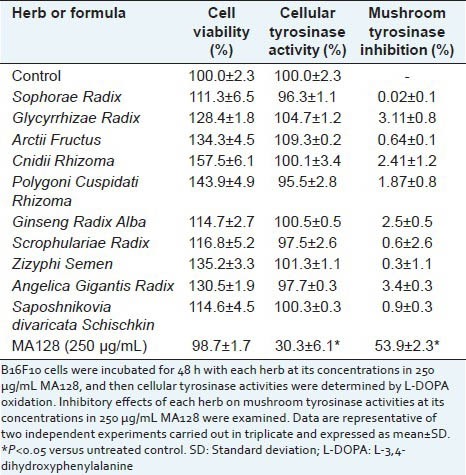
Many medicinal herbs have greater pharmacological efficacy when used together as a cocktail.[9,15,16] Therefore, we evaluated the potential synergistic anti-melanogenic activity of MA128 in comparison with the anti-melanogenic activities of single herbs. After cells were treated for 48 h with each herb at the individual concentrations included in 250 μg/mL MA128, cell viability and cellular tyrosinase activity were measured. As shown in Table 1, at these concentrations, none of the individual herbs caused cytotoxicity, rather increased cell proliferation in B16F10 cells. In addition, most herbs did not individually suppress cellular tyrosinase activity. To confirm the synergistic anti-melanogenic effect of MA128, inhibition of mushroom tyrosinase activity in a cell-free system was measured. Single herbs showed less than 5% inhibitory activity against mushroom tyrosinase, while MA128 had 53.9 ± 2.3% inhibitory effect. The sum of the individual activities was much lower than in MA128, suggesting synergism existed among the multiple herbs present in MA128.
Table 1.
Effects of single medicinal herb of MA128 on the cellular and mushroom tyrosinase activity
DISCUSSION
Melanin is synthesized in the melanosomes of melanocytes, transferred to neighboring keratinocytes through dendritic tips in melanocytes and eventually distributed throughout the epidermis.[17] Melanin synthesis is controlled by melanogenic enzymes including tyrosinase, TRP-1 and TRP-2, which are transcriptionally regulated by MITF. Since melanin is a key determinant of color and tissue tone, tyrosinase inhibitors could potentially treat abnormal pigmentation disorders and be used in skin-whitening cosmetics. Recently, due to the demand for complementary and alternative medicines with increased efficacy and less side effects, many researchers have attempted to identify clinically useful agents from natural resources that can inhibit melanin formation and tyrosinase activity.[7,18]
In this study, we demonstrated that MA128, a novel herbal medicine, reduces melanin synthesis by inhibition of tyrosinase activity and MITF expression via suppression of p38 phosphorylation. In addition, MA128 pretreatment blocked the α-MSH-induced increase in melanin content and tyrosinase activity through suppression of α-MSH-induced PKA and CREB phosphorylation. Treatment with individual herbs at the concentrations present in 250 μg/mL MA128 minimally inhibited tyrosinase activity, indicating synergistic effects on the inhibition of melanin synthesis without unwanted side effects, such as cytotoxicity. Several herbs in MA128, including Sophorae Radix, Glycyrrhizae Radix, Arctii Semen, Ginseng Radix Alba, Angelica Gigantis Radix and Saposhnikovia divaricata Schischkin, reportedly control melanogenesis; however, the effective doses were much higher than the doses in our experiments and potentially cytotoxic. For example, extract of Glycyrrhizae Radix inhibited tyrosinase activity by approximately 25% and 68% at 3.3 μg/mL and 333 μg/mL, respectively.[7] Sophorae Radix and Arctii Semen extract suppressed tyrosinase activity by approximately 25% at 333 and 500 μg/mL, respectively.[19,20,21] In ethanol extract of Ethanolic extract of Angelica Gigantis (AGE), melanin synthesis was significantly inhibited at doses of 5-30 μg/mL in a dose-dependent manner, but at 20, 25 and 30 μg/mL, cell viability was reduced by 10, 20 and 40%, respectively, compared with untreated control cells.[22] In contrast, MA128 is a relatively safe formula because at concentrations up to 1000 μg/mL, no cytotoxicity occurred in normal human keratinocytes [Figure 1a].
