Abstract
Background:
During the process of hepatic fibrosis, the activation of hepatic stellate cells (HSCs) is responsible for the increased formation and reduced degradation of extracellular matrix in the liver. By employing the hepatic stellate cell line, HSC-T6, it was found that the methanol extract of Limonium tetragonum, a halophyte living in salt marsh near south and western seashores of Korea significantly inhibited the proliferation of HSC-T6 cells.
Objective:
In the present study, we attempted to investigate the antifibrotic effects of the mathanolic extract of L. tetragonum (MELT) in the activated HSC-T6 cells.
Materials and Methods:
The proliferation of HSC-T6 was stimulated by culturing environment or platelet-derived growth factor (PDGF-BB) insult, and then the inhibitory activities of MELT were measured.
Results:
It was found that MELT suppressed the proliferation of the activated HSC-T6 in concentration- and time-dependent manners. The increased collagen deposition in the activated HSC-T6 cells was also decreased by the treatment of MELT. The maximal dose of MELT, however, had little effect on primary cultured rat hepatocytes. Wlammatory cytokine, tumor necrosis factor alpha (TNF-α) produced by lipopolysaccharide-stimulated RAW264.7 macrophages was inhibited by MELT.
Conclusion:
Collectively, the above results demonstrated that MELT suppressed HSCs proliferation but not in hepatocytes, implying that L. tetragonum may be useful candidates for developing therapeutic agents for the prevention and treatment of hepatic fibrosis.
Keywords: Antifibrotic, HSC-T6, halophyte, Limonium tetragonum, RAW264.7
INTRODUCTION
Halophytes, the naturally salt-tolerant plants growing in high salinity soil, have a long history of usage for alimentation, for their medicinal value, for their high salt contents. The edible young leaves and shoots of halophytes have been highly demanded by consumers for their salty taste and their high nutritional content which made it an ideal diet supplement. The potential of halophytes as neutraceuticals and/or functional foods are mainly due to the enriched natural antioxidants in halophytes. Halophytes are known to be equipped with powerful antioxidant defense system that can tolerate unfavorable environmental condition, such as high salinity. Enzymatic or nonenzymatic components in halophytes have ability to resist and decline internally generated reactive oxygen species (ROS).[1,2] Some of secondary metabolites including terpenoids (carotenoids, diterpenes, sesquiterpenes and essential oil) and phenolic compounds have been identified so far. These antioxidant components may delay the oxidation of cellular molecules and repair tissue injuries that lead to the prevention of cell death.[3] Recently, Salicornia herbacea has been on the spotlight in Korean industries (dietary, pharmaceutical and cosmetic) as new source of natural antioxidant due to its strong biological activities derived from high contents of useful polyphenols, minerals and vitamins. During search for new source with antioxidant and hepatoprotective activities from halophyte plants (salinity plants) living in foreshore or coastal sand in South Korea, it was found that the methanolic extract of Limonium tetragonum (MELT) showed great anti-proliferative activity in murine hepatic stellate cells, HSC-T6.
Limonium tetragonum (Thunb.) Bullock, which is also known as ‘halophytic carrot’ due to its long straight root looks like carrot is a biennial plant in the family of Plumbaginaceae. The roots and leaves of this plant (known as ‘Jin-Chi-Ye-Cao’ or ‘Bu-Xue-Cao’) have been used in folk medicine to treat uterine hemorrhage, oligomenorrhea, dysgalactia and tinnitus. Various biological activities of Limonium species have been reported including antioxidant, antibacterial, antifungal, antiviral and antileishmanial activities[4,5,6] derived from its active constituents such as myricetin, myricetin glycosides, tannins and caprolactam.[7,8] Amongst the compounds isolated from Plumbaginaceae family, plumbagin has been received most attention in pharmaceutical industry. Plumbagin is a simple hydroxyl-naphthoquinone generally extracted from the roots of Plumbago species. This compound has been ascribed with remarkable medicinal properties especially as potent anticancer agent.[9] Regarding tthe hepatoprotection of limonium species, L. sinensis was found to be hepatoprotective in carbon tetrachloride and beta-D-galactosamine intoxication in rats.[10] The flavonoids-enriched fraction of L. sinensis roots protected the injured rat liver through mitochondrial modulation related to increased expression of voltage-dependent anion channels (VDAC).[11]
Due to the lack of scientific evidences on bioactivity of L. tetragonum, in this study, our work was aimed at investigating antifibrotic activities L. tetragonum in HSCs as well as possible effects in hepatocytes and macrophages.
