Abstract
Plant defense against microbial pathogens and herbivores relies heavily on the induction of defense proteins and low molecular weight antibiotics. The signals between perception of the aggression, gene activation, and the subsequent biosynthesis of secondary compounds are assumed to be pentacylic oxylipin derivatives. The rapid, but transient, synthesis of cis-jasmonic acid was demonstrated after insect attack on a food plant and by microbial elicitor addition to plant suspension cultures. This effect is highly specific and not caused by a number of environmental stresses such as light, heavy metals, or cold or heat shock. Elicitation of Eschscholtzia cell cultures also led to a rapid alkalinization of the growth medium prior to jasmonate formation. Inhibition of this alkalinization process by the protein kinase inhibitor staurosporine also inhibited jasmonate formation. The induction of specific enzymes in the benzo[c]phenanthridine alkaloid pathway leading to the antimicrobial sanguinarine was induced to a qualitatively and quantitatively similar extent by fungal elicitor, methyl jasmonate, and its linolenic acid-derived precursor 12-oxophytodienoic acid. It is herein proposed that a second oxylipid cascade may exist in plants starting from linoleic acid via 15,16-dihydro-12-oxophytodienoic acid to 9,10-dihydrojasmonate. Experiments with synthetic trihomojasmonate demonstrated that beta-oxidation is not a prerequisite for biological activity and that 12-oxophytodienoic acid and derivatives are most likely fully active as signal transducers. Octadecanoic acid-derived compounds are essential elements in modulating the synthesis of antibiotic compounds and are thus integral to plant defense.