Abstract
Background:
Rostellularia diffusa is an unexplored medicinal plant used as brain tonic in traditional medicine system.
Objective:
This study was designed to investigate the antioxidant and anti-stress potential of R. diffusa by experimental animal models.
Materials and Methods:
The extracts of R. diffusa were subjected to preliminary phytochemical screening and high performance thin layer chromatography (HPTLC) finger printing analysis. The antioxidant potential of the extracts was found by different in vitro models. The anti-stress activity was investigated by using acetic acid induced writhing test, swimming endurance test, and restraint stress in experimental mice. Serum parameters such as glucose, triglyceride and cholesterol, oxidative stress parameter thiobarbituric acid reactive substance, antioxidant parameters such as reduced glutathione, superoxide dismutase and catalase and organ weights were evaluated after restraint stress in mice. Diazepam was used as reference standard to compare the anti-stress activity of plant extract.
Results:
High performance thin layer chromatography finger printing analysis revealed the presence of flavone compounds in both extracts. The extracts also showed good antioxidant property in different in vitro antioxidant models. Administration of extracts of R. diffusa decreased the number of wriths and immobility time when compared with control group in acetic acid-induced writhing test and swimming endurance test respectively in experimental mice. They also suppressed the restraint stress-induced alterations in serum parameters, oxidative stress, and antioxidant parameters in brain and also restored the organ weights in normal level.
Conclusion:
From these results, it has been concluded that the potential anti-stress activity of R. diffusa is through its adaptogenic and antioxidant properties.
Keywords: Antioxidants, brain tonic, restraint stress, stress, swimming endurance test
INTRODUCTION
Stress is a condition or feeling experienced when a person perceives that demands exceed the personal and social resources the individual is able to mobilize. Stress disturbs the normal physiological condition and results in a state of threatened homeostasis.[1] Endocrine response to stress is mediated by the secretion of glucocorticoids and catecholamines in order to cope with stress. Increased and prolonged stress is responsible for fatigue, reduced stamina, lowered mood and also involved in the etiopathogenesis of a variety of diseases ranging from psychiatric or behavioral disorders such as anxiety and depression, immunosuppression, cardiovascular diseases, endocrine disorders such as diabetes, increase in serum corticosterone levels, male impotency, cognitive dysfunction, and gastric ulcerations.[2,3,4] Moreover, during stressful conditions, the energy requirement of the body is increased, leading to increased production of reactive oxygen species (ROS) in the body resulting in the development of oxidative stress. These ROS can cause tissue damage by reacting with lipids, proteins and DNA resulting in various pathological conditions.[4,5]
Various measures are available to counteract the effects of stress, which include pharmacological and nonpharmacological methods.[2] Use of several anti-stress agents such as benzodiazepines such as diazepam, anxiolytics, certain central nervous system stimulants such as amphetamine and caffeine as well as some anabolic steroids, despite showing significant anti-stress activity against various models of stress, have not proved effective against chronic stress-induced adverse effects on immunity, behavior cognition, male sexual function, during pregnancy and lactation. In addition, the problem of incidence of toxicity, tolerance and physical dependence on their prolonged use limits the clinical utility of these drugs.[1,2,6] Therefore, there is a need for an effective herbal anti-stress agent in the therapy of stress-induced disorders.[1] The potential utility of safer and cheaper herbal medicines as anti-stress agents have been reported as they can withstand stress without altering the physiological functions of the body. Various herbs such as Withania somnifera, Asparagus racemosus, Ocimum sanctum, Tribulus terrestris, Piper longum are claimed to have immunomodulatory, adaptogenic, anabolic effects, and the ability to improve vital energy.[6]
Rostellularia diffusa (Willd.) (Synonyms: Justicia diffusa) is a medicinal plant (Family-Acanthaceae) common in forest undergrowth in waste places, especially in rock crevices [Figure 1]. It is found in Tirumala forests, Tirupati, Andhra Pradesh, India. It is having a very good potential as brain tonic, which is used traditionally in treating mental disorders for which stress is one of the cause.[7] Up to our knowledge until date no research work has been done on this plant. In order to support the traditional claim, the present work has been undertaken to investigate the anti-stress and antioxidant potential of R. diffusa in experimental animal model.
