Abstract
Background:
Azorella compacta is a rare yellow-green compact resinous cushion shrub growing from the high Andes of southern Perú to northwestern Argentina, and which is a producer of biologically active and unique diterpenoids.
Objective:
This study investigated the secondary metabolites present in a Peruvian sample of Azorella compacta and the evaluation of gastroprotective activity of the isolated compounds in a gastric- induced ulcer model in mice.
Material and Methods:
Six secondary metabolites (diterpenoids 1-6) present in the dichloromethane (DCM) extract of A. compacta growing in Perú were isolated by a combination of Sephadex LH-20 permeation and silica gel chromatography and their chemical structures were elucidated by spectroscopic methods (NMR) and molecular modeling. The gastroprotective activity of the new compound 1 was evaluated on the HCl/EtOH-induced gastric lesion model in mice and compared to the activity showed by the known compounds.
Results:
A new mulinane diterpene along with five known diterpenoids have been isolated from a Peruvian sample of A. compacta and the gastroprotective results show that compound 1 is less active than the other known mulinane diterpenoids isolated.
Conclusions:
A. compacta growing in Perú showed the presence of the new mulinane 1, which was poorly active in the HCl/EtOH-induced gastric lesion model in mice. Indeed, the activity was lower than other diterpenoids (2-6) showing an oxygenated function at C-16 or/and C-20, which confirm the role of an oxygenated group (OH or carboxylic acid) for the gastroprotective activity of mulinane compounds.
Keywords: Apiaceae, azorella compacta, diterpenoids, gastroprotective activity, mulinanes
INTRODUCTION
Terrestrial flora has formed the basis of our traditional medicine systems and even today invokes tremendous interest in the scientific world. There is an optimism placed on natural products in the search of new drugs because the world of the plants represents an untapped reservoir of natural compounds waiting for us. The Azorella genus is well known for a wide range of secondary metabolites such as diterpenoids, triterpenoids and flavonoids present in several species of the genus.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17] In particular Andean species of the genus Azorella, Bolax, Mulinum and Laretia are recognized for the production of diterpenoids having unique mulinane and azorellane skeletons.[1] These diterpenoids have displayed a wide variety of interesting biological activities, including trypanosomicidal,[2] trichomonacidal,[3] toxoplasmocidal,[4] antiplasmodial,[5] antibacterial,[6,7] antiviral,[8] spermicidal,[9] cytotoxic,[10,11] antihyperglycemic,[12] antitubercular,[13,14] antiinflammatory and analgesic activities.[18,19] Azorella compacta known as “llareta” is distributed in the Andes mountains of southern Peru, northeastern Chile, Bolivia, southern Ecuador and northwestern Argentina among boulders in the High-Andean belt at altitudes between 3800-4000 and 4800-5200 m. Llareta is a compact cushion shrub of extremely slow growth (only 1 cm in 20 years).[20] The Andean people utilized this plant since precolombian times for the treatment of colds, pains, diabetes, asthma, bronchitis, womb complaints, gastric disorders, backache, wounds, and altitude sickness.[21] As a continuation of our current chemical studies on the Azorella genus, we report in this work the isolation of five known diterpenoids along with a new mulinane diterpenoid from a sample of A. compacta growing in Perú. Furthermore, the gastroprotective activity of the new isolated diterpenoid against the HCl/ethanol-induced gastric ulcer test in mice and comparison with the known compounds is reported.
MATERIAL AND METHODS
General procedures
Aluminum-coated silica gel thin layer chromatography (TLC, Kieselgel F254) was developed using n-hexane/EtOAc mixtures (1:0; 9:1; 7:3 and 1:1 v/v) as solvent systems while the spots were revealed by spraying the plates with H2SO4-MeOH (5:95, v/v) and heating at 120°C. Silica gel (Kieselgel 60, Merck 0.063-0.200 mm) and Sephadex (LH-20) were used for open column chromatography (CC). Technical solvents used in bulk plant extraction were previously distilled and dried according to standard procedures.
Instrumentation
A Bruker Avance AM-400 spectrometer equipped with 5 mm probes was used for all NMR experiments. Compounds were dissolved in CDCl3 with tetramethylsilane (TMS) as internal standard. 1H-NMR spectrum was obtained at 400.13 MHz;[13] C-NMR spectrum at 100.61 MHz. IR spectra was recorded on a Vector 22 FT-IR spectrometer. Mass spectra were recorded on a MAT 95XP Thermo Finnigan model spectrometer with an accelerating voltage of 3kV and ionization energy of 70 eV. The temperature of the ion source was maintained at 250°C. Optical rotations were obtained in CHCl3 on a Polax-2L ATAGO, polarimeter.