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa H., Clark W. G., Psenak M., Coscia C. J. Purification and characterization of dihydrobenzophenanthridine oxidase from elicited Sanguinaria canadensis cell cultures. Arch Biochem Biophys. 1992 Nov 15;299(1):1–7. doi: 10.1016/0003-9861(92)90236-p. [DOI] [PubMed] [Google Scholar]
- Choi D., Bostock R. M., Avdiushko S., Hildebrand D. F. Lipid-derived signals that discriminate wound- and pathogen-responsive isoprenoid pathways in plants: methyl jasmonate and the fungal elicitor arachidonic acid induce different 3-hydroxy-3-methylglutaryl-coenzyme A reductase genes and antimicrobial isoprenoids in Solanum tuberosum L. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2329–2333. doi: 10.1073/pnas.91.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline S. D., Coscia C. J. Stimulation of Sanguinarine Production by Combined Fungal Elicitation and Hormonal Deprivation in Cell Suspension Cultures of Papaver bracteatum. Plant Physiol. 1988 Jan;86(1):161–165. doi: 10.1104/pp.86.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn E. E. Biosynthesis of cyanogenic glycosides. Naturwissenschaften. 1979 Jan;66(1):28–34. doi: 10.1007/BF00369352. [DOI] [PubMed] [Google Scholar]
- Cosio E. G., Frey T., Verduyn R., van Boom J., Ebel J. High-affinity binding of a synthetic heptaglucoside and fungal glucan phytoalexin elicitors to soybean membranes. FEBS Lett. 1990 Oct 1;271(1-2):223–226. doi: 10.1016/0014-5793(90)80411-b. [DOI] [PubMed] [Google Scholar]