Figure 1.

Whole plant of Rostellularia diffusa
MATERIALS AND METHODS
Chemicals and drugs
Nitro blue tetrazolium (NBT), nicotinamide adenine dinucleotide (NADH), sodium lauryl sulfate, Bradford reagent, Griess reagent, Potassium dihydrogen phosphate, Phenazine methosulphate, sodium pyrophosphate, 5, 51-dithio-bis-2-nitrobenzoic acid, trichloroacetic acid (TCA), thiobarbituric acid (TBA), sodium dihydrogen phosphate, Disodium hydrogen phosphate, 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma life sciences, Bangalore. All other chemicals used were of analytical grade with high purity.
Animals
Male swiss albino mice (25 ± 5 g) were selected for the study. The animals were housed in clean polypropylene cages under hygienic and standard environmental conditions at 22°C ± 2°C, 12:12 h light: Dark cycle and 60 ± 5% relative humidity with free access to standard laboratory food and water ad libitum (SaiDurga Feeds and Foods, Bangalore). Mice were habituated to laboratory conditions for 1 week before the test. All the experiments were carried out during the light period (08:00-16:00) and conducted in accordance with the guidelines given by the committee for the purpose of control and supervision of experiments on animals (CPCSEA), New Delhi (India) and the Institutional Animal Ethics Committee (1220/a/08/CPCSEA) approved the experimental protocol.
Plant material and preparation of extract
The whole plant of R. diffusa was collected from the rock crevices in Tirumala forests, Tirupati, A.P, India in the month of August 2012 and was authenticated by Dr. K. Madhava Chetty, Professor and Head, Department of Botany, S. V. University, Tirupati and voucher specimen number was lodged (ANCP-MP-COL-01/13) and preserved in the herbarium, which was retained in our lab for future reference. Whole plant of R. diffusa was shade dried and coarsely powdered. The 500 g of the powdered plant material was defatted with petroleum ether (60-80°C) using a soxhlet extractor and then it is successively extracted with CHCl3 and 70% ethanol each for 72 h and the extracts obtained from both the solvents were filtered and concentrated using rota evaporator (Medika Instrument). The yield of the extracts was found to be 10.9% and 11.5%, respectively.
Preliminary phytochemical screening
Both hydroalcoholic (HAERD) and chloroform (CERD) extracts of R. diffusa were screened for the presence of carbohydrates, proteins, alkaloids, flavonoids, glycosides, triterpenoids, tannins and phenolic compounds, fats and fixed oils using the standard procedures.[8]
High performance thin layer chromatography
Both the extracts of R. diffusa were then subjected to high performance thin layer chromatography (HPTLC) for identification of specific phytoconstituents.[8] The HAERD and CERD were dissolved in HPTLC grade chloroform and ethanol, which were used for sample application on precoated silica gel GF254 aluminum sheets. A number of solvent systems were tried for both extracts, but the satisfactory resolution was obtained in the solvent benzene: Chloroform (1:4) (double elution) for CERD and ethylacetate: formicacid: Acetic acid: water (100:11:11:26) for HAERD. The samples (5 μL-10 mg/5 ml) were spotted in the form of bands of width 8 mm with a 100 μL sample using a Hamilton syringe on silica gel, which was precoated on aluminum plate GF254 plates (5 × 10 cm-E. MERCK KGaA) with the help of Linomat five applicator attached to CAMAG HPTLC system, which was programmed through WIN CATS software, CAMAG USA. The mobile phase consisted of benzene: Chloroform (1:4) (double elution) for CERD and ethylacetate: formicacid: acetic acid: water (100:11:11:26) for HAERD and 15 ml of mobile phase was used for chromatography run. The linear ascending development was carried out in a (5 × 10 cm) twin through the glass chamber saturated with the mobile phase. The developed plate was dried by oven at 60°C to evaporate solvents from the plate. The plate was kept in photo-documentation chamber (CAMAG REPROSTAR 3) and captured the images under ultraviolet (UV) light at 254 and 366 nm, respectively. The Rf values and finger print data were recorded by WIN CATS software.