Plant material
Specimens were collected in 2012 at “Patapampa” (4980 m Arequipa, Perú) and identified as Azorella compacta [Figure 1] by the expert botanist Prof. Fatima Cáceres. A voucher specimen (N° AC031112) is kept at the Herbarium of the Departamento de Biología, Universidad Nacional de San Agustin (Arequipa, Perú).
Figure 1.

Photograph of A. compacta from Patapampa Perú
Extraction and isolation
The aerial parts of A. compacta (400 g) were extracted with DCM (2 l) three times for 7 days. The concentrated DCM extract (10.5 g) was submitted to an open silica gel column (10 × 70 cm) using n-hexane/EtOAc mixtures (100:0 to 0:100% v/v) to give five fractions. Fraction 1 (100% n-hexane; 950 mg) was subjected on a Sephadex LH-20 column (7 × 70 cm, n-hexane: DCM: MeOH; 3:1:1 v/v/v) followed by silicagel column chromatography (4 × 50 cm) using as mobile phase only n-hexane to afford 1 (20 mg). Fraction 2 (n-hexane: EtOAc; 8:2; 2.2 g) was permeated through Sephadex LH-20 (7 × 70 cm, n-hexane: DCM: MeOH; 3:1:1 v/v/v) to afford two fraction 2A and 2B. Fraction 2A (200 mg) was submitted to a silica gel column (4 × 30 cm) using n-hexane/EtOAc mixtures (1:0 to 9:1 v/v) to yield 1 (10 mg) and 2[22] (mulin-11,13-dien-20-oic acid; 50 mg). Fraction 2B (1.8 g) was rechromatographed on silica gel (4 × 60cm) using mixtures of n-hexane/EtOAc of increasing polarity as eluents (1:0 to 8:2 v/v) to give 2 (1.1 g) and 3[3] (13α-hydroxyazorellane; 350 mg). Fraction 3 (n-hexane: EtOAc; 6:4; 3.5 g) was permeated on Sephadex LH-20 (7 × 70 cm; n-hexane: DCM: MeOH; 3:2:1 v/v/v) and then was purified over silica gel using open column chromatography (4 × 60 cm) and n-hexane/EtOAc as eluents (0:1 to 0:10 v/v) to yield 3 (100 mg), 4[23] (mulinic acid; 300 mg), 5[24] (mulinolic acid; 1g) and 6[25] (azorellanol; 1.1g). Fraction 4 (n-hexane: EtOAc; 3:7; 1.5 g) and fraction 5 (100% EtOAc; 1.0 g) were permeated on Sephadex LH-20 (7 × 70 cm; MeOH). No presence of diterpenoids or other metabolites were observed in this last polar fraction. The chemical structures of the diterpenoids are shown in Figure 2.
Figure 2.
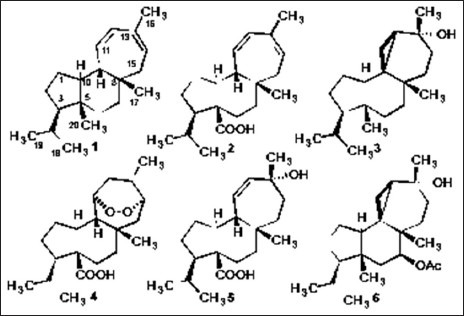
Chemical structures of compounds 1-6
Computational procedures
Conformational analysis of 1a and 1b was carried out using a protocol described in previous publications[26,27] First, a Monte Carlo search was done using the MMFF94 molecular mechanics force field through the SPARTAN 02 program.[28] Next, lower-energy conformers were obtained and then reoptimized using DFT at the B3LYP/6-31G (d) level of theory (GAUSSIAN 09 program).[29] Finally, NOE distances were calculated via GAUSSIAN 09.