- Dittrich H., Kutchan T. M. Molecular cloning, expression, and induction of berberine bridge enzyme, an enzyme essential to the formation of benzophenanthridine alkaloids in the response of plants to pathogenic attack. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9969–9973. doi: 10.1073/pnas.88.22.9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
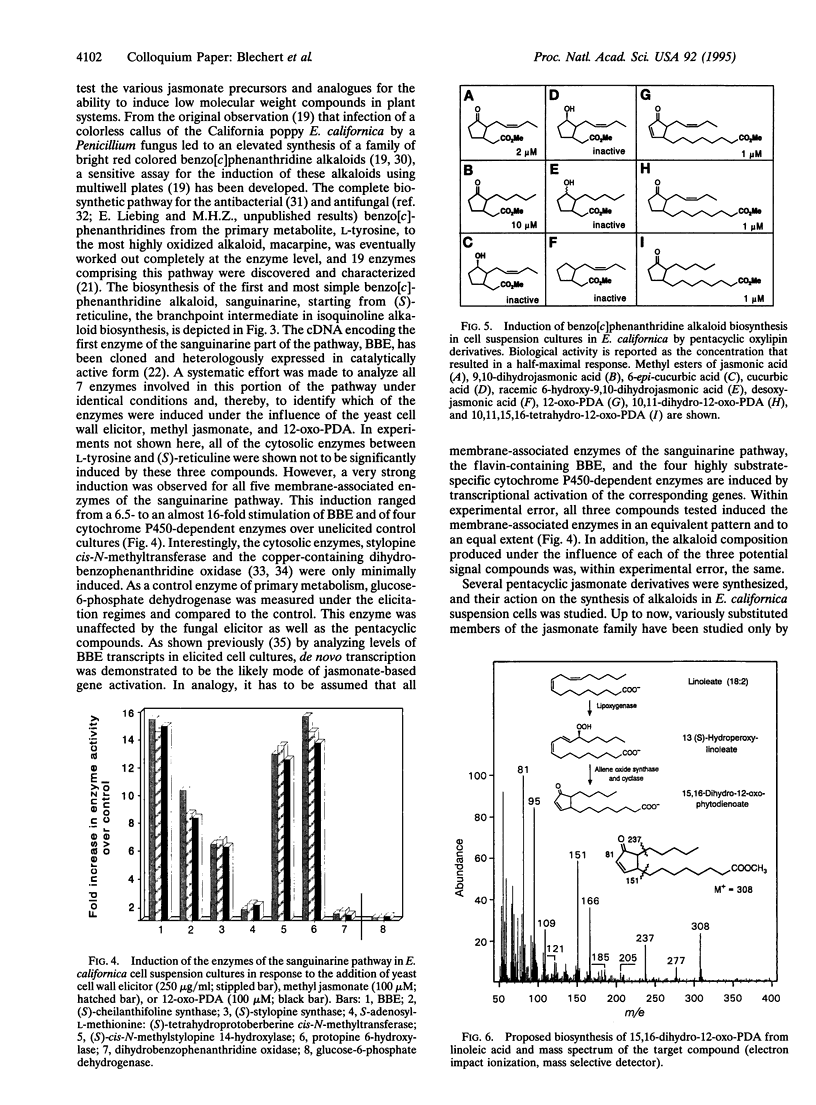
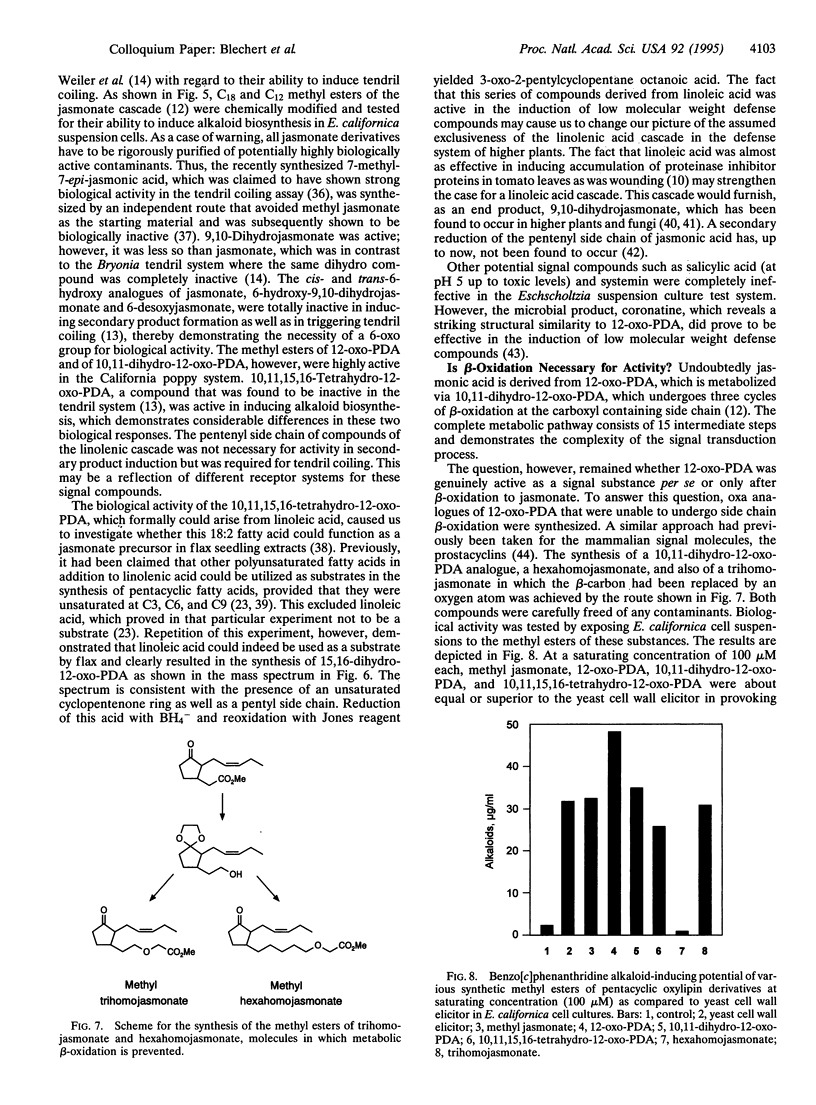
- Dittrich H., Kutchan T. M., Zenk M. H. The jasmonate precursor, 12-oxo-phytodienoic acid, induces phytoalexin synthesis in Petroselinum crispum cell cultures. FEBS Lett. 1992 Aug 31;309(1):33–36. doi: 10.1016/0014-5793(92)80733-w. [DOI] [PubMed] [Google Scholar]
- Dzink J. L., Socransky S. S. Comparative in vitro activity of sanguinarine against oral microbial isolates. Antimicrob Agents Chemother. 1985 Apr;27(4):663–665. doi: 10.1128/aac.27.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
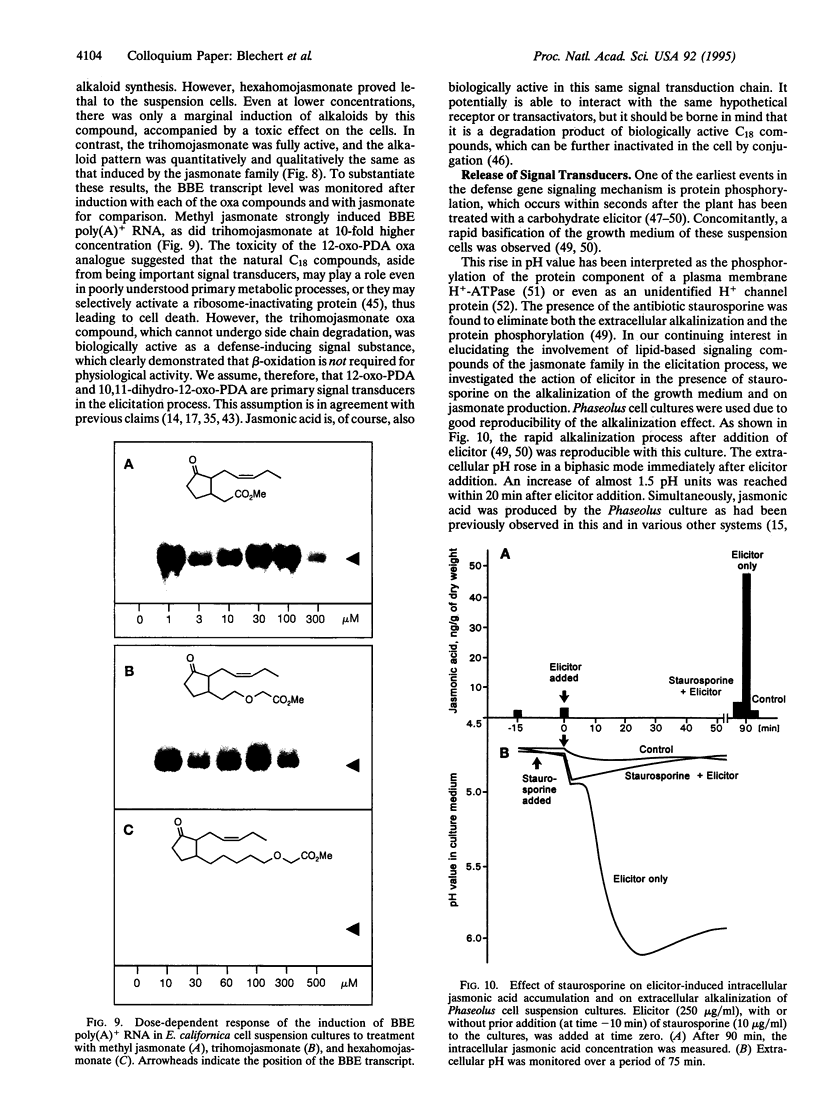
- Farmer E. E., Pearce G., Ryan C. A. In vitro phosphorylation of plant plasma membrane proteins in response to the proteinase inhibitor inducing factor. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1539–1542. doi: 10.1073/pnas.86.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Octadecanoid Precursors of Jasmonic Acid Activate the Synthesis of Wound-Inducible Proteinase Inhibitors. Plant Cell. 1992 Feb;4(2):129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Octadecanoid-derived signals in plants. Trends Cell Biol. 1992 Aug;2(8):236–241. doi: 10.1016/0962-8924(92)90311-a. [DOI] [PubMed] [Google Scholar]