In vitro antioxidant activity
Determination of reducing power
The reducing power of both extracts of R. diffusa was studied by adopting the method of Oyaizu.[9] About 2.5 ml of different concentrations of the plant extract (10-500 μg/ml) were mixed with 2.5 ml each of phosphate buffer (0.2 M, pH 6.6) and 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 min, then rapidly cooled, mixed with 2.5 ml of 10% TCA and centrifuged at 3000 rpm for 10 min. About 2.5 ml of the supernatant was taken and 2.5 ml distilled water and 0.5 ml of 0.1% ferric chloride was added to it, mixed well and allowed to stand for 10 min and the absorbance was measured at 700 nm. Vitamin-C was used as standard.
2,2-diphenyl-1-picrylhydrazyl assay
The free radical scavenging activity of both extracts of R. diffusa were measured in terms of hydrogen donating or radical scavenging ability using the stable radical DPPH. A 0.004% DPPH solution in methanol was prepared and 4 ml of this solution was added to 1 ml of sample extract solution in water at different concentrations (10-500 mg/ml). Left it for 30 min at room temperature for the reduction of the DPPH free radical and the absorbance was measured at 517 nm. The procedure was repeated for vitamin-C, which was used as standard. The antioxidant activity of the extracts was expressed as IC50, which is the inhibitory concentration required to scavenge 50% of DPPH free radicals.[10] The percentage inhibition was calculated from the following equation:
% inhibition = Ablank − (Asample/Ablank) ×100
Peroxide radical scavenging activity
The peroxide radical scavenging activities of both extracts of R. diffusa were carried out by adapting the method of Ruch et al.[11] 4 mM of H2O2 solution in phosphate buffered saline was prepared. 0.6 ml of this solution was added to 1 ml of extract solution at different concentrations (10-500 μg/ml). This solution was incubated for 10 min at room temperature and the absorbance was measured at 230 nm. Vitamin-C was used as standard. The antioxidant activity of the extracts was expressed as IC50, which is the inhibitory concentration required to scavenge 50% of peroxide free radicals. The percentage inhibition was calculated from the following equation:
% inhibition = Ablank− (Asample/Ablank) ×100
Nitric oxide radical scavenging activity
Nitric oxide (NO) was spontaneously generated from sodium nitroprusside in aqueous solution at physiological pH, which interacts with oxygen to produce nitrite ions that can be estimated by the use of Griess reagent. To 1 ml of extract solution at different concentrations (10-500 μg/ml), 1 ml of 10 mM sodium nitroprusside was added. It was incubated for 150 min at room temperature and 0.5 ml of Griess reagent was added and the absorbance of the chromophore formed during the deionization of nitrite with sulfanilamide and subsequent coupling with napthyl ethylene diamine dihydrochloride was measured at 546 nm. Vitamin-C was used as standard. The antioxidant activity of the extracts was expressed as IC50, which is the inhibitory concentration required to scavenge 50% of NO radicals.[12] The percentage inhibition was calculated from the following equation:
% inhibition = Ablank − (Asample/Ablank) ×100
Superoxide radical scavenging activity
This activity was determined by the NBT reduction method. To 1 ml of extract solution at different concentrations (10-500 μg/ml), I ml each of 60 μM phenazine metho sulfate and 450 μM NADH and incubate it for 5 min at 25°C and the percentage inhibition of superoxide generation was evaluated by measuring the absorbance values. Vitamin-C was used as standard. The antioxidant activity of the extracts was expressed as IC50, which is the inhibitory concentration required to scavenge 50% of superoxide radicals.[13] The percentage inhibition was calculated from the following equation:
% inhibition = Ablank − (Asample/Ablank) ×100
Acute oral toxicity study
The acute oral toxicity study was performed according to the method described by Lorke.[14] HAERD and CERD up to a dose of 2000 mg/kg did not produce any signs of toxicity and mortality. Based on this the doses for CERD and HAERD for further experimental study were selected.