Gastroprotective activity
Animals
Swiss albino mice (30 ± 3 g) were purchased at the Instituto de Salud Pública de Chile in Santiago-Chile. Mice were fed on certified Champion diet with free access to water under standard conditions of 12-h dark-light cycle and at 20°C room temperature. The protocols were followed according to the recommendations of the Canadian Council on Animal Care with the ethical guidelines for investigations in conscious animals and were approved by the Universidad de Chile Animal Use and Care Committee (certificate approved on July 2, 2010 by Dr. Nicolas Giuliani).[30]
The gastroprotective activity of the diterpene 1 was evaluated in the HCl/EtOH-induced lesion model as described previously.[31,32] Mice were randomly distributed into groups of seven animals each and fasted for 12 h with free access to water prior to the experiment. Diterpenoid 1 and lansoprazole were suspended in Tween 80 (1% solution), and was administered intragastrically to mice at a dose of 20 mg/kg in a 10 ml/kg vol. Fifty minutes after administration of 1, lansoprazole or 1% Tween 80 (10 ml/kg), all groups were orally treated with 0.2 ml of a solution containing 0.3 M HCl/60% ethanol solution (HCl/EtOH) for gastric lesion induction. Animals were sacrificed 1 h after the administration of HCl/EtOH; the stomachs were excised and inflated by saline injection (1 ml). The ulcerated stomachs were fixed in 5% formalin for 30 min and opened along the greater curvature. Gastric damage visible to the naked eye was observed in the gastric mucosa as elongated black-red lines, parallel to the long axis of the stomach similar to the HCl/EtOH-induced lesions in rats. The length (mm) of each lesion was measured, and the lesion index was expressed as the sum of the length of all lesions.
Statistical analysis
Results are presented as the mean ± sem. In all experiments, statistical differences between treatments and their respective control were determined by one-way analysis of variance (ANOVA) followed by Dunnet's pair-wise test. The level of significance was set at P < 0.01. GraphPad Prism 4 for Windows was used for all statistical tests.
RESULTS
The dichloromethane extract of the leaves of A. compacta afforded the new mulinane diterpenoid 1 and the known compounds mulin-11,13-dien-20-oic acid 2, 13α-hydroxyazorellane 3, mulinic acid 4, mulinolic acid 5, and azorellanol 6, by Sephadex LH-20 permeation and open column silica gel chromatography. The structures of the known diterpenoids 2-6 and the new compound 1 were elucidated by comparison of their NMR data with those found in previous works.[3,5,6,7,22,23,24]
DISCUSSION
Structural elucidation of the new compound 1
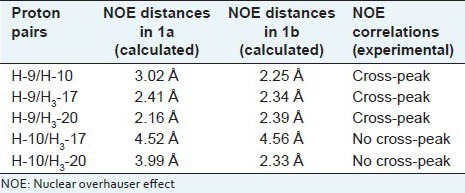
The structure of 1 was mainly elucidated by NMR spectroscopy as follows: The 1H-NMR spectrum of 1 indicated resonances for a diene moiety at δH 5.64 (1H, d, J = 12.7, H-12), 5.52 (1H, dd, J = 12.7; 6.4, H-11), 5.48 (1H, d, J = 8.1, H-14) along with four methyl groups at δH 1.80 s, 0.93 d (6.6), 0.87 s, 0.84 d (6.6) and 0.74 s. These data were similar to the resonances of the mulinane diterpenoids particularly mulin-11,13-dien-20-oic acid, which Loyola et al. earlier reported.[22] The major difference was the presence of an additional methyl group in 1 in place of a carboxylic group in 2. DEPT 135 and 13C-NMR data confirmed the presence of that methyl group (δC 11.8) instead of carboxylic acid (δC 182.1). 13C-NMR data and low-resolution mass spectrometry indicated the molecular formula C20H32 for 1. The structure of 1 was determined as mulin-11, 13-diene. 1D and 2D NMR allowed the correct assignments of all 1H NMR signals for compound 1. In the HMBC spectrum, the proton signal at δH 1.33 (H-10) showed long-range correlations with the carbon signals at δC 11.8 (C-20), 24.1 (C-1) and 44.3 (C-5), and the proton signal at δH 0.72 (H3-20) had cross peaks with δC 57.8 (C-3), 44.3 (C-5), 35.9 (C-6) and 54.6 (C-10) indicating that δC 11.8 to be the C-20 signal. The other known signals were deduced by comparison with the spectra of 2.[22] The relative stereochemistry of 1 was assigned on the basis of 2D NOESY experiment. In the NOESY spectrum, correlations between δH 0.87 (H3-17) and δH 2.02 (H-9), and δH 0.73 (H3-20) and δH 2.02 (H-9) were observed, indicating that they are on the same molecular face of 1. No NOESY correlation was detected between δH 1.33 (H-10) and δH 0.87 (H3-17) and δH 0.73 (H3-20). Surprisingly, a strong NOE interaction was observed between δH 2.02 (H-9) and δH 1.33 (H-10). The last one data is inconsistent with the relative stereochemistry for mulinane diterpenoids. To clarify this data, molecular modeling was used to examine the two possible structures of 1, corresponding to the α (1a) and β (1b) orientations of H-10. Conformational distribution using a Monte Carlo search was carried out to define stable conformations within a 10 kcal/mol window. Low-energy conformers were then submitted to further optimization using B3LYP functional with a 6-31G(d) basis set. Two optimized structures 1a and 1b were obtained [Figure 3], in which the distances between H-9 and H-10, H-17 and H-20 were calculated. In both structures the distances between H-9 and H-10 led to a cross peak in the NOESY spectrum [see Table 1]. However, the NOESY spectrum of 1 showed the expected cross peaks which are consistent with the configuration of the optimized structure 1a and the relative configuration reported for mulinane-type diterpenoids.[3,5,6,7,22,23,24] The spectroscopic and physical data for new compound 1 is presented below.