MATERIALS AND METHODS
Plant material and sample preparation
The whole plants of L. tetragonum were collected from the foreshore in Sinan-gun, Korea in July 2013. A voucher specimen (GNP-70) has been deposited in the laboratory of pharmacognosy, college of life sciences and natural resources, Gyeongnam National University of Science and Technology. The aerial parts of L. tetragonum were dried using freezing-dryer, and then extracted three times with methanol. The methanolic extract was filtered and evaporated in vacuum. The dried extract of L. tetragonum was weighted accurately and dissolved in dimethylsulfoxide (DMSO) for cell culture. Our preliminary study showed that DMSO at a final concentration of 0.1% in media did not affect the cell viability.
Culture of HSC-T6 hepatic stellate cells
An immortalized rat hepatic stellate cell line, HSC-T6 was kindly provided by Prof. SL Friedman (Columbia University, New York). HSC-T6 cells were maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 100 IU/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Assessment of cell viability
For the assay, hepatocytes or HSC-T6 cells were seeded in 48-well plates at a density of 5 × 104 cells/ml and incubated for 24 h. Cells were treated with the sample to be tested at the concentration as indicated for 24 h or 48 h. Inhibitory effect on the proliferation was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were incubated with 2 mg/mL of MTT for 2 h. Reduction of MTT to formazan was assessed in an ELISA plate reader at 450 nm. Data were expressed as the mean of three independent experiments.
Measurement of cell proliferation
Cell proliferation was assessed by bromodeoxyuridine (BrdU) incorporation using a colorimetric ELISA Kit (Roche Diagnostics, GmbH, Mannheim, Germany). HSC-T6 cells were plated and treated as described for the MTT assay. At the end of each treatment period, the medium was discarded and the cell pellet was used for assay according to the manufacturer's instructions.
Measurement of collagen content
The collagen content was quantified by the Sirius Red-based colorimetric assay.[12] Briefly, after treatment, the cultured HSC-T6 cells were washed with PBS, followed by fixation with Bouin's fluid for 1 h. After fixation, the fixation fluid was removed and the culture dishes were washed by immersion in running tap water for 15 min. The culture dishes were air dried and stained by Sirius Red dye reagent for 1 h under mild shaking on a shaker. Thereafter, the solution was removed and the cultures were washed with 0.01 N HCl to remove non-bound dye. The stained material was dissolved in 0.1 N NaOH and the absorbance was measured at 550 nm against 0.1 N NaOH as a blank.
Determination of NO production
RAW264.7 cells were seeded in 48 well plates at a density of 5 × 105 cells/ml and incubated for 24 h. Then, the cell culture was washed and the medium was replaced with Griess medium to remove any trace of phenol red, and treated with sample to be tested for 1 h before exposure to 100 ng/ml of lipopolysaccharide (LPS). After 24 h incubation, nitrite in culture medium was measured to assess NO production in RAW264.7 cells using Griess reagent. The absorbance at 550 nm was measured on a microplate reader and the concentration was determined using nitrite standard curve.
Determination of TNF-a production
RAW264.7 cells were plated overnight in 12 well plates at a density of 5 × 105 cells/ml. The cells were treated with the samples to be tested for 1 h before exposure to 100 ng/mL of LPS. After incubation for 24 h, the supernatants were collected and used for cytokine measurement. The concentration of TNF-a in the culture medium was determined by a mouse TNF enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences, San Jose, CA, USA).