- Felix G., Grosskopf D. G., Regenass M., Boller T. Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8831–8834. doi: 10.1073/pnas.88.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Regenass M., Spanu P., Boller T. The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labeling with [33P]phosphate. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):952–956. doi: 10.1073/pnas.91.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Grimes H. D. Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6745–6749. doi: 10.1073/pnas.88.15.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
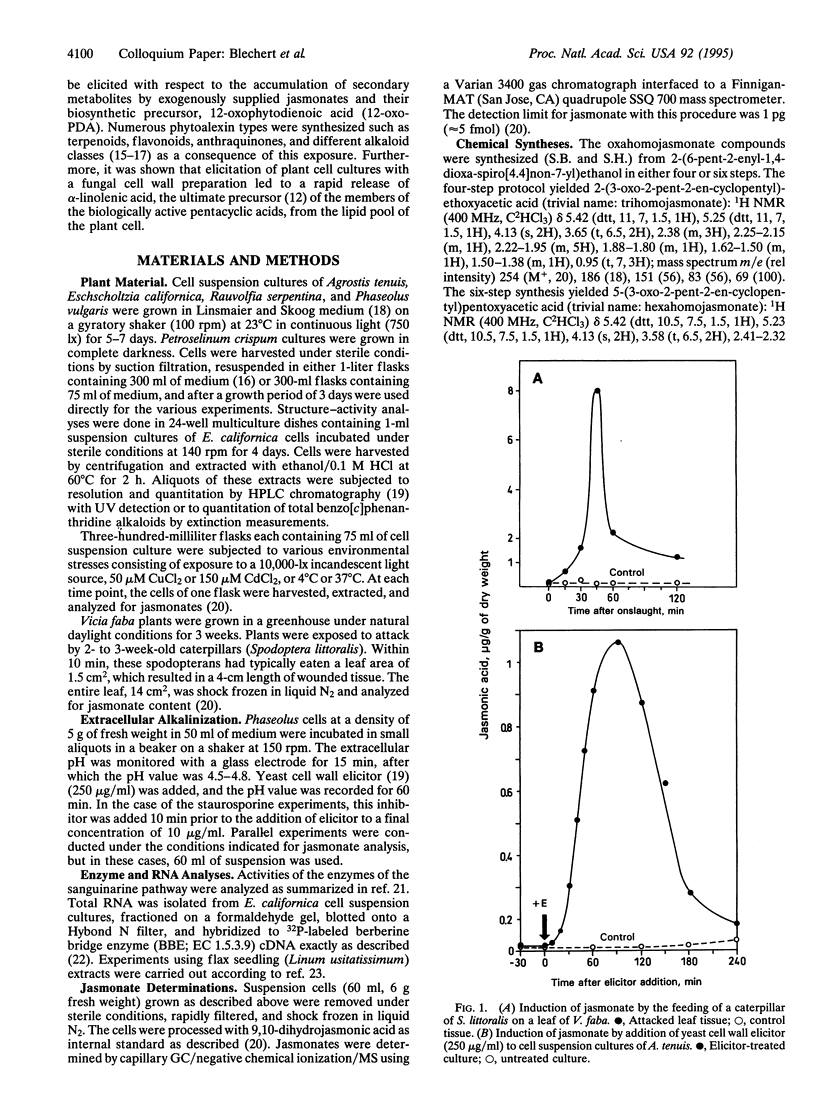
- Gundlach H., Müller M. J., Kutchan T. M., Zenk M. H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagendoorn M. J., Poortinga A. M., Sang H. W., van der Plas L. H., van Walraven H. S. Effect of Elicitors on the Plasmamembrane of Petunia hybrida Cell Suspensions : Role of DeltapH in Signal Transduction. Plant Physiol. 1991 Aug;96(4):1261–1267. doi: 10.1104/pp.96.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
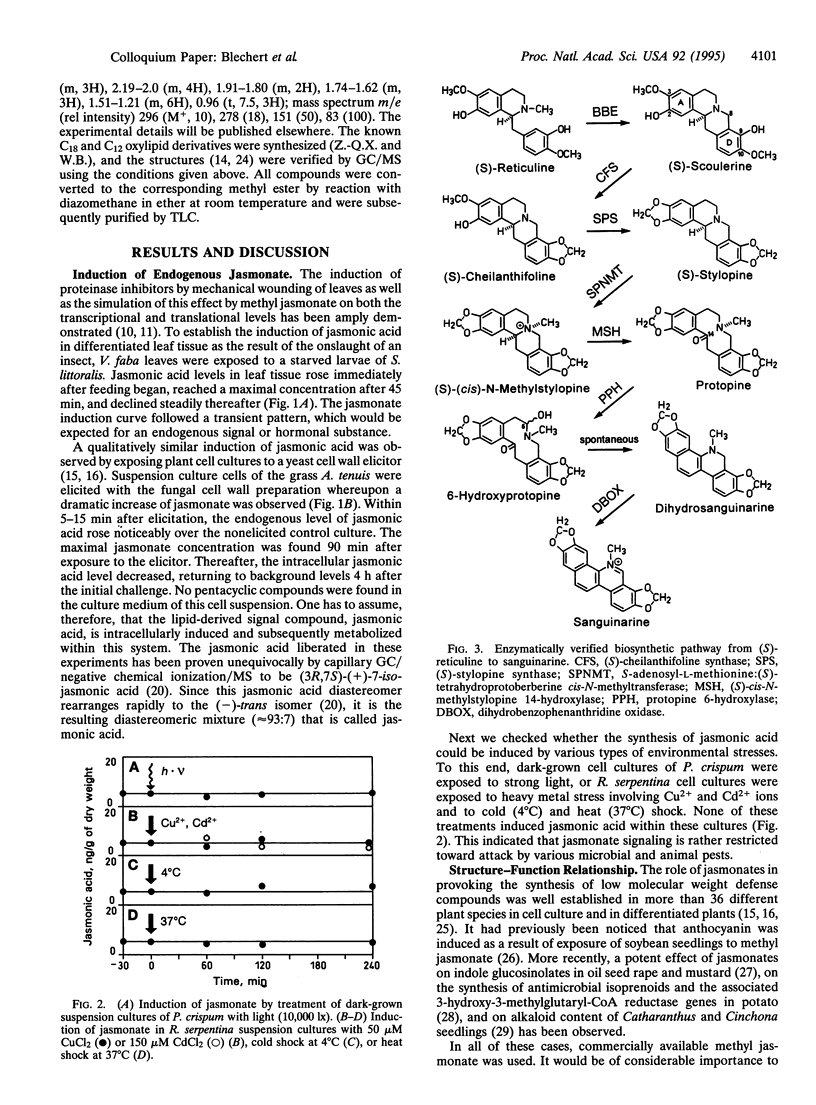
- Hain R., Bieseler B., Kindl H., Schröder G., Stöcker R. Expression of a stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytoalexin resveratrol. Plant Mol Biol. 1990 Aug;15(2):325–335. doi: 10.1007/BF00036918. [DOI] [PubMed] [Google Scholar]
- Hain R., Reif H. J., Krause E., Langebartels R., Kindl H., Vornam B., Wiese W., Schmelzer E., Schreier P. H., Stöcker R. H. Disease resistance results from foreign phytoalexin expression in a novel plant. Nature. 1993 Jan 14;361(6408):153–156. doi: 10.1038/361153a0. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Gardner H. W. Oxylipin pathway to jasmonates: biochemistry and biological significance. Biochim Biophys Acta. 1992 Nov 11;1165(1):1–18. doi: 10.1016/0005-2760(92)90069-8. [DOI] [PubMed] [Google Scholar]
