Abstract
Background:
So-Cheong-Ryong-Tang (SCRT), herbal medicine, has been used for the control of respiratory disease in East Asian countries. However, its therapeutic mechanisms, especially an inhibitory effect on inflammatory cell infiltration and airway remodeling in allergic asthma are unclear.
Objective:
The present study investigated the mechanism of antiasthmatic effects of SCRT in allergic asthma in mice.
Materials and Methods:
We investigated the influence of SCRT on levels of interleukin-17 (IL-17), granulocyte/macrophage colony-stimulating factor (GM-CSF), IL-4, and interferon gamma (IFN-γ) in bronchoalveolar lavage fluid (BALF), ovalbumin (OVA)-specific IgE in serum, and histopathological changes in allergen-induced asthma.
Results:
So-Cheong-Ryong-Tang decreased levels of IL-17 and GM-CSF in BALF. IL-4, a Th2-driven cytokine, was also decreased by SCRT, but IFN-γ, a Th1-driven cytokine, was not changed. Levels of OVA-specific IgE in serum were also decreased by SCRT. With SCRT treatment, histopathological findings showed reduced tendency of inflammatory cell infiltration, and prevention from airway remodeling such as epithelial hyperplasia.
Conclusion:
In this study, we firstly demonstrated that regulation of IL-17 and GM-CSF production may be one of the mechanism contributed to a reduction of inflammatory cell infiltration and prevention from airway remodeling.
Keywords: Asthma, granulocyte/macrophage colony-stimulating factor, herbal medicine, interleukin-17, So-Cheong-Ryong-Tang
INTRODUCTION
So-Cheong-Ryong-Tang (SCRT; Chinese name, Xiao-Qing-Long-Tang; Japanese name, Sho-Seiryu-To) is a well-known traditional herbal medicine for allergic rhinitis, bronchitis, bronchial asthma[1,2] and widely used for the control of respiratory diseases in Korea. However, its therapeutic mechanisms, especially an inhibitory effect on inflammatory cell infiltration and airway remodeling in allergic asthma are unclear. Recently, SCRT was known to have an antiallergic activity on airway inflammation in a mouse model.[3] In addition, in vitro analysis shows several antiallergic activity of SCRT including inhibition of histamine release,[4,5] proliferation of eosinophils and basophils.[6,7] Interestingly, SCRT was shown to decrease the expression of the interleukin-4 (IL-4) mRNA, which suppresses Th2 cell development,[1,2] which have relevance to our previous report.[8] These evidences indicate SCRT would exert various immuno-suppressive effects in vivo and in vitro although the molecular mechanism has not been clearly demonstrated.
Throughout the world, asthma is a serious public health problem affecting people of all ages, and is an inflammatory disease of the airways, which may be worsened by numerous extrinsic factors, and continuous exposure to allergens activates asthma.[9] Allergic asthma is characterized by reversible airway obstruction, increased mucus production, infiltration of eosinophils, and nonspecific airway hyper-responsiveness, and its development is interposed by the over-expression of Th2-or Th1-mediated cytokines, including IL-4, IL-5, IL-8, and tumor necrosis factor-α.[10,11]
Interleukin-17, a pro-inflammatory cytokine, inducts neutrophils into the airway via the release of IL-8 from bronchial epithelial cells[12] and arbitrates macrophage accumulation during allergen-induced airway inflammation. It also expends its physiological action by acting directly on airway macrophages and promoting their recruitment and survival[13] and it is possibly associated with the recruitment of eosinophils into the airway.[14,15] It has been demonstrated that IL-17 level was increased in bronchoalveolar lavage fluid (BALF), sputum and blood from patients with asthma.[16,17]
In vitro, IL-17 activates fibroblasts and macrophages for the secretion of granulocyte/macrophage colony-stimulating factor (GM-CSF).[16] GM-CSF plays a fundamental role in the function of eosinophils in allergic asthma.[18] In allergic airway, the concentration of GM-CSF increases.[19,20]
However, the pathophysiological mechanism of asthma is still unclear in despite of the increasing prevalence of asthma, and current treatments are not sufficient.
