Abstract
Uterine leiomyomatosis is a common disease in women; however, intravenous leiomyomatosis with intracaval and intracardiac tumor extension is rare. We sought to analyze the clinical and echocardiographic features of intracardiac leiomyomatosis.
From January 2003 through July 2012, 7 women (age range, 24–59 yr) underwent surgical resection of histopathologically diagnosed intracardiac leiomyomas at our hospital. Most of the patients had histories of hysterectomy or uterine leiomyoma. We retrospectively analyzed their preoperative echocardiograms. We found that the tumors had no stalks, did not adhere to the wall of the right side of the heart, were highly mobile, and moved back and forth in the right atrium near the tricuspid orifice. All tumors originated from the inferior vena cava and had borders well demarcated from that structure's wall. Most of the masses extended into the inferior vena cava and right atrium through the right internal and common iliac veins. Computed tomograms revealed pelvic tumors and contiguous filling defects in 6 patients.
When echocardiograms reveal a right-sided cardiac mass that originates from the inferior vena cava, particularly in women who have a history of hysterectomy or uterine leiomyoma, intracardiac leiomyomatosis should be suspected. If the mass has no stalk and freely moves within the inferior vena cava and right-sided cardiac chambers without attachment to the endothelial surface or endocardium, intracardiac leiomyomatosis should be diagnosed. We discuss our findings and briefly review the relevant medical literature.
Keywords: Diagnosis, differential; diagnostic imaging/methods; heart neoplasms/pathology/secondary/surgery; leiomyoma/diagnosis/ultrastructure; leiomyomatosis/complications/pathology; neoplasm invasiveness; uterine neoplasms/pathology/ultrastructure; vena cava, inferior/pathology
Intravenous leiomyoma (IVL) is a rare, histologically benign smooth-muscle-cell tumor that occurs only in women. This neoplasm occupies vascular spaces from the intrauterine venules to the systemic veins, including the iliac vein and inferior vena cava (IVC), and it does not invade the tissue. The mass can extend into the right heart chambers and pulmonary arteries.1,2 Its extrauterine involvement occurs in approximately 30% of cases, and intracardiac extension accounts for about 10%.3–5 This extension of IVL into the right side of the heart is called intracardiac leiomyomatosis (ICL).
The diagnosis of ICL can be overlooked. Echocardiography, abdominal ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) are available for detection and diagnosis. Echocardiography is important in the initial diagnosis of ICL. To our knowledge, the literature about ICL chiefly comprises case reports, and the authors of the few case series have not in general discussed the echocardiographic characteristics and extending pathways of ICL. We retrospectively studied the cases of 7 patients with ICL who underwent successful tumor resection in our hospital. We outlined the echocardiographic characteristics of the tumors and analyzed their clinical features, confirmed the extending pathways by means of CT reports, and studied the surgical and pathologic results. We discuss the echocardiographic diagnosis of ICL and briefly review the pertinent medical literature.
Patients and Methods
We reviewed our hospital's clinical database and identified 7 women who had undergone surgical resection of ICL tumors from January 2003 through July 2012. The echocardiographic images included parasternal, apical, and subcostal views. In addition, M-mode, pulsed and continuous-wave Doppler, and color-flow Doppler images were available. The cine loops of 3 consecutive cardiac cycles had been stored digitally for offline analysis. We retrospectively analyzed the patients' clinical and imaging records.
Results
Table I shows the clinical characteristics of the 7 ICL patients. Their mean age was 42.1 ± 10 years (range, 24–59 yr). Patients 2, 6, and 7 had histories of hysterectomy. The other 4 patients had uterine leiomyomas or pelvic masses diagnosed ultrasonographically. Patients 1, 2, 5, and 7 had presented with exertional dyspnea, and Patient 6 had presented with bilateral edema of the lower legs. Patient 4 had presented with abdominal discomfort related to uterine leiomyomas, and her cardiac tumor was detected preoperatively on echocardiography. Patient 3 had no subjective symptoms; her cardiac mass was detected during routine echocardiographic examination.
TABLE I.
Clinical and Echocardiographic Characteristics of the 7 Patients with Intracardiac Leiomyomatosis

Echocardiographic Characteristics. In the echocardiographic parasternal 4-chamber and aortic short-axis views, tumors were seen in the right atrium in 6 of the 7 patients. These tumors had medium echogenicity, no stalk, and no adhesion with the wall of the right side of the heart. They were highly mobile and moved back and forth in the right atrium near the tricuspid orifice (Figs. 1 and 2). In Patient 5, the tumor extended from the right atrium into the right ventricle and pulmonary arteries (Fig. 3). The subcostal view showed that all the tumors originated from the IVC and extended into the right-sided heart chambers. The masses had borders well demarcated from the wall of the IVC, enabling them to wander within the IVC and right side of the heart. Patients 4 and 5 had mild tricuspid regurgitation, and Patient 7 had moderate-to-severe regurgitation.