- Kombrink E., Hahlbrock K. Responses of cultured parsley cells to elicitors from phytopathogenic fungi : timing and dose dependency of elicitor-induced reactions. Plant Physiol. 1986 May;81(1):216–221. doi: 10.1104/pp.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher E. A., Bate N. J., Ni W., Elkind Y., Dixon R. A., Lamb C. J. Increased disease susceptibility of transgenic tobacco plants with suppressed levels of preformed phenylpropanoid products. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7802–7806. doi: 10.1073/pnas.91.16.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M. J., Brodschelm W. Quantification of jasmonic acid by capillary gas chromatography-negative chemical ionization-mass spectrometry. Anal Biochem. 1994 May 1;218(2):425–435. doi: 10.1006/abio.1994.1202. [DOI] [PubMed] [Google Scholar]
- Mueller M. J., Brodschelm W., Spannagl E., Zenk M. H. Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7490–7494. doi: 10.1073/pnas.90.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe S., Reinbothe C., Lehmann J., Becker W., Apel K., Parthier B. JIP60, a methyl jasmonate-induced ribosome-inactivating protein involved in plant stress reactions. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7012–7016. doi: 10.1073/pnas.91.15.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuballa W., Schillinger E., Stürzebecher C. S., Vorbrüggen H. Synthesis of a new chemically and metabolically stable prostacyclin analogue with high and long-lasting oral activity. J Med Chem. 1986 Mar;29(3):313–315. doi: 10.1021/jm00153a001. [DOI] [PubMed] [Google Scholar]