Only 5-10% of patients with asthma were accounted for severe asthma, but it was reported that a considerable measure of the health care costs associated with this disease, and patients with this severe asthma are particularly difficult to treat.[21,22,23,24] Current therapies incompletely control symptoms of asthma and even intensive care having little outcome on health care utilization.[24]
Consequently, effort should be made to identify new remedies, preferably of natural origin, for palliating these disorders. We previously reported the strong possibility of SCRT as a complementary or alternative drug to western drug also demonstrated that regulation of Th1/Th2 imbalance may be one of the mechanism contributed to the treatment for respiratory disease by SCRT.[8] In this study, we investigated the mechanisms for the decrease of infiltration of inflammatory cells and prevention of airway remodeling through changes of several cytokines.
MATERIALS AND METHODS
Animal
Eight week-old female Balb/c mice (Orient Bio, Korea) were housed in polypropylene cages at 24°C ± 3°C under 12 h light and dark cycle for 2 weeks prior to the experiment. Mice used in this experiment were fed with standard pellet food and distilled water ad libitum. All experiments were approved by Institutional Animal Care committee of Seoul National University and conducted in accordance with the guidelines of Seoul National University.
Preparation of So-Cheong-Ryong-Tang and reagent
Eight species of dried medicinal herbs composing SCRT were supplied by H-Max Pharmaceutics Ltd., (Seoul, Korea), and were authenticated by one of the authors (SI Cho, an experienced pharmacognosist) at the School of Korean Medicine, Pusan National University, where voucher specimens (No. SKM-SCRT-201-208) were deposited. As shown in Table 1, SCRT was composed of eight species of medicinal herbs and the mixture of ingredients was boiled in 1300 ml of distilled water using herbal solution heater (Dae Woong, Seoul, Korea) for 3 h to make the final volume of 500 ml. The extract was centrifuged at 2200×g for 20 min, and then it was filtered using Whattman filter paper No. 3. The filtrate was condensed using Vacuum Evaporator (EYELA, Tokyo, Japan) and then stored in refrigerator at −20°C until use. Ovalbumin (Grade V) purified from hen egg white was purchased from Sigma-Aldrich (St. Louis, MO, USA). DetoxiGel column (Pierce, New York, USA) was used to detoxify the ovalbumin (OVA) before use.
Table 1.
Components and standard materials of SCRT
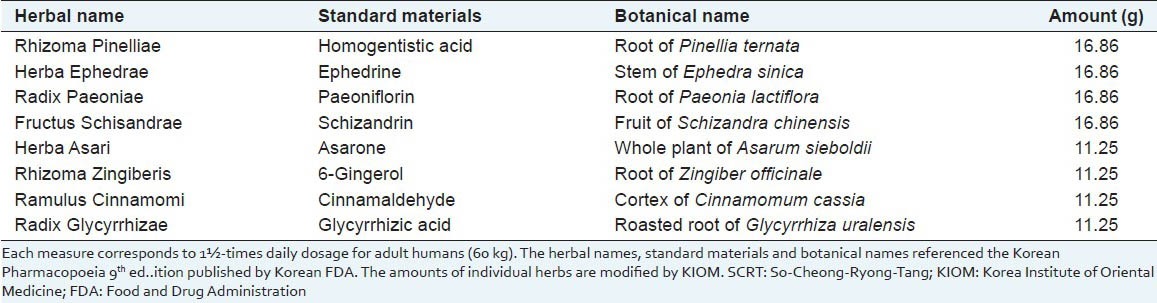
Induction of asthma and categorization of experimental animals
A volume of 100 ml of phosphate buffered saline (PBS) emulsion having 100 μg of OVA and 2 mg of alum were injected to the experimental mice intraperitoneally for 3 consecutive days. In 2 weeks, mice were anesthetized with intraperitoneal injection of ketamine (100 mg/kg) and lompun (10 mg/kg), and were applied intranasal instillation of 30 μl of PBS having 25 μg of OVA for 2 days. Three days after the intranasal instillation of OVA, intranasal instillation was conducted again for 2 days. SCRT was given every morning for 6 consecutive days [Figure 1]. The dosage of SCRT was 4 times higher than that of adult human. The dosage of SCRT administration was ascertained considering basal metabolic rates and body weights of experimental animals in our previous experiments.[8]
Figure 1.