Figure 3.
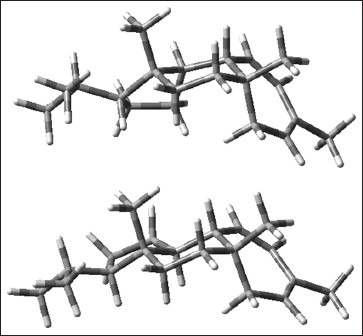
Low-energy structures for 1a and 1b using a Monte Carlo-guided search and geometry optimization calculations at the DFT B3LYP/6-31G(d) level of theory
Table 1.
Calculated distances of key proton pairs for low-energy structures 1a and 1b
Mulin-11,13-diene (1): White oil; FT-IR νmax: 3090, 1440, 1260, 1125 cm-1; HREIMS: Calcd. for C20H32 [M+•] 272.2504, found: 272.2473; EI-MS: m/z (rel. int. %): C20H32 272 [M+•] (4), 257 (2), 247 (4), 245 (68), 242 (13), 227 (24), 191 (66), 175 (19), 163 (12), 161 (28), 149 (33), 147 (33), 136 (27), 135 (43), 133 (25), 123 (31), 121 (83), 119 (41), 117 (9), 109 (51), 107 (100), 105 (48), 95 (54), 91 (48), 85 (26), 82 (47), 79 (53), 77 (35), 69 (44), 67 (46), 55 (64), 53 (27), 43 (48), 41 (61). [1]H NMR (400.13 MHz, CDCl3): 1.62 m; 1.22 m (H-1); 1.82 m; 1.26 m (H-2); 1.00 m (H-3); 1.50 m (H-4); 1.66 m; 1.40 m (H-6); 2.74 brd (16.9); 1.56 m (H-7); 2.02 dd (6.4;8.8) (H-9); 1.33 m (H-10); 5.52 dd (6.4; 12.7) (H-11); 5.63 d (12.7) (H-12); 5.48 d (8.1) (H-14); 1.63 m; 1.23 m (H-15); 1.8 s (H-16); 0.87 s (H-17); 0.84 d (6.6) (H-18); 0.92 d (6.6) (H-19); 0.73 s (H-20).[13] C NMR (100.61 MHz, CDCl3): 24.1 t (C-1); 28.5 t (C-2); 57.8 d (C-3); 31.1 d (C-4); 44.3 s (C-5); 35.9 t (C-6); 36.4 t (C-7); 34.9 s (C-8); 48.9 d (C-9); 54.6 d (C-10); 133.7 d (C-11); 127.6 d (C-12); 131.5 s (C-13); 125.5 d (C-14); 39.0 t (C-15); 25.8 q (C-16); 27.3 q (C-17); 23.1 q (C-18); 22.4 q (C-19); 11.8 q (C-20).
Gastroprotective activity
The effect of mulin-11, 13-diene 1 on the model of gastric ulcers induced by HCl/EtOH in mice is shown in Table 2. A single oral administration of 1 and lansoprazole at 20 mg/kg inhibited the appearance of gastric lesions by 18% and 68%, respectively.
Table 2.