In accordance with previous reports that activation of p38 MAPK is essential for melanin synthesis via up-regulation of MITF expression to promote tyrosinase transcription,[10,23,24] MA128 treatment dose-dependently decreased p38 phosphorylation, inhibiting MITF expression, tyrosinase activity and melanin synthesis [Figure 2].
In HPLC PDA analysis, six marker components (liquiritin, nodakenin, icariin, glycyrrhizin, decursin and decursinol angelate) were identified in MA and MA128.[11,12] In previous studies, topical liquiritin treatment was shown to be effective in the management of melasma, an asymmetrical brown or greyish-brown facial hypermelanosis.[25] In human epidermal melanocytes, icariin reportedly inhibited tyrosinase activity and melanogenesis but exerted potent melanogenic activities in murine B16 melanoma cells.[26] Decursin and decursinol angelate were shown to retain potent inhibitory effects on melanin synthesis.[27] These results suggest that MA128 exerts anti-melanogenic effects via these active components.
Our research group recently reported that MA and MA128 exhibited therapeutic properties for the treatment of AD.[12] In particular, postinflammatory hyper-pigmentation in AD mice was also significantly improved in the MA and MA128 treatment groups, confirming that MA128 inhibits pigmentation. Collectively, these results suggest that MA128 represents a safe herbal medicine for controlling pigmentation disorders and for use in cosmetics
ACKNOWLEDGMENTS
This work has been supported by the a Grant K13050 awarded to Korea Institute of Oriental Medicine (KIOM) from Ministry of Education, Science and Technology (MEST), Republic of Korea.
Footnotes
Source of Support: This work has been supported by the a Grant K13050 awarded to Korea Institute of Oriental Medicine (KIOM) from Ministry of Education, Science and Technology (MEST), Republic of Korea
Conflict of Interest: None declared.
REFERENCES
- 1.Costin GE, Hearing VJ. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–94. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 2.Kim YJ, Kang KS, Yokozawa T. The anti-melanogenic effect of pycnogenol by its anti-oxidative actions. Food Chem Toxicol. 2008;46:2466–71. doi: 10.1016/j.fct.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP, et al. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol. 1998;142:827–35. doi: 10.1083/jcb.142.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naish-Byfield S, Riley PA. Tyrosinase kinetics: Failure of acceleration in oxidation of ring-blocked monohydric phenol substrate. Pigment Cell Res. 1998;11:94–7. doi: 10.1111/j.1600-0749.1998.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 5.Chang TS. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009;10:2440–75. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagawa M, Kawai K, Kawai K. Contact allergy to kojic acid in skin care products. Contact Dermatitis. 1995;32:9–13. doi: 10.1111/j.1600-0536.1995.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee KT, Kim BJ, Kim JH, Heo MY, Kim HP. Biological screening of 100 plant extracts for cosmetic use (I): Inhibitory activities of tyrosinase and DOPA auto-oxidation. Int J Cosmet Sci. 1997;19:291–8. doi: 10.1046/j.1467-2494.1997.171725.x. [DOI] [PubMed] [Google Scholar]
- 8.Ye Y, Chu JH, Wang H, Xu H, Chou GX, Leung AK, et al. Involvement of p38 MAPK signaling pathway in the anti-melanogenic effect of San-bai-tang, a Chinese herbal formula, in B16 cells. J Ethnopharmacol. 2010;132:533–5. doi: 10.1016/j.jep.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Kim A, Yim NH, Im M, Jung YP, Liang C, Cho WK, et al. Ssanghwa-tang, an oriental herbal cocktail, exerts anti-melanogenic activity by suppression of the p38 MAPK and PKA signaling pathways in B16F10 cells. BMC Complement Altern Med. 2013;13:214. doi: 10.1186/1472-6882-13-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsang TF, Ye Y, Tai WC, Chou GX, Leung AK, Yu ZL, et al. Inhibition of the p38 and PKA signaling pathways is associated with the anti-melanogenic activity of Qian-wang-hong-bai-san, a Chinese herbal formula, in B16 cells. J Ethnopharmacol. 