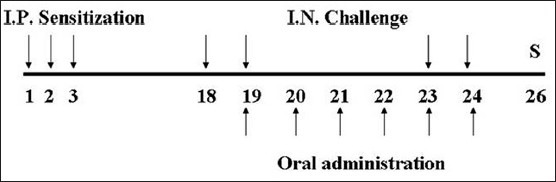
Experimental schedule. So-Cheong-Ryong-Tang (SCRT) and control group were sensitized intraperitoneally at days 1, 2, and 3 and challenged intranasally at days 18, 19, 23, and 24. Animals were administrated with SCRT from days 19 to 24. All animals were sacrificed at day 26. I.P.: Intraperitoneal; I.N.: Intranasal; S: Sacrifice
The experimental groups as follows. (1) Normal: Neither sensitized nor challenged, fed with D/W. (2) Control: Sensitized and challenged with OVA, fed with D/W. (3) SCRT: Sensitized and challenged with OVA, fed with SCRT.
Cytokine detection in bronchoalveolar lavage fluid
A lethal dose of ketamine and lompun was injected to mice intraperitoneally to sacrifice. BALF was collected via insertion of silicon tube to the trachea. Mice were given 1.8 ml of PBS into lung and >1.5 ml of buffer was retrieved consistently. To detect cytokines, BALF was centrifuged at 1100×g for 10 min. Supernatants were obtained and stored at 4°C.
Interleukin-4, interferon gamma (IFN-γ), IL-17, and GM-CSF levels in BALF were measured according to the method of Chen et al.[25] Briefly, we added BALF or serially diluted cytokine standards in 96-well micro plate (Millipore, Billerica, MA, USA), antibody-linked fluorescent bead and secondary antibody biotin-conjugated were added, and then incubated at room temperature (RT) for 2 h. After washing, streptavidin-phycoerythrin was added, and samples were incubated at RT for 1 h. After washing, the samples were replaced in a 5-ml tube (SPL, Gyoungi-do, Korea), and 300 μl of assay buffer was added. Fluorescence from micro plate was detected using flow cytometry instrument (BD, Franklin Lakes, NJ, USA). Data were analyzed using FlowCytomix Pro software (Bender Medsystems, Vienna, Austria) and expressed as picogram per milliliter.
Ovalbumin-specific IgE detection in serum
The OVA-specific IgE level in serum was measured by ELISA method. Blood samples obtained from mice were placed in the refrigerator at 4°C overnight and centrifuged at 2800×g for 10 min, and sera were obtained. For ELISA, 96-micro well plates (Nunc, Rochester, NY, USA) were coated with 50 μg/ml of detoxified OVA (Grade V, Sigma-Aldrich), which was dissolved in blocking solution (1% skim milk and 0.05% Tween 20 in PBS) at RT for 3 h, and again incubated at 4°C overnight. After washing the plates, blocking solution was added to the plates, and the plates were incubated at RT for 1 h. After washing the plates again, 100 μl of sequentially diluted serum was added in each well of the plates, and the plates were incubated at RT for 2 h. The serum which had been added to the first well was diluted in a ratio of 1:50 with blocking solution. After washing the plates again, alkaline phosphatase-conjugated anti-mouse immunoglobulin was added to each well of the plates, and the plates were incubated at RT for 1 h. After incubation of the plates, p-NPP (Amresco, Solon, OH, USA) was added. A microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) was used to measure the optical density at 405 nm. Secondary antibody used was rat anti-mouse IgE antibody (Southern Biotech, Cat# 1130-04, 1: 1000). From another experiment, we obtained an OVA-specific serum, and it was used as a standard serum. All the titers of antibodies were evaluated from the relative values to this serum.