RESULTS
The effects of MELT on the viability of hepatocytes
To exclude the nonselective cytotoxicity of candidates, the effect of MELT on the viability of primary cultured rat hepatocytes was examined using MTT assay. Hepatocytes were intact up to 48 h in response to various doses of MELT [Figure 1]. No cytotoxicity induced by the treatment of MELT itself was observed in the concentration range of 1-50 μg/ml.
Figure 1.
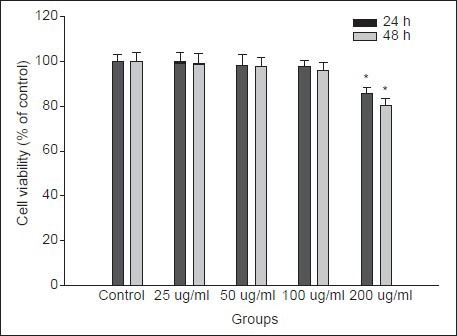
Effect of MELT on cell viability in primary cultured rat hepatocytes. Cells were incubated with MELT at the concentrations of 1–200 μg/ml for 48 h. Cell viability was measured by MTT assay. The percent of cell viability (%) was calculated as 100 × (absorbance of compound-treated/absorbance of control). Data are expressed as the mean ± SD of three independent experiments, each performed using triplicate wells
The effects of MELT on the proliferation of HSCs
The effects of MELT on the cell integrity in HSC-T6 cells were examined using MTT assay. The HSC-T6 cells were treated with 25-100 μg/ml of MELT for 24 and 48 h. The significant cellular loss was observed from the concentration of 50 μg/ml at both incubation times [Figure 2a]. To confirm the inhibitory effect of MELT on cellular proliferation, the BrdU incorporation in HSC-T6 cells was measured. Treatment of HSC-T6 cells with MELT resulted in time- and concentration-dependent growth arrest of cells [Figure 2b]. Platelet-derived growth factor (PDGF) produced by activated macrophages is a potent stimulator of HSCs. The proliferation of HSC-T6 was greatly increased by PDGF-BB insult. The pre-treatment of HSC-T6 with MELT for 1 h suppressed significantly cell proliferation induced by PDGF-BB [Figure 3a and b].
Figure 2.
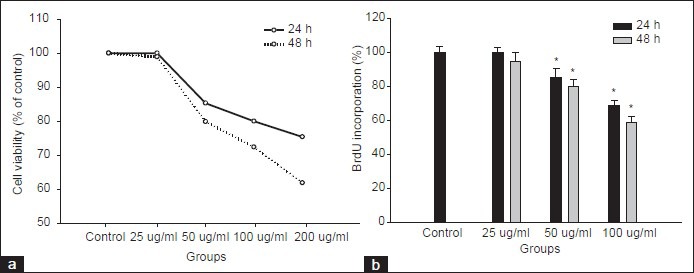
Effect of MELT on cell proliferation and integrity in HSC-T6 cells. HSC-T6 cells were incubated with MELT at the concentrations of 1–100 -g/ml for 24 and 48 h. (a) Cell viability was measured by the MTT assay. Cell proliferation was measured by the BrdU incorporation assay (b). *P < 0.01 compared with non-treated control
Figure 3.