- Tanahashi T., Zenk M. H. New hydroxylated benzo[c]phenanthridine alkaloids from Eschscholtzia californica cell suspension cultures. J Nat Prod. 1990 May-Jun;53(3):579–586. doi: 10.1021/np50069a007. [DOI] [PubMed] [Google Scholar]
- Vick B. A., Feng P., Zimmerman D. C. Formation of 12-[18-O]oxo-cis-10, cis-15-phytodienoic acid from 13[18-O]hydroperoxylinolenic acid by hydroperoxide cyclase. Lipids. 1980 Jun;15(6):468–471. doi: 10.1007/BF02534074. [DOI] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Biosynthesis of jasmonic Acid by several plant species. Plant Physiol. 1984 Jun;75(2):458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Substrate specificity for the synthesis of cyclic Fatty acids by a flaxseed extract. Plant Physiol. 1979 Mar;63(3):490–494. doi: 10.1104/pp.63.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler E. W., Kutchan T. M., Gorba T., Brodschelm W., Niesel U., Bublitz F. The Pseudomonas phytotoxin coronatine mimics octadecanoid signalling molecules of higher plants. FEBS Lett. 1994 May 23;345(1):9–13. doi: 10.1016/0014-5793(94)00411-0. [DOI] [PubMed] [Google Scholar]
- Williams D. H., Stone M. J., Hauck P. R., Rahman S. K. Why are secondary metabolites (natural products) biosynthesized? J Nat Prod. 1989 Nov-Dec;52(6):1189–1208. doi: 10.1021/np50066a001. [DOI] [PubMed] [Google Scholar]
- Xia Z. Q., Zenk M. H. Metabolism of jasmonic Acid in suspension cultures of eschscholtzia. Planta Med. 1993 Dec;59(6):575–575. doi: 10.1055/s-2006-959770. [DOI] [PubMed] [Google Scholar]