Gastroprotective effect of compound 1 and lansoprazole at 20 mg/kg on HCl/EtOH-induced gastric lesions in mice
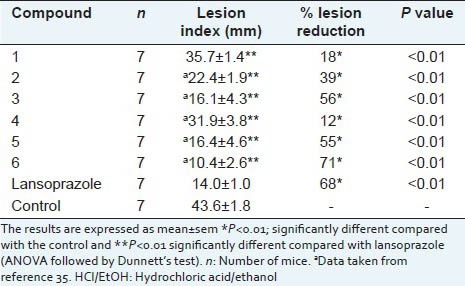
The imbalance between aggressive (chemical agents, gastric acid, pepsin, bile, lysolecithin, pancreatic enzymes, stress and drugs) and protective factors (mucus, PGs, bicarbonate, fast epithelial cell renewal, sulfhydryl compounds and gastric blood flow) in the gastric mucosa may lead to gastric damage.[33] On the other hands, various natural compounds from medicinal plants are a source of gastroprotective agents.[34,35,36,37] In a previous study, several mulinane and azorellane diterpenoids showed gastroprotective activity at 20 mg/kg on the HCl/EtOH-induced gastric lesion model in mice.[38] Among the compounds studied, azorellanol (6), 13β-hydroxyazorellane and mulin-11,13-dien-18-acetoxy-16,20-dioic acid showed the greater gastroprotective effect, being as active as lansoprazole and reducing the gastric lesions by at least 69%. The same study demonstrated that in the mulinane diterpenoid series, the best activity was related to the presence of an additional carboxylic acid. In our study, the hydrocarbon 1 was less active than lansoprazole reducing the gastric lesions by 18%. Clearly, the same trend was observed for the gastroprotective activity when compared to the natural compounds mulin-11,13-dien-20-ol (26%), mulin-11,13-dien-20-oic acid (39%) and mulin-11,13-dien-18-acetoxy-16, 20-dioic acid (73%). Therefore, according to the results, it is suggested that the gastroprotective effect increases with the degree of oxidation at both carbons C-16 and C-20 in mulinane diterpenoids.
CONCLUSION
To the best of our knowledge, no previous studies on the chemistry of A. compacta samples growing in Perú were published, thus this is the first report of secondary metabolites from the Peruvian plant and the presence of the new mulinane compound 1 in the leaves, which was fully elucidated using NMR and molecular modeling techniques. Furthermore, the gastroprotective activity against ulcers in mice was tested for this new compound which was low (18%) compared to the positive control lanzoprasole (68%) and other known mulinanes isolated which indeed confirm the role of an oxygenated group (OH or carboxylic acid) at C-16 and C-20 for the gastroprotective activity of mulinane diterpenoids.
ACKNOWLEDGMENTS
Financial support by FONDECYT DE INICIACION N° 11110241 is gratefully acknowledged.
Footnotes
Source of Support: FONDECYT DE INICIACIÓN N° 11110241
Conflict of Interest: None declared.
REFERENCES
- 1.Loyola LA, Bórquez J, Morales G, San-Martín A, Darias J. Madreporanone, a unique diterpene with a novel skeleton from Azorella madreporica. Tetrahedron Lett. 2002;43:6359–62. [Google Scholar]
- 2.Neira I, Pobleta L, Porcille P, Silva P, Araya J, Bórquez J, et al. Activity of diterpenoids isolated from Azorella compacta (Llareta) on Trypanosoma cruzi amastigotes. Bol Chil Parasitol. 1998;53:9–13. [PubMed] [Google Scholar]
- 3.Loyola LA, Borquez J, Morales G, Araya J, Gonzalez J, Neira I, et al. Diterpenoids from Azorella yareta and their trichomonicidal activities. Phytochemistry. 2001;56:1177–80. doi: 10.1016/s0031-9422(00)00380-0. [DOI] [PubMed] [Google Scholar]
- 4.Loyola LA, Borquez J, Morales G, Araya J, Gonzalez J, Neira I, et al. Azorellane diterpenoids from Laretia acaulis and its toxoplasmacidal activity. J Chil Chem Soc. 2001;46:9–13. [Google Scholar]