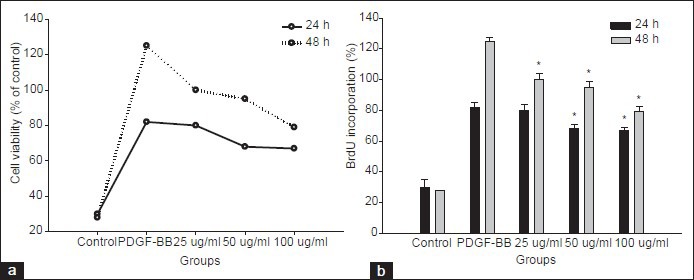
Effect of MELT on PDGF-BB-induced cell proliferation in HSC-T6 cells. HSC-T6 cells were pre-treated with MELT for 30 min followed by PDGF-BB (10 ng/ml) treatment. Cells were further incubated for 24 and 48 h. Cell viability was measured by the MTT assay (a). Cell proliferation was measured by the BrdU incorporation assay (b). *P < 0.01 compared with PDGF-BB-treated only
The effects of MELT on collagen deposition in HSCs
Excessive production and deposition collagen is one of characteristic features observed in the activated HSCs. To assess the effect of MELT on extracellular matrix (ECM) production, the collagen produced in the activated HSC-T6 cells was measured. The amount of collagen produced in HSC-T6 was reduced by the treatment of MELT in a concentration-dependent manner. The treatment of MELT reduced the collagen level by 23.5 ± 3.0%, 30.7 ± 2.2% and 35.2 ± 1.5% over untreated control cells [Figure 4].
Figure 4.
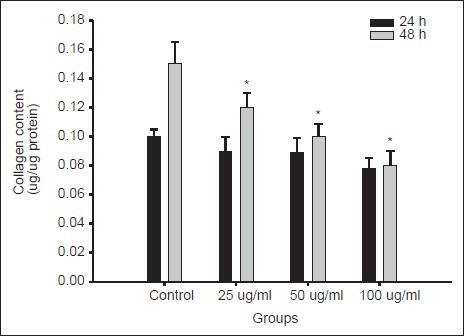
Effects of MELT on collagen deposition in HSC-T6 cells. HSC-T6 cells were incubated with MELT at the concentrations of 25–100 μg/ml for 24 and 48 h. The collagen content was measured by the Sirius Red-based colorimetric assay. Collagen content (mg/mg protein) was calibrated from a standard curve based on reference standard. Data are presented as the mean ± SD of three independent experiments, each performed using triplicate wells *P < 0.01 compared with non-treated control
The effects of MELT on the production of NO and TNF-α in macrophages
Necrosis and apoptosis related hepatic cell death is well related with liver inflammation originating from the products of activated macrophages such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6).[13,14] To understand the possible role of MELT in hepatic fibrosis, the immunomodulatory function was tested. As shown in Figure 5, the pretreatment of MELT significantly attenuated the production of nitric oxide and TNF-α in LPS-activated RAW264.7 macrophage cells. No cytotoxicity of MELT was found in the concentration range of 1–100 μg/ml.
Figure 5.
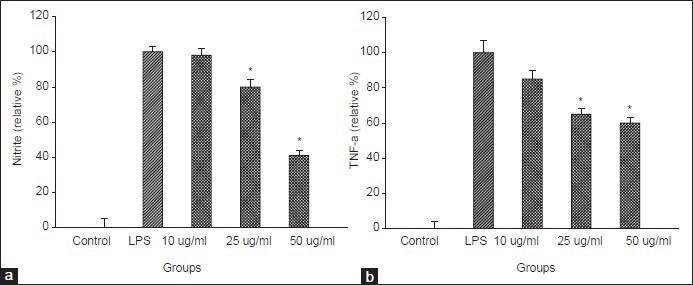
Inhibitory effect of MELT on the production of nitrite and proinflammatory cytokine, TNF-α induced by LPS treatment in RAW264.7 cells. RAW264.7 cells were treated with MELT for 30 min followed by LPS (100 ng/ml) treatment. The content of nitrite was measured by Griess assay, TNF-α by ELISA assay. *P < 0.01 compared with LPS-only treated cells
DISCUSSION AND CONCLUSION
Hepatic fibrosis is well characterized by the increased proliferation, and excessive production and deposition of ECM, and HSCs are considered to play pivotal roles responsible for fibrosis.[15,16,17,18] As a consequence of liver injury caused by persistent viral and helminthic infection, autoimmune, alcoholism, metabolic agents, HSCs undergo phenotypic transformation from vitamin A-storing quiescent cells into myofibroblast-like proliferative.[15,18] The activated HSCs produce a huge amount of ECM, primarily fibrillar collagens I and III,[19] and express alpha-smooth muscle actin that subsequently lead to hepatic fibrosis.[15] The accumulation of ECM proteins disturbs the liver architecture by forming a fibrous scar and the subsequent development of cirrhosis with nodules of regenerating hepatocytes, which often leads to progressive loss of liver function.[20] Hence, antifibrogenic therapy that suppresses the activation of HSCs has been preferentially considered as an attractive target to prevent the pathological progression to cirrhosis in chronic liver diseases.[21,22]