Anti-stress activity
Chemical induced stress in mice
The mice were randomly divided into eight groups of six animals each. Group I received normal saline (10 ml/kg p.o.) for 7-day and served as control. Groups II, III, and IV received different doses (100, 200, and 300 mg/kg p.o) of HAERD for 7-day. Groups V, VI, and VII received different doses (100, 200, and 300 mg/kg p.o) of CERD for 7-day. Group VIII received diazepam (2 mg/kg i.p.) for 7-day and served as positive control. On day 7, 1 h after the drug treatment, writhings were induced in all the groups by giving 0.1 ml of 6% (v/v) glacial acetic acid i.p. and the number of writhes was recorded in all the groups for 20 min.[15,16]
Swimming endurance test
The mice were randomly divided into eight groups of six animals each and the animals were treated for 7-day same as in the above model. On day 7, 1 h after the administration of extracts, all the animals were subjected to swimming stress by keeping them in a polypropylene vessel of dimension 40 × 40 × 30 cm with a water level of 20 cm and the immobility time of each mouse was recorded for 30 min.[15,16]
Restraint stress in mice
Mice were randomly divided into five groups of six animals each (from the results of the writhing and swimming test, among the three different doses (100, 200, and 300 mg/kg), 300 mg/kg was found to be effective. Hence, only that particular dose was selected for this model). Groups I and II received normal saline (10 ml/kg p.o.) for 15-day and served as control and stress control respectively. Groups III and IV received 300 mg/kg p.o. of HAERD and CERD respectively for 15-day. Group V received diazepam (2 mg/kg i.p.) for 15-day and served as positive control. On the 15th day, 1 h after the last treatment, the forelimbs and hind limbs of mice in all groups except control group were tied using adhesive tape for 2 h to induce stress by immobilizing them.[17] The adhesive tapes were removed after 2 h and blood was collected from retro orbital plexus of all the animals. The mice were then sacrificed under ether anesthesia and their brain, adrenal glands and spleen were isolated and weighed.
Assessment of biochemical parameters
The blood samples were centrifuged (3000 rpm for 20 min) and the serum obtained was separated out and used for the estimation of blood glucose, total cholesterol, and triglycerides using commercial kits. The isolated mouse brains were rinsed with 0.9% ice-cold normal saline and processed to get 10% homogenate in cold phosphate buffer using glass Teflon homogenizer. The homogenates obtained were used for the estimation of TBA reactive substance (TBARS),[18] reduced glutathione (GSH),[19] superoxide dismutase (SOD),[20] Catalase,[21] protein[22] and nitrite levels.[23]
Statistical analysis
Results were expressed as mean ± standard deviation and analyzed using Graph Pad Prism version 5.1 GraphPad Software, Inc using one-way analysis of variance followed by Dunnett's posttest. P < 0.05 was considered to be significant.
RESULTS
Preliminary phytochemical screening
The preliminary phytochemical analysis revealed the presence of alkaloids, carbohydrates, saponins, phenolic compounds, and flavonoids.
High performance thin layer chromatography finger print analysis of extracts of Rostellularia diffusa
High performance thin layer chromatography fingerprinting analysis of extracts of whole plant of R. diffusa revealed several peaks and was recorded in Table 1. HPTLC profile under UV 366 and 254 nm was recorded in the Figure 2. The CERD revealed seven spots with Rf values in the range of 0.02-0.96. HAERD showed 1 peak with Rf value 0.26 and purity of the sample was confirmed by comparing the absorption spectra at start, middle, and end position of the band. Appearance of blue color under UV examination confirmed the presence of flavone components in both extracts. The intensity of blue color is more in the CERD with the Rf value of 0.25.
Table 1.
HPTLC profile of chloroform and hydroalcoholic extract of Rostellularia diffusa
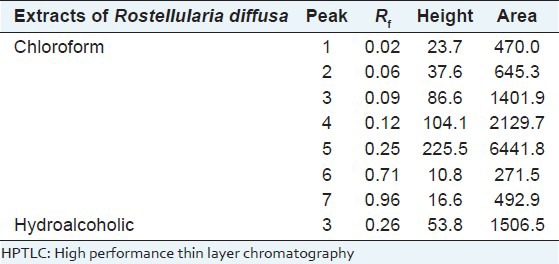
Figure 2.