- 5.Loyola LA, Borquez J, Morales G, San-Martin A, Darias J, Flores N, et al. Mulinane-type diterpenoids from Azorella compacta display antiplasmodial activity. Phytochemistry. 2004;65:1931–5. doi: 10.1016/j.phytochem.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Wächter GA, Matooq G, Hoffmann JJ, Maiese WM, Singh MP, Montenegro G, et al. Antibacterial diterpenoid acids from Azorella madreporica. J Nat Prod. 1999;62:1319–21. doi: 10.1021/np990134u. [DOI] [PubMed] [Google Scholar]
- 7.Areche C, Vaca I, Loyola LA, Bórquez J, Rovirosa J, San-Martín A. Diterpenoids from Azorella and their Antibacterial Activity. Planta Méd. 2010;76:1749–51. doi: 10.1055/s-0030-1249835. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Malek S, Bastien JW, Mahler WF, Jia Q, Reinecke MG, Robinson WE, Jr, et al. Drug leads from the Kallawaya herbalists of Bolivia. Background, rationale, protocol and anti-HIVactivity. J Ethopharmacol. 1996;50:157–66. doi: 10.1016/0378-8741(96)01380-3. [DOI] [PubMed] [Google Scholar]
- 9.Morales P, Kong M, Pizarro E, Pasten C, Morales G, Borquez J, et al. Effect of azorellanone, a diterpene from Azorella yareta Hauman on human sperm physiology. J Androl. 2003;24:364–70. doi: 10.1002/j.1939-4640.2003.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 10.Mongelli E, Desmarchelier C, Coussio J, Ciccia G. Biological studies of Bolax gummifera, a plant of the Falkland Islands used as a treatment of wounds. J Ethnopharmacol. 1997;56:117–21. doi: 10.1016/s0378-8741(97)01516-x. [DOI] [PubMed] [Google Scholar]
- 11.Mongelli E, Pampero S, Coussio J, Salomon H, Ciccia G. Cytotoxic and DNA interaction activities of extracts from medical plants used in Argentina. J Ethnopharmacol. 2000;71:145–51. doi: 10.1016/s0378-8741(99)00195-6. [DOI] [PubMed] [Google Scholar]
- 12.Fuentes NL, Sagua H, Morales G, Borquez J, San-Martín A, Soto J, et al. Experimental antihyperglycemic effect of diterpenoids of llareta Azorella compacta (Umbelliferae) Phil in rats. Phytother Res. 2005;19:713–6. doi: 10.1002/ptr.1740. [DOI] [PubMed] [Google Scholar]
- 13.Wächter GA, Franzblau SG, Montenegro G, Suarez E, Fortunato RH, Saavedra E, et al. new antitubercular mulinanediterpenoid from Azorella madreporica Clos. J Nat Prod. 1998;61:965–8. doi: 10.1021/np980066w. [DOI] [PubMed] [Google Scholar]
- 14.Molina-Salinas GM, Bórquez J, Ardiles A, Said-Fernández S, Loyola LA, San-Martín A, et al. Antituberculosis activity of natural and semisynthetic azorellane and mulinane diterpenoids. Fitoterapia. 2010;81:50–4. doi: 10.1016/j.fitote.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Loyola LA, Borquez J, Morales G, San-Martin A. Mulinol, A diterpenoid from Azorella compacta. Phytochemistry. 1997;45:1465–7. [Google Scholar]
- 16.Loyola LA, Borquez J, Morales G, San-Martin A. 11,12-epoxy-mulin-13-en-20-oic acid, a diterpenoid from Azorella compacta. Phytochemistry. 1998;49:1091–3. [Google Scholar]
- 17.Araya JE, Neira I, da Silva S, Mortara RA, Manque P, Cordero E, et al. Diterpenoids from Azorella compacta (Umbelliferae) active on Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 2003;98:413–8. doi: 10.1590/s0074-02762003000300022. [DOI] [PubMed] [Google Scholar]
- 18.Delporte C, Backhouse N, Salinas P, San-Martin A, Borquez J. Pharmacotoxicological study of diterpenoids. Bioorg Med Chem. 2003;11:1187–90. doi: 10.1016/s0968-0896(02)00645-4. [DOI] [PubMed] [Google Scholar]
- 19.Borquez J, Loyola LA, Morales G, San-Martín A, Roldan R, Marquez N, et al. Azorellane diterpenoids from Laretia acaulis inhibit nuclear factor-kappa B activity. Phytother Res. 2007;21:1082–6. doi: 10.1002/ptr.2218. [DOI] [PubMed] [Google Scholar]