Histopathologic examination of the lung
The lung and trachea were resected and fixed overnight in Bouin's solution. Specimens were dehydrated in alcoholic series, embedded in paraffin wax, and then sectioned with 5-μm thickness. Sections were placed on glass slide, stained with hematoxylin and eosin, and examined under the light mircoscope.
Statistical analysis
For data analysis, comparing groups for statistical differences were made with the Mann-Whitney's U-test. SPSS version 11.5 (SPSS Inc., Chicago, IL, USA) was the statistical analysis software package that was used for the data analysis. The data in this manuscript are expressed mean ± standard error of the mean. A value of P < 0.05 was considered to be statistical significance.
RESULTS
The effect of So-Cheong-Ryong-Tang on interleukin-17 level in bronchoalveolar lavage fluid
The mice of the control group showed increased levels of IL-17 (26.08 ± 9.18 pg/ml) in BALF, compared with these of the normal group. When SCRT was orally administered to these control mice, IL-17 levels were decreased markedly (11.26 ± 1.56 pg/ml) [Figure 2a]. The control group also showed increased levels of GM-CSF (19.33 ± 5.35 pg/ml). GM-CSF levels were decreased by administration of SCRT (8.46 ± 2.42 pg/ml) [Figure 2b].
Figure 2.
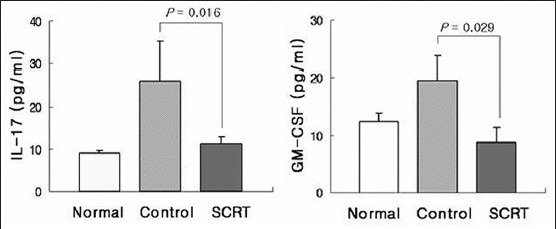
Influences of So-Cheong-Ryong-Tang on levels of interleukin-17 and granulocyte/macrophage colony-stimulating factor. Results are presented as mean ± standard error of the mean P value is represented as compared with control group
The effect of So-Cheong-Ryong-Tang on interleukin-17 level in bronchoalveolar lavage fluid
In the control group, IL-4 levels in BALF were increased (36.57 ± 3.37 pg/ml). These increased levels of IL-4 were significantly decreased by SCRT (15.47 ± 9.06 pg/ml) [Figure 3a]. On the other hand, IFN-γ levels were decreased in the control group (7.90 ± 1.61 pg/ml), and administration of SCRT did not affect decreased levels of IFN-γ (10.15 ± 2.50) [Figure 3b].
Figure 3.
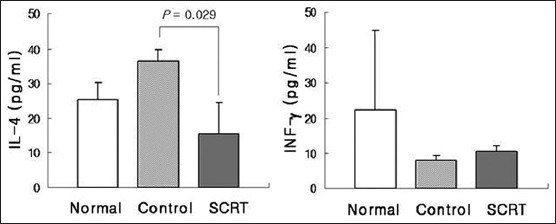
Influences of So-Cheong-Ryong-Tang on levels of interleukin-4 and interferon gamma. Results are presented as mean ± standard error of the mean P value is represented as compared with control group
The effect of So-Cheong-Ryong-Tang on ovalbumin-specific IgE level in serum
Ovalbumin-specific IgE levels were increased markedly in control group. Administration of SCRT decreased >50% of IgE levels elevated in control group [Figure 4].
Figure 4.