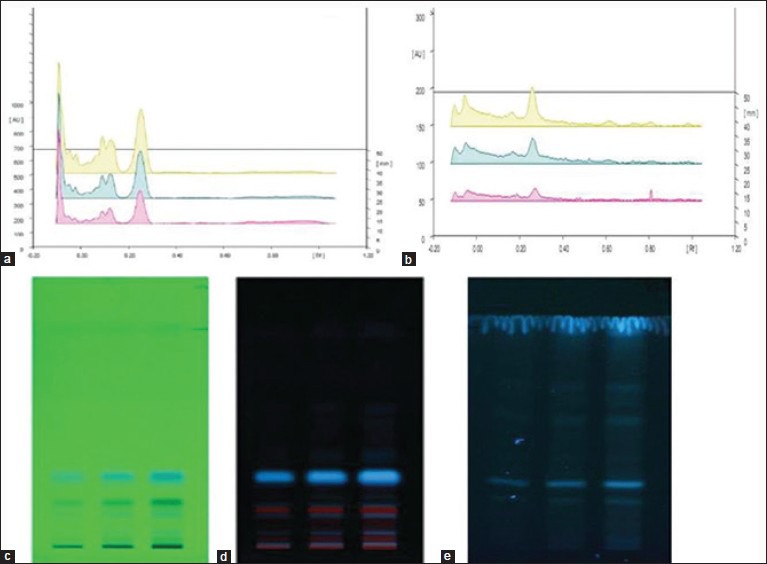
High performance thin layer chromatography (HPTLC) profile of Rostellularia diffusa (a) HPTLC scanning of CERD, (b) HPTLC scanning of HAERD, (c and d) HPTLC profile of CERD at 254 and 366 nm, (e) HPTLC profile of HAERD at 366 nm
In vitro antioxidant activity
Several concentrations ranging from 10 to 500 μg/ml of HAERD and CERD were tested for their antioxidant activity in different in vitro models. It was observed that the test compounds scavenged the free radicals in a concentration-dependent manner in all the models. The reducing power of HAERD and CERD was very potent and increases with concentration and it is comparable with that of vitamin-C. The IC50 values of HAERD, CERD and vitamin-C in DPPH scavenging activity were found to be 82.2, 138.5 and 27.1 μg/ml, in peroxide scavenging activity were 69.3, 67.4 and 63.1 μg/ml, in NO scavenging activity were 77.1, 83.9 and 60.3 μg/ml and in superoxide scavenging activity were 92.8, 125.2 and 69.8 μg/ml, respectively.
Effect of Rostellularia diffusa against chemical induced stress
Seven-day pretreatment of both HAERD and CERD of whole plant of R. diffusa significantly and dose dependently reduced the number of acetic acid-induced writhing in mice compared to the vehicle control group. The percentage inhibition in a number of writhes was found to be 73.4%, 78.4% and 87.3% in 100, 200 and 300 mg/kg of HAERD and 56.9%, 85.9% and 88.6% in 100, 200 and 300 mg/kg of CERD, respectively. Diazepam produced 94.9% inhibition in a number of writhes [Table 2].
Table 2.
Effect of Rostellularia diffusa on chemical induced stress and swimming endurance test
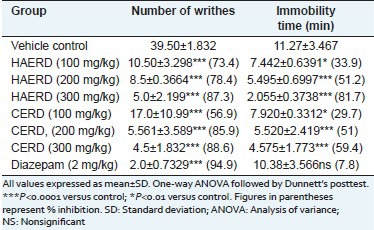
Effect of Rostellularia diffusa in swimming endurance test
In the swimming endurance test, 7-day pretreatment of all the three doses (100, 200 and 300 mg/kg) of both HAERD and CERD significantly reduced the immobility time as compared to the vehicle control group. Diazepam nonsignificantly reduced the immobility time compared to the vehicle control group. The percentage inhibition in immobility time was found to be 33.9, 51.2 and 81.7% in 100, 200 and 300 mg/kg of HAERD and 29.7, 51 and 59.4 with 100, 200 and 300 mg/kg of CERD, respectively. Diazepam produced 7.8% inhibition in immobility time [Table 2].