Fig. 1.

Patient 7. Two-dimensional transthoracic echocardiogram shows a tumor (T) within the right atrium (RA) and inferior vena cava (IVC). The stalkless tumor has borders well demarcated from the wall of the RA and IVC, and no adhesion to the walls of the heart or vein.
Supplemental motion image (1.4MB, mp4) is available for Figure 1.
Fig. 2.

Patient 6. Two-dimensional transthoracic echocardiograms show a freely moving tumor (T) A) within the right atrium (RA) and B) within the inferior vena cava (IVC) (arrowheads) without adhesion to the walls of the vein.
LA = left atrium; LV = left ventricle; RV = right ventricle
Fig. 3.
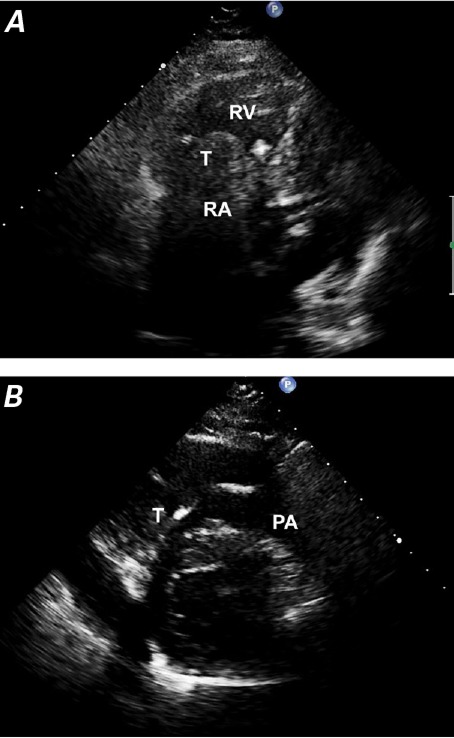
Patient 5. Two-dimensional transthoracic echocardiograms show A) a mobile, solid tumor (T) within the right atrium (RA) that extends into the right ventricle (RV) and B) pulmonary artery (PA).
Confirmation of Extending Pathways. In all 7 patients, CT revealed the overall shape of the tumors and the extending pathways of venous spread. Six patients had pelvic tumors and a contiguous filling defect. In Patients 1, 3, 4, and 7, the tumor extended into the IVC and right atrium through the right internal and common iliac veins (Fig. 4). In Patient 5, the tumor extended into the IVC and right side of the heart through the bilateral ovarian vein, bilateral internal iliac vein, and common iliac veins. In Patient 6, the mass extended into the right atrium through the right ovarian vein, right renal vein, and IVC (Fig. 5). In Patient 2, who had no pelvic tumor, the mass extended to the right atrium from the right internal iliac vein, right common iliac veins, and IVC.
Fig. 4.
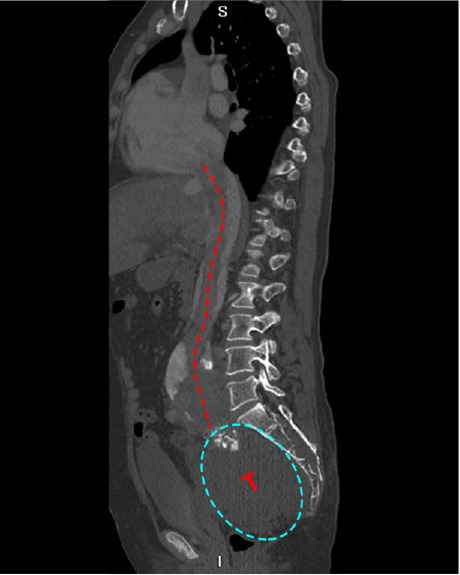
Patient 7. Computed tomogram shows a hypodense pelvic tumor (T) that extends from the right iliac vein into the inferior vena cava and right atrium, suggesting a continuous intravenous lesion.
Fig. 5.

Patient 6. Computed tomogram shows a pelvic tumor (T), an elliptical low-attenuation contiguous filling defect, and another low-attenuation contiguous filling defect, this within the inferior vena cava above the right renal vein.