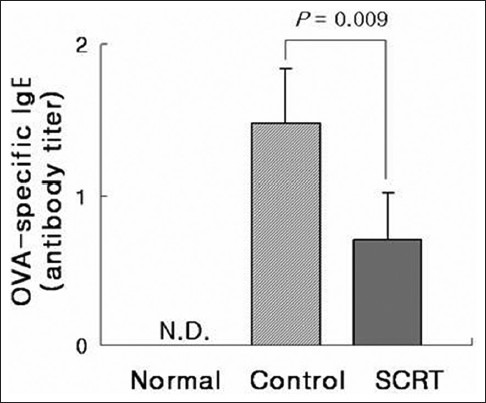
Influence of So-Cheong-Ryong-Tang on ovalbumin-specific IgE production in serum. Results are presented as mean ± standard error of the mean P value is represented as compared with control group. N.D.: Not detectable
The effect of So-Cheong-Ryong-Tang on airway inflammation and hyperplasia
In the control group, trachea and lung sections revealed the infiltration of inflammatory cells including neutrophils and eosinophils into the connective tissue between blood vessels, bronchiole and alveolus. And airway remodeling such as epithelial hyperplasia was also observed. Few inflammatory cells were observed in the normal group. Administration of SCRT suppressed inflammatory cell infiltration and epithelial hyperplasia [Figure 5]. Inflammatory cells were also significantly increased in BALF of control mice and administration of SCRT decreased total cell number including polymorphonuclear cells, macrophages, and lymphocytes (data not shown).
Figure 5.
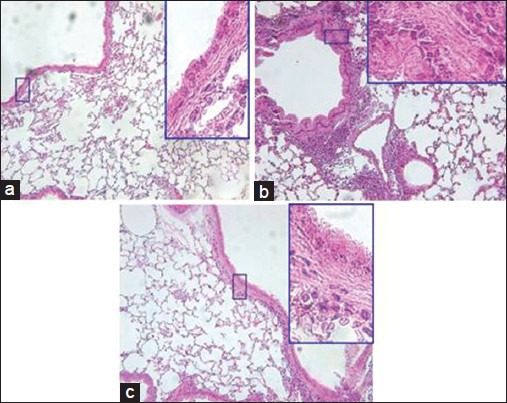
Influences of So-Cheong-Ryong-Tang (SCRT) on inflammatory cell infiltration of asthmatic airway and histological change of lung tissue. The tissue was stained with H and E, and was observed (×200). Highly magnified figures (×1000) are inserted to show details of airway wall structures. (a) Normal, (b) control, (c) SCRT
DISCUSSION
The infiltration and prolonged survival of inflammatory cells are an essential step in the pathogenesis of asthma. IL-17, a pro-inflammatory cytokine, recruits neutrophils, macrophages and eosinophils into the airway.[12,13,14,15] It is well-known that IL-17 activates fibroblasts and macrophages with subsequent secretion of GM-CSF and it is also known that eosinophils are the potential source of IL-17 within the asthmatic airway.[16] In allergic asthma, GM-CSF can regulate the adhesion and chemotaxis of human eosinophils.[18] It has been demonstrated that GM-CSF is of critical importance to promote survival, maturation and function of eosinophils,[26,27,28] and it has also demonstrated that by in vitro activation, eosinophils produce and release GM-CSF.[29] It has been well established that the expressions of IL-17 and GM-CSF increase in the airway tissue of patients with asthma.[16,17]
Although SCRT has been traditionally used to treat allergic disease such as allergic rhinitis, bronchitis, and bronchial asthma in Asian traditional medicine,[1,30] the mechanism of pharmaceutical effect has not been clearly reported yet. In recent, in vivo and in vitro studies showed antiallergic activity of SCRT in which many kinds of immune cells and cytokines is regulated by treatment of SCRT.[2,3,7,31,32]
Our data showed that oral administration of SCRT decreased levels of IL-17 and GM-CSF, which had been elevated in allergen-induced asthma. In SCRT group, histopathological findings showed reduced tendency of inflammatory cell infiltration, and prevention from airway remodeling such as epithelial hyperplasia. The regulation of IL-17, known to activate fibroblasts,[16] and GM-CSF can be a possible mechanism through which SCRT restores several alterations in asthma including histopathological changes.