2012;141:622–8. doi: 10.1016/j.jep.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 11.Kim DS, Kim SH, Kim BK, Yang MC, Ma JY. Antiasthmatic effects of herbal complex MA and its fermented product MA128. Evid Based Complement Alternat Med 2012. 2012:769508. doi: 10.1155/2012/769508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung TH, Kang TJ, Cho WK, Im GY, Lee GS, Yang MC, et al. Effectiveness of the Novel Herbal Medicine, KIOM-MA, and Its Bioconversion Product, KIOM-MA128, on the treatment of atopic dermatitis. Evid Based Complement Alternat Med 2012. 2012:762918. doi: 10.1155/2012/762918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye Y, Chou GX, Mu DD, Wang H, Chu JH, Leung AK, et al. Screening of Chinese herbal medicines for antityrosinase activity in a cell free system and B16 cells. J Ethnopharmacol. 2010;129:387–90. doi: 10.1016/j.jep.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Buscà R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–9. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- 15.Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell. 2007;130:769–74. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiyohara H, Matsumoto T, Yamada H. Combination Effects of Herbs in a Multi-herbal Formula: Expression of Juzen-taiho-to's Immuno-modulatory Activity on the Intestinal Immune System. Evid Based Complement Alternat Med. 2004;1:83–91. doi: 10.1093/ecam/neh004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schallreuter KU, Kothari S, Chavan B, Spencer JD. Regulation of melanogenesis – Controversies and new concepts. Exp Dermatol. 2008;17:395–404. doi: 10.1111/j.1600-0625.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu W, Gao J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J Investig Dermatol Symp Proc. 2008;13:20–4. doi: 10.1038/jidsymp.2008.8. [DOI] [PubMed] [Google Scholar]
- 19.Hyun SK, Lee WH, Jeong da M, Kim Y, Choi JS. Inhibitory effects of kurarinol, kuraridinol, and trifolirhizin from Sophora flavescens on tyrosinase and melanin synthesis. Biol Pharm Bull. 2008;31:154–8. doi: 10.1248/bpb.31.154. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Son KH, Chang HW, Kang SS, Kim HP. Tyrosinase inhibitory prenylated flavonoids from Sophora flavescens. Biol Pharm Bull. 2003;26:1348–50. doi: 10.1248/bpb.26.1348. [DOI] [PubMed] [Google Scholar]
- 21.Park H, Song KH, Jung PM, Kim JE, Ro H, Kim MY, et al. Inhibitory effect of arctigenin from fructus arctii extract on melanin synthesis via repression of tyrosinase expression. Evid Based Complement Alternat Med 2013. 2013:965312. doi: 10.1155/2013/965312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv N, Koo JH, Yoon HY, Yu J, Kim KA, Choi IW, et al. Effect of Angelica gigas extract on melanogenesis in B16 melanoma cells. Int J Mol Med. 2007;20:763–7. [PubMed] [Google Scholar]
- 23.Smalley K, Eisen T. The involvement of p38 mitogen-activated protein kinase in the alpha-melanocyte stimulating hormone (alpha-MSH)-induced melanogenic and anti-proliferative effects in B16 murine melanoma cells. FEBS Lett. 2000;476:198–202. doi: 10.1016/s0014-5793(00)01726-9. [DOI] [PubMed] [Google Scholar]
- 24.Singh SK, Sarkar C, Mallick S, Saha B, Bera R, Bhadra R. Human placental lipid induces melanogenesis through p38 MAPK in B16F10 mouse melanoma. Pigment Cell Res. 2005;18:113–21. doi: 10.1111/j.1600-0749.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 25.Amer M, Metwalli M. Topical liquiritin improves melasma. Int J Dermatol. 2000;39:299–301. doi: 10.1046/j.1365-4362.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 26.Ye Y, Chou GX, Wang H, Chu JH, Yu ZL. Flavonoids, apigenin and icariin exert potent melanogenic activities in murine B16 melanoma cells. Phytomedicine. 2010;18:32–5. doi: 10.1016/j.phymed.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Lee K, Lee JH, Boovanahalli SK, Choi Y, Choo SJ, Yoo ID, et al. Synthesis of (S)-(+)-decursin and its analogues as potent inhibitors of melanin formation in B16 murine melanoma cells. Eur J Med Chem. 2010;45:5567–75. doi: 10.1016/j.ejmech.2010.09.006. [DOI] [PubMed] [Google Scholar]