Surgical Resection. Six patients had been placed under general anesthesia and had undergone single-staged thoracoabdominal surgical resection. Our hospital's departments of gynecology, cardiac surgery, and vascular surgery were involved. The pelvic masses had been excised by the gynecologic team. The tumors within the veins and heart were upwardly dislodged when the right atrium was opened. Patient 1 had undergone a 2-staged procedure: resection of the intracardiac component (with laparotomy for intra-abdominal vascular control), followed by resection of the remaining intra-abdominal tumor. All of the operations were successful.
Histopathologic Analysis. Each pathologic report confirmed ICL. Each mass microscopically resembled classical leiomyomata and had the same histopathologic features: a spindle cell tumor, size consistency, reduced karyokinesis, and a thick mesenchymal wall vessel (Fig. 6).
Fig. 6.

Patient 4. Photomicrograph shows a spindle cell tumor, size consistency, reduced karyokinesis, and a thick mesenchymal wall vessel—classic histopathologic features of intracardiac leiomyomatosis (H & E, orig. ×100).
Discussion
The intracardiac extension of IVL tumors has been documented for some time. In 1907, Mirabeau6 and Dürck7 independently produced what appear to be the first reports of ICL. To our knowledge, only a few other reports about ICL have been published, chiefly single cases,8–11 and our case series might be the first in which the diagnostic usefulness of echocardiography is discussed.
When a patient's cardiac clinical status changes, echocardiography is typically chosen as the first method of imaging. Echocardiography is noninvasive and has no known risks or side effects. It is valuable in detecting and characterizing the relationship of a tumor to the cardiac chambers and valves, revealing the presence of attachment sites, and suggesting effects on valvular function.12
The 7 patients in our hospital were proved histopathologically after surgery to have had ICL. The following echocardiographic evidence should alert clinicians to the possibility of ICL in female patients: a right atrial mass with caval involvement, and an intravascular and intra-atrial mass not attached to the endothelial surface or endocardium, but instead freely mobile within the IVC and right-sided cardiac chambers. These masses are typically long and serpentine, and they can resemble “walking-stick heads” or “snakeheads.”
Two patients in our study had received the misdiagnosis of myxoma in another hospital before they underwent cardiac surgery in our hospital. If a mass is seen in the right atrium but the course is not traced back to the IVC, it might be misdiagnosed as right atrial myxoma, because myxoma is characteristically highly mobile. However, myxomas are usually confined within one cardiac chamber with no prolapse into the IVC and are frequently attached to the interatrial septum.13 Therefore, identifying the point of origin and the site of attachment helps in differentiating ICL from myxoma. When the intra-atrial tumor is observed to originate from the IVC, the differential diagnosis includes right atrial metastatic tumor, thrombus, and primary leiomyosarcoma. Metastatic tumors such as renal cell carcinoma, hepatoma, adrenal tumor, and uterine leiomyosarcoma can extend into the right-sided cardiac chambers via the IVC. These tumors are typically fixed and have a mass-like appearance, rather than that of a venous cast of long, thin, mobile structures with a primary tumor present.14 Thrombus is clearly identifiable throughout the cardiac cycle, adjacent to asynergic myocardium or regions where there is stasis of blood. Thrombus generally develops in the presence of an acute postoperative state, indwelling central catheters, or chronic debility, and especially at the junction of the superior vena cava and right atrium.15 Primary leiomyosarcoma of the IVC invading the right atrium can have an appearance similar to that of ICL, but it rarely involves the ovarian vein. Unlike ICL, which is located in the vessel lumen, a caval sarcoma originates from the mural wall of the vessel.16
Imaging methods such as CT and MRI are chosen for their multiplanar capabilities and large fields of view. Although ICL is histologically benign, it can be biologically and clinically aggressive and take several recognized pathways of venous spread. Accordingly, CT or MRI can aid in diagnosis and in determining the extent of tumor involvement.
Lam and colleagues2 reported that ICL can follow 2 distinct routes into the systemic venous circulation: chiefly the uterine vein, and sometimes the ovarian vein. Growth in the uterine vein can extend into the internal iliac veins, common iliac veins, and IVC. In contrast, growth in the ovarian vein can extend into the subphrenic portion of the IVC and bypass the iliac veins. Right ovarian vein involvement can extend directly to the IVC; left ovarian vein involvement extends to the left renal vein before reaching the IVC. In our ICL patients, the extending pathways were consistent with those in previous reports: 5 of the tumors extended into the IVC and right side of the heart through the uterine vein, and two grew into those areas through the ovarian vein. One of these two (in Patient 6) was a special case: the mass extended into the right atrium through the right ovarian vein, right renal vein, and IVC. The right ovarian vein connected to the right renal vein as a congenital anomaly.