This study shows that SCRT decreased the number of inflammatory cells through the regulation of IL-17 and GM-CSF. These results can be explained by two possibilities: First, SCRT modulates GM-CSF through the regulation of IL-17, and second, it regulates the two cytokines through the system that interacts independently [Figure 6].
Figure 6.
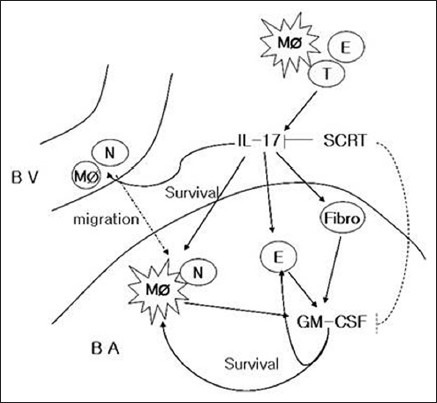
So-Cheong-Ryong-Tang modulates inflammatory cell infiltration and prevents airway remodeling via regulation of interleukin-17 and granulocyte/macrophage colony-stimulating factor in allergic asthma. BV: Bood vessel; BA: Bronchoalveolar space; MØ: Macrophage; N: Neutophil; E: Eosinophil; Fibro: Fibroblast; T: T-cell
It has been reported that SCRT reduces elevated levels of IL-4 in allergic asthma.[3,33] However, the influence of SCRT on the production of IFN-γ remains controversial. Our results indicate that SCRT decrease the production of IL-4, and does not affect that of IFN-γ. In addition, SCRT also lowered production of OVA-specific IgE level, which was increased by induction of asthma. These results imply that SCRT can balance Th1/Th2 ratio through down-regulation of Th2 reaction.
CONCLUSION
We firstly demonstrated that regulation of IL-17 and GM-CSF production may be one of the mechanism contributed to a reduction of inflammatory cell infiltration and prevention from airway remodeling. Further studies are required to elucidate the exact mechanism through which SCRT regulates the production of IL-17 and GM-CSF.
ACKNOWLEDGMENT
This work was generously supported by the Bio-Scientific Research Grant funded by the Pusan National University (PNU, Bio-Scientific Research Grant) (PNU-2010-101-259).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ko E, Rho S, Cho C, Choi H, Ko S, Lee Y, et al. So-Cheong- Ryong-Tang, tradititional Korean medicine, suppresses Th2 lineage development. Biol Pharm Bull. 2004;27:739–43. doi: 10.1248/bpb.27.739. [DOI] [PubMed] [Google Scholar]
- 2.Ko E, Rho S, Lee EJ, Seo YH, Cho C, Lee Y, et al. Traditional Korean medicine (SCRT) modulate Th1/Th2 specific cytokine production in mice CD4+T cell. J Ethnopharmacol. 2004;92:121–8. doi: 10.1016/j.jep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Nagai T, Arai Y, Emori M, Nunome SY, Yabe T, Takeda T, et al. Anti-allergic activity of a Kampo (Japanese herbal) medicine “Sho-seiryu-to (Xiao-Qing-Long-Tang)” on airway inflammation in a mouse model. Int Immunopharmacol. 2004;4:1353–65. doi: 10.1016/j.intimp.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi M, Mase A, Iizuka A, Yuzurihara M, Ishige A, Amagaya S, et al. Further pharmacological study on Sho-seiryu-to as an antiallergic. Methods Find Exp Clin Pharmacol. 1997;19:707–13. [PubMed] [Google Scholar]
- 5.Sakaguchi M, Iizuka A, Yuzurihara M, Ishige A, Komatsu Y, Matsumiya T, et al. Pharmacological characteristics of Sho-seiryu-to, an antiallergic Kampo medicine without effects on histamine H1 receptors and muscarinic cholinergic system in the brain. Methods Find Exp Clin Pharmacol. 1996;18:41–7. [PubMed] [Google Scholar]