Effect of Rostellularia diffusa on different biochemical parameters in blood in restraint stress model
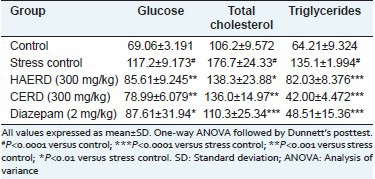
Restraint stress adversely affected the blood concentration of various biochemical parameters. The induction of restraint stress led to a significant rise in blood glucose, total cholesterol, and triglycerides as compared with the vehicle control group [Table 3]. 15-day pretreatment with HAERD (300 mg/kg) and CERD (300 mg/kg) reduced the levels of blood glucose, total cholesterol, and triglycerides significantly compared to the stress control group. Diazepam (2 mg/kg) also reduced the levels of glucose, total cholesterol, and triglycerides significantly compared to the stress control group.
Table 3.
Effect of Rostellularia diffusa on different biochemical parameters in blood after restraint stress
Effect of Rostellularia diffusa on different oxidative stress parameters in restraint stress model
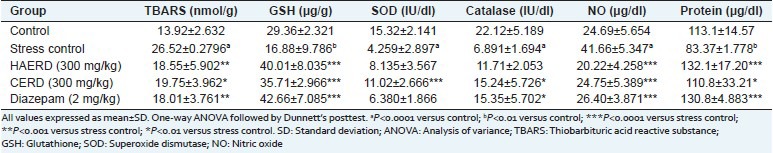
Restraint stress affected the levels of various oxidative stress parameters in the brain. Stress elevated the levels of lipid peroxidation (TBARS) and NO and reduced the levels of protein, GSH, catalase, and SOD significantly [Table 4] as compared with the vehicle control group. 15-day pretreatment with HAERD (300 mg/kg) and CERD (300 mg/kg) significantly reduced the levels of lipid peroxidation (TBARS) and NO and increased the levels of protein and GSH significantly. CERD (300 mg/kg) significantly increased the levels of SOD and catalase also but HAERD (300 mg/kg) shown nonsignificant rise in SOD and catalase. Diazepam (2 mg/kg) reduced the levels of lipid peroxidation (TBARS) and NO and increased the levels of catalase, protein and GSH significantly and SOD nonsignificantly.
Table 4.
Effect of Rostellularia diffusa on different biochemical parameters in brain after restraint stress
Effect of Rostellularia diffusa on organ weights in restraint stress model
Restraint stress caused a significant increase in the weight of adrenal glands and a significant decrease in the weight of the brain and spleen as compared to vehicle control group [Table 5]. 15-day pretreatment of both HAERD and CERD and Diazepam significantly suppressed the stress-induced alterations in weights of adrenal glands, spleen, and brain.
Table 5.
Effect of Rostellularia diffusa on organ weights after restraint stress
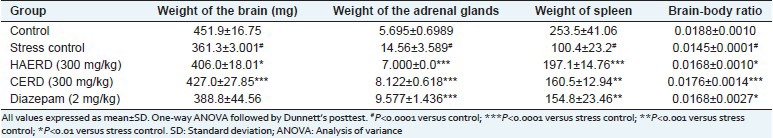
Effect of Rostellularia diffusa on the brain-body ratio in restraint stress model
All the three treatments (HAERD, CERD and Diazepam) significantly increased the stress-induced decrease in the brain-body ratio [Table 5].
DISCUSSION
In this study, the anti-stress activity of two different (hydroalcoholic and chloroform) extracts of R. diffusa (100, 200 and 300 mg/kg) has been evaluated using various acute stress experimental models such as acetic acid induced writhing test, swimming endurance test, and restraint stress. Acetic acid induced writhing test and swimming endurance test are the known physical stress models for the evaluation of anti-stress activity.
In swimming endurance paradigm, mice forced to swim in water in a restricted space from which they cannot escape assume a characteristic immobile posture which reflects a state of tiredness, fatigue, reduced stamina and mental depression. These symptoms represent the intense stress condition. It has been well demonstrated that drugs with anti-stress activity increase swimming endurance (or reduce immobility time). The same result has also been reported by many other researchers.[24,25] The results of the swimming endurance test in the present study have shown that there is an increase in swimming endurance which indicates clearly that both the hydro alcoholic and chloroform extracts of R. diffusa have anti-stress properties. Higher activity was observed with high doses (200 and 300 mg/kg) of both the extracts.