Prior to evaluation of anti-proliferative effects of MELT on HSCs, the cytotoxicity of MELT in primary cultured rat hepatocytes were tested to rule out nonselectivity of the candidates. Our data showed that the hepatocytes were intact by the treatment of MELT up to 48 h at the concentration range of 1–100 μg/ml. Slight cytototoxicity was found at the highest concentration (200 μg/ml). Antiproliferative activities of MELT were measured in HSC-T6 cells, an immortalized rat hepatic stellate cell line. HSC-T6 retains major features of activated stellate cells, including expression of desmin, α-smooth muscle actin, and glial fibrillary acidic protein, and it can esterify retinol into retinyl esters.[23] The activation of HSCs can be promoted by addition of serum, cytokines and other factors,[21,24] and also can be induced by the specific culturing conditions in vitro. Culturing HSCs on uncoated plastic plates is known to cause spontaneous activation leading to myoblastic phenotype, mimicking the process seen in vivo. The accelerated proliferation of HSCs by culturing on plastic plate was found to be attenuated by the treatment of MELT in MTT and BrdU incorporation assay. The proliferation of HSCs was decreased up to 62.5 ± 2.1% over untreated control by the treatment with MELT for 48 h at the concentration of 100 μg/ml [Figure 2]. The content of deposited collagen in HSCs was also decreased significantly by the treatment of MELT in dose-dependent manner [Figure 4].
During liver fibrogenesis, enormous amounts of macrophages are infiltrated into the damaged region of liver, where the pro-fibrogenic cytokines such as interleukin-1, TNF-α and PDGF released by macrophages activates HSCs.[22,25] Autocrine and paracrine signaling via PDGF promotes myofibroblast proliferation and chemotaxis, also stimulates collagen production in HSCs.[26,27,28,29] Upon the binding of PDGF-BB to PDGF receptor, the receptor undergoes dimerization and autophosphorylation on tyrosine residues in intracellular domain. This resulted in the activation of specific signaling pathway including mitogen-activated protein kinase (MAPK) which leads to proliferative and fibrogenic HSCs.[30] In our study, proliferative rate of HSC-T6 were greatly increased by PDGF-BB treatment. The pre-treatment of HSC-T6 with MELT suppressed PDGF-BB-induced cell proliferation in concentration-dependent manners [Figure 3].
It has been known that there are two classified groups of macrophages with the opposite role in inflammation, M1 and M2. Especially, M1 macrophages (pro-inflammatory) polarized by cytokines from Th1 cells might positively regulate hepatic fibrosis.[31,32] TNF-α produced by macrophages activates NF-κB signaling via the activation of IκB-kinase complex. Several in vitro studies have proposed that HSC activation is associated with the elevation of (nuclear factor-κB) NF-κB activity.[33,34] Also in acute and chronic liver diseases in vivo, the elevated hepatic level of TNF-α was observed demonstrating the crucial role of TNF-α associated NF-kB signaling in the activation of HSCs.[35] In this regard, anti-TNF strategy is believed to be beneficial for treating hepatic fibrosis. In our experimental system, in RAW264.7 macrophage cells, the increased production of nitrite or TNF-α in culture media induced by LPS insult was slightly reduced by the pre-treatment of MELT but not significant demonstrating high specificity of MELT on HSCs [Figure 5].