- 6.Yamahara J, Yamada T, Kimura H, Sawada T, Fujimura H. Biologically active principles of crude drugs. II. Anti-allergic principles in “Shoseiryu-To” anti-inflammatory properties of paeoniflorin and its derivatives. J Pharmacobiodyn. 1982;5:921–9. doi: 10.1248/bpb1978.5.921. [DOI] [PubMed] [Google Scholar]
- 7.Tanno Y, Shindoh Y, Takishima T. Modulation of human basophil growth in vitro by xiao-qing-long-tang (syo-seiryu-to), chai-pu-tang (saiboku-to), qing-fei-tang (seihai-to), baicalein and ketotifen. Am J Chin Med. 1989;17:45–50. doi: 10.1142/S0192415X89000085. [DOI] [PubMed] [Google Scholar]
- 8.Jung S, Cho SJ, Moon KI, Kim HW, Kim BY, Cho SI. Effects of socheongryong-tang on immunoglobulin production in asthmatic mice. Korean J Herbology. 2008;23:23–8. [Google Scholar]
- 9.Agarwal R, Gupta D. Severe asthma and fungi: Current evidence. Med Mycol. 2011;(49 Suppl 1):S150–7. doi: 10.3109/13693786.2010.504752. [DOI] [PubMed] [Google Scholar]
- 10.Corrigan CJ, Kay AB. The roles of inflammatory cells in the pathogenesis of asthma and of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1991;143:1165–8. doi: 10.1164/ajrccm/143.5_Pt_1.1165. [DOI] [PubMed] [Google Scholar]
- 11.Corry DB. Emerging immune targets for the therapy of allergic asthma. Nat Rev Drug Discov. 2002;1:55–64. doi: 10.1038/nrd702. [DOI] [PubMed] [Google Scholar]
- 12.Barczyk A, Pierzchala W, Sozañska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–33. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 13.Sergejeva S, Ivanov S, Lötvall J, Lindén A. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am J Respir Cell Mol Biol. 2005;33:248–53. doi: 10.1165/rcmb.2004-0213OC. [DOI] [PubMed] [Google Scholar]
- 14.Shen F, Zhao MW, He B, Wang YZ, Yao WZ. The levels and clinical implications of induced sputum interleukin-17 in chronic obstructive pulmonary disease and asthma. Zhonghua Nei Ke Za Zhi. 2004;43:888–90. [PubMed] [Google Scholar]
- 15.Zhou QT, Sun YC, Yao WZ. Characteristics of the airway inflammation and the relationship to interleukin-17 in severe asthma. Zhonghua Jie He He Hu Xi Za Zhi. 2005;28:630–4. [PubMed] [Google Scholar]
- 16.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–8. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 17.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, et al. Airway remodeling-associated mediators in moderate to severe asthma: Effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111:1293–8. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 18.Ip WK, Wong CK, Wang CB, Tian YP, Lam CW. Interleukin-3, -5, and granulocyte macrophage colony-stimulating factor induce adhesion and chemotaxis of human eosinophils via p38 mitogen-activated protein kinase and nuclear factor kappaB. Immunopharmacol Immunotoxicol. 2005;27:371–93. doi: 10.1080/08923970500240925. [DOI] [PubMed] [Google Scholar]
- 19.Alam R, York J, Boyars M, Stafford S, Grant JA, Lee J, et al. Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am J Respir Crit Care Med. 1996;153:1398–404. doi: 10.1164/ajrccm.153.4.8616572. [DOI] [PubMed] [Google Scholar]
- 20.Bodey KJ, Semper AE, Redington AE, Madden J, Teran LM, Holgate ST, et al. Cytokine profiles of BAL T cells and T-cell clones obtained from human asthmatic airways after local allergen challenge. Allergy. 1999;54:1083–93. doi: 10.1034/j.1398-9995.1999.00889.x. [DOI] [PubMed] [Google Scholar]