In acetic acid induced writhing test, the administration of acetic acid caused hyperalgesic effects on the pain pathway which results in an increase in the number of writhes indicating stress development. The results of acetic acid induced writhing test in the present study show the decrease in the number of writhes with the treatment which indicates clearly that all the doses of both (hydro alcoholic and chloroform) the extracts of R. diffusa can play a significant role in the inhibition of pain processes, which shows that the extracts have anti-stress property. Several other studies have also been reported that the plants having anti-stress property will reduce the number of writhes.[2,26]
Under stressful conditions, blood glucose and lipid levels will get increased. The mechanism by which stress raises the levels of glucose, serum cholesterol and triglycerides is likely to be related to the enhanced activity of hypothalamic-pituitary adrenal axis during stress, resulting in increased secretion of adrenocorticotropic hormone (ACTH) and corticosteroids into the circulation. Release of ACTH in stress stimulates the adrenals to increase the production of catecholamines. They mobilize the stored fat, lipids and carbohydrate reserves from adipose tissues which lead to elevated levels of blood glucose, cholesterol, and triglycerides since adrenaline. Hyper secretion of cortisol helps the maintenance of internal homeostasis through gluconeogenesis and lipogenesis. This results in increased blood glucose and lipid levels.[2]
In this study, when the mice were subjected to restraint stress, the blood glucose, total cholesterol, and triglyceride levels were increased. A number of studies have reported the same result.[2,24,26] Stress-induced rise in blood glucose, total cholesterol and triglyceride levels were significantly reduced by both extracts of R. diffusa at a dose of 300 mg/kg b.w. indicating their anti-stress potential.
The nervous system is extremely sensitive to lipid peroxidation following restraint stress as it has high oxygen tension and is rich in oxidizable substrates and low in antioxidant capacity. After restraint stress, it was found that lipid peroxidation (TBARS) and NO levels were highly increased in the stress control group as a result of oxidative damage. The similar results were found by many other researchers.[2] Both the extracts of R. diffusa significantly reduced the TBARS and NO levels indicating that the extracts have anti-stress activity through reduction of oxidative stress. Restraint stress also caused the depletion of GSH, SOD, catalase and protein content in mouse brain. Both extracts of R. diffusa significantly increased the reduced GSH levels and protein content, but the reduced SOD and catalase levels were significantly increased only by the chloroform extract. The antioxidant property of the extracts was further confirmed by in vitro antioxidant study, which shows that the protective role of these extracts is through an antioxidant mechanism.
In this study, it also shows that the adrenal gland weights were significantly increased and spleen weights were significantly decreased in stress control group after restraint stress. Similar findings were observed by other researchers.[24,27]
Stress induces adreno-medullary response to release adrenaline which in turn stimulates β2 receptors on the pituitary gland. It leads to greater release of ACTH that can stimulate the adrenal medulla and cortex resulting in the excess release of adrenaline and corticosterone, respectively. The adrenal hypertrophy (increase in adrenal gland weight) takes place in response to the secretion of ACTH from the pituitary for increased corticosterone from cortical cells to combat stress. Spleen contracts during stress and releases more amount of blood red blood cell into circulation; hence, its weight decreases.[24] Pretreatment with R. diffusa prevented the stress-induced increase in weight of adrenal glands and stress-induced decrease in spleen and brain weight and significantly increased the stress-induced decrease in the brain-body ratio, indicating the protective effect of R. diffusa against stress.
In this study, HPTLC finger printing analysis of CERD and HAERD revealed the presence of flavones. Hence, the anti-stress activity of R. diffusa attributes to their adaptogenic and antioxidant properties which seem to be due to the presence of flavones in high amounts.