In summary, our data demonstrate that MELT reduced HSCs proliferation and subsequent collagen deposit induced by culturing environmental stimulation or PDGF-BB treatment. Although further mechanistic studies are required to confirm this effect, L. tetragonum might be useful candidate for developing therapeutic agents for the prevention and treatment of hepatic fibrosis. We plan to make a further study identifying active constituents responsible for antifibrotic activity of L. tetragonum, as well as investigating the detailed action mechanism of active constituents.
ACKNOWLEDGEMENTS
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ009804)” Rural Development Administration, Republic of Korea.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol Biochem. 2007;45:244–9. doi: 10.1016/j.plaphy.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Ksouri R, Megdiche W, Falleh H, Trabelsi N, Boulaaba M, Smaoui A, et al. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C R Biol. 2008;331:865–73. doi: 10.1016/j.crvi.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Tepe B, Sokmen A. Screening of the antioxidative properties and total phenolic contents of three endemic Tanacetum subspecies from Turkish flora. Bioresour Technol. 2007;98:3076–9. doi: 10.1016/j.biortech.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Kuo YC, Lin LC, Tsai WJ, Chou CJ, Kung SH, Ho YH. Samarangenin B from Limonium sinense suppresses herpes simplex virus type 1 replication in Vero cells by regulation of viral macromolecular synthesis. Antimicrob Agents Chemother. 2002;46:2854–64. doi: 10.1128/AAC.46.9.2854-2864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nostro A, Filocamo A, Giovannini A, Catania S, Costa C, Marino A, et al. Antimicrobial activity and phenolic content of natural site and micropropagated Limonium avei (De Not.) Brullo and Erben plant extracts. Nat Prod Res. 2012;26:2132–6. doi: 10.1080/14786419.2011.628669. [DOI] [PubMed] [Google Scholar]
- 6.Smirnova GV, Vysochina GI, Muzyka NG, Samoĭlova ZIu, Kukushkina TA, Oktiabr'skiĭ ON. The antioxidant characteristics of medicinal plant extracts from Western Siberia. Prikl Biokhim Mikrobiol. 2009;45:705–9. [PubMed] [Google Scholar]
- 7.Kozhamkulova ZA, Radwan MM, Zhusupova GE, Abilov ZZh, Rahadilova SN, Ross SA. Gmelinoside I, a new flavonol glycoside from Limonium gmelinii. Nat Prod Commun. 2010;5:1061–2. [PubMed] [Google Scholar]
- 8.Murray AP, Rodriguez S, Frontera MA, Tomas MA, Mulet MC. Antioxidant metabolites from Limonium brasiliense (Boiss.) Kuntze. Z Naturforsch C. 2004;59:477–80. doi: 10.1515/znc-2004-7-804. [DOI] [PubMed] [Google Scholar]
- 9.Padhye S, Dandawate P, Yusufi M, Ahmad A, Sarkar FH. Perspectives on medicinal properties of plumbagin and its analogs. Med Res Rev. 2012;32:1131–58. doi: 10.1002/med.20235. [DOI] [PubMed] [Google Scholar]
- 10.Chaung SS, Lin CC, Lin J, Yu KH, Hsu YF, Yen MH. The hepatoprotective effects of Limonium sinense against carbon tetrachloride and beta-D-galactosamine intoxication in rats. Phytother Res. 2003;17:784–91. doi: 10.1002/ptr.1236. [DOI] [PubMed] [Google Scholar]
- 11.Tang XH, Gao J, Chen J, Xu LZ, Tang YH, Zhao XN, et al. Mitochondrial modulation is involved in the hepatoprotection of Limonium sinense extract against liver damage in mice. J Ethnopharmacol. 2008;120:427–31. doi: 10.1016/j.jep.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Tullberg-Reinert H, Jundt G. In situ measurement of collagen synthesis by human bone cells with a sirius red-based colorimetric microassay: Effects of transforming growth factor beta2 and ascorbic acid 2-phosphate. Histochem Cell Biol. 1999;112:271–6. doi: 10.1007/s004180050447. [DOI] [PubMed] [Google Scholar]
- 13.Shi J, Kokubo Y, Wake K. Expression of P-selectin on hepatic endothelia and platelets promoting neutrophil removal by liver macrophages. Blood. 1998;92:520–8. [PubMed] [Google Scholar]