- 21.Kukulj S, Serdarevic M, Popovic-Grle S. Pharmacotherapy of severe asthma. Lijec Vjesn. 2013;135:268–73. [PubMed] [Google Scholar]
- 22.Peters JB, Rijssenbeek-Nouwens LH, Bron AO, Fieten KB, Weersink EJ, Bel EH, et al. Health status measurement in patients with severe asthma. Respir Med. 2014;108:278–86. doi: 10.1016/j.rmed.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Wysocki K, Park SY, Bleecker E, Busse W, Castro M, Chung KF, et al. Characterization of factors associated with systemic corticosteroid use in severe asthma: Data from the Severe Asthma Research Program. J Allergy Clin Immunol. 2014;133:915–8. doi: 10.1016/j.jaci.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen R, Lowe L, Wilson JD, Crowther E, Tzeggai K, Bishop JE, et al. Simultaneous Quantification of Six Human Cytokines in a Single Sample Using Microparticle-based Flow Cytometric Technology. Clin Chem. 1999;45:1693–694. [PubMed] [Google Scholar]
- 26.Caldenhoven E, van Dijk TB, Tijmensen A, Raaijmakers JA, Lammers JW, Koenderman L, et al. Differential activation of functionally distinct STAT5 proteins by IL-5 and GM-CSF during eosinophil and neutrophil differentiation from human CD34+ hematopoietic stem cells. Stem Cells. 1998;16:397–403. doi: 10.1002/stem.160397. [DOI] [PubMed] [Google Scholar]
- 27.Esnault S, Kelly EA, Schwantes EA, Liu LY, DeLain LP, Hauer JA, et al. Identification of genes expressed by human airway eosinophils after an in vivo allergen challenge. PLoS One. 2013;8:e67560. doi: 10.1371/journal.pone.0067560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esnault S, Malter JS. GM-CSF regulation in eosinophils. Arch Immunol Ther Exp (Warsz) 2002;50:121–30. [PubMed] [Google Scholar]
- 29.Esnault S, Malter JS. Granulocyte macrophage-colony-stimulating factor mRNA is stabilized in airway eosinophils and peripheral blood eosinophils activated by TNF-alpha plus fibronectin. J Immunol. 2001;166:4658–63. doi: 10.4049/jimmunol.166.7.4658. [DOI] [PubMed] [Google Scholar]
- 30.Amagaya S, Iizuka A, Makino B, Kubo M, Komatsu Y, Cheng FC, et al. General pharmacological properties of Sho-seiryu-to (TJ-19) extracts. Phytomedicine. 2001;8:338–47. doi: 10.1078/0944-7113-00061. [DOI] [PubMed] [Google Scholar]
- 31.Chang JS, Yeh CF, Wang KC, Shieh DE, Yen MH, Chiang LC. Xiao-Qing-Long-Tang (Sho-seiryu-to) inhibited cytopathic effect of human respiratory syncytial virus in cell lines of human respiratory tract. J Ethnopharmacol. 2013;147:481–7. doi: 10.1016/j.jep.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 32.Wang SD, Lin LJ, Chen CL, Lee SC, Lin CC, Wang JY, et al. Xiao-Qing-Long-Tang attenuates allergic airway inflammation and remodeling in repetitive Dermatogoides pteronyssinus challenged chronic asthmatic mice model. J Ethnopharmacol. 2012;142:531–8. doi: 10.1016/j.jep.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda Y, Kaneko A, Yamamoto M, Ishige A, Sasaki H. Possible involvement of suppression of Th2 differentiation in the anti-allergic effect of Sho-seiryu-to in mice. Jpn J Pharmacol. 2002;90:328–36. doi: 10.1254/jjp.90.328. [DOI] [PubMed] [Google Scholar]