From the pharmacological studies, it is concluded that both the chloroform and hydroalcoholic extracts of R. diffusa exhibited anti-stress activity by scavenging free radicals in different in vitro antioxidant models, by holding back the physical strength in chemical induced stress and swimming stress models and by preventing the stress-induced alterations in biochemical parameters in the brain, serum parameters and in organ weights of mice. In this study, the phytochemical investigation of both chloroform and hydroalcoholic extracts of R. diffusa revealed that they are rich in flavones and it was further confirmed by HPTLC. Though further work to characterize the this chemical constituent and quantitative estimation with marker compounds is also necessary, this data can also be considered along with the other values for fixing standards to this plant.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Habbu PV, Mahadevan KM, Kulkarni PV, Daulatsingh C, Veerapur VP, Shastry RA. Adaptogenic and in vitro antioxidant activity of flavanoids and other fractions of Argyreia speciosa (Burm.f) Boj. in acute and chronic stress paradigms in rodents. Indian J Exp Biol. 2010;48:53–60. [PubMed] [Google Scholar]
- 2.Nade VS, Kawale LA, Naik RA, Yadav AV. Adaptogenic effect of Morus alba on chronic footshock-induced stress in rats. Indian J Pharmacol. 2009;41:246–51. doi: 10.4103/0253-7613.59921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh AK, Dhamanigi SS, Asad M. Anti-stress activity of hydro-alcoholic extract of Eugenia caryophyllus buds (clove) Indian J Pharmacol. 2009;41:28–31. doi: 10.4103/0253-7613.48889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi T, Sah SP, Singh A. Antistress activity of ethanolic extract of Asparagus racemosus Willd roots in mice. Indian J Exp Biol. 2012;50:419–24. [PubMed] [Google Scholar]
- 5.Koppula S, Kopalli SR, Sreemantula S. Adaptogenic and nootropic activities of aqueous extracts of Carum carvi Linn (Caraway) fruit: An experimental study in wistar rats. Aust J Med Herbalism. 2009;21:72–8. [Google Scholar]
- 6.Desai SK, Desai SM, Navdeep S, Arya P, Pooja T. Antistress activity of Boerhaavia diffusa root extract and a polyherbal formulation containing Boerhaavia diffusa using cold restraint stress model. Int J Pharm Pharm Sci. 2011;3:130–2. [Google Scholar]
- 7.Chetty KM, Sivaji K, Rao KT. Andhra Pradesh, India. Tirupati: Students Offset Printers; 2008. A Textbook of Flowering Plants of Chittor District; p. 262. [Google Scholar]
- 8.Harbone JB, Baxter HH. Washington: Taylor and Francis; 1993. Phytochemical Dictionary: A Hand Book of Bioactive Compound from Plants; pp. 237–40. [Google Scholar]
- 9.Oyaizu M. Studies on products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 10.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–8. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–8. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 12.Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994;201:748–55. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- 13.Misra HP, Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972;247:188–92. [PubMed] [Google Scholar]
- 14.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–87. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 15.Nimbkar SR, Patki VP, Patki MP. Pharmacological evaluation of antistress and androgenic activity of polyherbal formulation ‘AP-3000’ containing Panax ginseng. Indian Drugs. 2001;38:27. [Google Scholar]
- 16.Kulkarni MP, Juvekar AR. Attenuation of acute and chronic restraint stress-induced perturbations in experimental animals by Nelumbo nucifera Gaertn. Indian J Pharm Sci. 2008;70:327–32. doi: 10.4103/0250-474X.42982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharya D, Sur TK. The effect of Panax ginseng and diazepam on norepinephrine levels of whole brain and hypothalamus during stress. Indian J Pharmacol. 1991;31:124. [Google Scholar]
- 18.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 21.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 2. NY: Verlag Chemic Acad Press Inc; 1974. pp. 673–85. [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 24.Debnath J, Prakash T, Karki R, Kotresha D, Sharma P. An experimental evaluation of anti-stress effects of Terminalia chebula. J Physiol Biomed Sci. 2011;24:13–9. [Google Scholar]
- 25.Anju L. Adaptogenic and anti-stress activity of Ocimum sanctum in mice. Res J Pharm Biol Chem Sci. 2011;2:670–8. [Google Scholar]
- 26.Patel NB, Galani VJ, Patel BG. Antistress activity of Argyreia speciosa roots in experimental animals. J Ayurveda Integr Med. 2011;2:129–36. doi: 10.4103/0975-9476.85551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azmathulla S, Hule A, Naik SR. Evaluation of adaptogenic activity profile of herbal preparation. Indian J Exp Biol. 2006;44:574–9. [PubMed] [Google Scholar]