- 14.Kiso K, Ueno S, Fukuda M, Ichi I, Kobayashi K, Sakai T, et al. The role of Kupffer cells in carbon tetrachloride intoxication in mice. Biol Pharm Bull. 2012;35:980–3. doi: 10.1248/bpb.35.980. [DOI] [PubMed] [Google Scholar]
- 15.Friedman SL. Hepatic fibrosis-overview. Toxicology. 2008;254:120–9. doi: 10.1016/j.tox.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Gieling RG, Burt AD, Mann DA. Fibrosis and cirrhosis reversibility-molecular mechanisms. Clin Liver Dis. 2008;12:915–37. doi: 10.1016/j.cld.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Kisseleva T, Brennerk DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood) 2008;233:109–22. doi: 10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- 18.Popov Y, Schuppan D. Targeting liver fibrosis: Strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–306. doi: 10.1002/hep.23123. [DOI] [PubMed] [Google Scholar]
- 19.Friedman SL. Liver fibrosis – from bench to beside. J Hepatol. 2003;38:S38–53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 20.Ginès P, Cabrera J, Guevara M, Morillas R, Ruiz del Arbol L, Solàe R, et al. Consensus document on the treatment of ascites, dilutional hyponatremia and hepatorenal syndrome in liver cirrhosis. Gastroenterol Hepatol. 2004;27:535–44. doi: 10.1016/s0210-5705(03)70522-6. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Zern MA. Hepatic stellate cells: A target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665–72. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]
- 22.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, et al. An immortalized rat liver stellate cell line (HSC-T6): A new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882–93. [PubMed] [Google Scholar]
- 24.Chen YW, Li DG, Wu JX, Chen YW, Lu HM. Tetrandrine inhibits activation of rat hepatic stellate cells stimulated by transforming growth factor-b in vitro via upregulation of Smad 7. J Ethnopharmacol. 2005;100:299–305. doi: 10.1016/j.jep.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–56. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 26.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–73. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: Molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol. 2005;45:605–28. doi: 10.1146/annurev.pharmtox.45.120403.095906. [DOI] [PubMed] [Google Scholar]
- 28.Luttenberger T, Schmid-Kotsas A, Menke A, Siech M, Beger H, Adler G, et al. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: Implications in pathogenesis of pancreas fibrosis. Lab Invest. 2000;80:47–55. doi: 10.1038/labinvest.3780007. [DOI] [PubMed] [Google Scholar]
- 29.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21:397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 30.Claesson-Welsh L. Signal transduction by the PDGF receptors. Prog Growth Factor Res. 1994;5:37–54. doi: 10.1016/0955-2235(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 31.Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: Agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267–88. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manuelpillai U, Lourensz D, Vaghjiani V, Tchongue J, Lacey D, Tee JY, et al. Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS One. 2012;7:e38631. doi: 10.1371/journal.pone.0038631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellerbrand C, Jobin C, Iimuro Y, Licato L, Sartor RB, Brenner DA. Inhibition of NFkappaB in activated rat hepatic stellate cells by proteasome inhibitors and an IkappaB super-repressor. Hepatology. 1998;27:1285–95. doi: 10.1002/hep.510270514. [DOI] [PubMed] [Google Scholar]
- 34.Lang A, Schoonhoven R, Tuvia S, Brenner DA, Rippe RA. Nuclear factor kappaB in proliferation, activation, and apoptosis in rat hepatic stellate cells. J Hepatol. 2000;33:49–58. doi: 10.1016/s0168-8278(00)80159-2. [DOI] [PubMed] [Google Scholar]
- 35.Robinson SM, Mann DA. Role of nuclear factor kappaB in liver health and disease. Clin Sci (Lond) 2010;118:691–705. doi: 10.1042/CS20090549. [DOI] [PubMed] [Google Scholar]