- 20.Wickens GE. Llareta (Azorella compacta, Umbelliferae): A review. Econ Bot. 1995;49:207–12. [Google Scholar]
- 21.Wickens GE. Vegetation and ethnobotany of the Atacama Desert and adjacent Andes in northern Chile. Opera Bot. 1993;121:291–307. [Google Scholar]
- 22.Loyola LA, Borquez J, Morales G, San-Martin A. Diterpenoids from Azorella compacta. Phytochemistry. 1997;44:649–51. doi: 10.1016/j.phytochem.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Loyola LA, Morales G, Rodriguez B, Jimenez-Barbero J, De la Torre MC, Perales A, et al. Mulinic and isomulinic acids. Rearranged diterpenes with a new carbon skeleton from mulinum crassifolium. Tetrahedron. 1990;46:5413–20. [Google Scholar]
- 24.Loyola LA, Borquez J, Morales G, San-Martin A. Mulinolic acid, a diterpenoid from Mulinum crassifolium. Phytochemistry. 1996;43:165–8. [Google Scholar]
- 25.Loyola LA, Borquez J, Morales G, San-Martin A, Manriquez V, Wittke O. Azorellanol: A diterpenoid with a new carbon skeleton from Azorella compacta. Tetrahedron. 1998;54:15533–40. [Google Scholar]
- 26.Areche C, San-Martin A, Rovirosa J, Soto-Delgado J, Contreras R. An unusual halogenated meroditerpenoid from Stypopodium flabelliforme: Studies by NMR spectroscopic and computational methods. Phytochemistry. 2009;70:1315–20. doi: 10.1016/j.phytochem.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Areche C, San-Martin A, Rovirosa J, Muñoz M, Hernandez-Barragan A, Bucio MA, et al. Stereostructure reassignment and absolute configuration of isoepitaondiol, a meroditerpenoid from Stypopodium flabelliforme. J Nat Prod. 2010;73:79–82. doi: 10.1021/np900553p. [DOI] [PubMed] [Google Scholar]
- 28.Irvine, CA: Wavefunction Inc; 2002. Spartan 02. [Google Scholar]
- 29.Wallingford, CT: Gaussian, Inc; 2009. Gaussian 09. [Google Scholar]
- 30.Olfert ED, Cross BM, McWilliam AA. Ottawa, Ontario: Canadian Council on Animal Care; 1993. Guide to the care and use of experimental animals. [Google Scholar]
- 31.Quesada L, Areche C, Astudillo L, Gutierrez M, Sepulveda B, San-Martin A. Biological activity of isoflavonoids from Azorella madrepórica. Nat Prod Commun. 2012;7:1187–8. [PubMed] [Google Scholar]
- 32.Areche C, San-Martin A, Rovirosa J, Sepulveda B. Gastroprotective activity of epitaondiol and sargaol. Nat Prod Commun. 2011;6:1073–4. [PubMed] [Google Scholar]
- 33.Mozsik GY, Abdel-Salam OM, Szolcsanyi J. Budapest, Hungary: Hungarian Academy of Sciences; 1997. Capsaicin-sensitive afferent nerves in Gastric mucosal damage and protection; pp. 3–22. [Google Scholar]
- 34.Lewis DA, Hanson D. Antiulcer drugs of plant origin. In: Ellis GP, West GB, editors. Progress in Medicinal Chemistry. Amsterdam: Elsevier Science; 1991. pp. 201–31. [DOI] [PubMed] [Google Scholar]
- 35.Tundis R, Loizzo MR, Bonesi M, Menichini F, Conforti F, Statti G, et al. Natural Products as gastroprotective and antiulcer agents: Recents developments. Nat Prod Commun. 2008;3:2129–44. [Google Scholar]
- 36.Mota KS, Dias GE, Pinto ME, Luiz-Ferreira A, Souza-Brito AR, Hiruma-Lima CA, et al. Flavonoids with gastroprotective activity. Molecules. 2009;14:979–1012. doi: 10.3390/molecules14030979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Awaad AS, El-Meligy RM, Soliman GA. Natural products in treatment of ulcerative colitis and peptic ulcer. J Saudi Chem Soc. 2013;17:101–24. [Google Scholar]
- 38.Areche C, Rojas-Alvarez F, Campos-Briones C, Lima C, Perez EG, Sepulveda B. Further mulinane diterpenoids from Azorella compacta. J Pharm Pharmacol. 2013;65:1231–8. doi: 10.1111/jphp.12083. [DOI] [PubMed] [Google Scholar]