Intracardiac leiomyomatosis occurs only in women. The clinical characteristics of ICL are usually similar to those of typical uterine leiomyomas and relate to the obstructive effect of the tumor on the tricuspid orifice and the disturbance of venous return. Symptoms can include dyspnea on exertion, syncopal episodes, pulmonary embolism, or sudden death.5,17,18 In our study, 4 patients presented with exertional dyspnea, and one of the 7 (14.3%) was asymptomatic. Approximately 10% of ICL patients are reportedly asymptomatic. In this circumstance, a diagnosis is made when abnormal physical examination findings prompt further investigation, or when discovery is incidental during investigation into an unrelated medical problem. Successful treatment relies on a multidisciplinary surgical approach with a single- or 2-stage procedure, involving resection of the pelvic tumor and upward dislodgment of the intracardiac and intravascular tumor by opening the right atrium.
When echocardiograms reveal a right-sided cardiac mass originating from the IVC in women—particularly in those with histories of hysterectomy or uterine leiomyoma—ICL should be suspected. If the tumor has no stalk and freely moves within the IVC and right-sided cardiac chambers without attachment to the endothelial surface or endocardium, ICL should be diagnosed.
Acknowledgments
We thank Sonya E. Fogg, MLS, Manager, Library and Learning Resource Center, Texas Heart Institute, for researching and confirming our historical references.
Footnotes
From: Departments of Ultrasound (Drs. Jiang, Li, Su, Sun, J. Yang, and Y. Yang) and Radiology (Drs. Shen and Zhang), Beijing Anzhen Hospital, Capital Medical University, Beijing 100029, People's Republic of China
References
- 1.Clement PB. Intravenous leiomyomatosis of the uterus. Pathol Annu. 1988;23(Pt 2):153–83. [PubMed] [Google Scholar]
- 2.Lam PM, Lo KW, Yu MY, Wong WS, Lau JY, Arifi AA, Cheung TH. Intravenous leiomyomatosis: two cases with different routes of tumor extension. J Vasc Surg. 2004;39(2):465–9. doi: 10.1016/j.jvs.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Castelli P, Caronno R, Piffaretti G, Tozzi M. Intravenous uterine leiomyomatosis with right heart extension: successful two-stage surgical removal. Ann Vasc Surg. 2006;20(3):405–7. doi: 10.1007/s10016-006-9024-0. [DOI] [PubMed] [Google Scholar]
- 4.Mulvany NJ, Slavin JL, Ostor AG, Fortune DW. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 22 cases. Int J Gynecol Pathol. 1994;13(1):1–9. doi: 10.1097/00004347-199401000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Baca Lopez FM, Martinez-Enriquez A, Castrejon-Aivar FJ, Ruanova-Leon D, Yanez-Gutierrez L. Echocardiographic study of an intravenous leiomyoma: case report and review of the literature. Echocardiography. 2003;20(8):723–5. doi: 10.1111/j.0742-2822.2003.02152.x. [DOI] [PubMed] [Google Scholar]
- 6.Mirabeau S. Berichte aus Gynäkologische Gesellschaften und Krankenhäusern [in German] Zentralbl Gynäkol. 1907;31(Pt 2):1604–6. Available from: http://books.google.com/books?oe=UTF-8&id=RjwDAAAAYAAJ&q=1604#v=snippet&q=1604&f=false [cited 2014 Sep 10] [Google Scholar]
- 7.Dürck H. Ueber ein Kontinvierlich durch die entere Hohlvene in das Herz vor wachsendes: Fibromyom des uterus [in German] Münchener Med Wochenschr. 1907;54(2):1154. Available from: http://babel.hathitrust.org/cgi/pt?id=uc1.$c 59405;view=1up;seq=512 [cited 2014 Sep 10] [Google Scholar]
- 8.Singh T, Lamont PM, Otton GR, Thomson DS. Intravenous leiomyomatosis with intracardiac extension: first reported case in Australia. Heart Lung Circ. 2010;19(1):50–2. doi: 10.1016/j.hlc.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Peters PJ, Reinhardt S. The echocardiographic evaluation of intracardiac masses: a review. J Am Soc Echocardiogr. 2006;19(2):230–40. doi: 10.1016/j.echo.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Leitman M, Kuperstein R, Medalion B, Stamler A, Porat E, Rosenblatt S et al. A highly unusual right atrial mass presented in two women. Eur J Echocardiogr. 2008;9(6):833–4. doi: 10.1093/ejechocard/jen173. [DOI] [PubMed] [Google Scholar]
- 11.Nishizawa J, Matsumoto M, Sugita T, Matsuyama K, Tokuda Y, Yoshida K et al. Intravenous leiomyomatosis extending into the right ventricle associated with pulmonary metastasis and extensive arteriovenous fistula. J Am Coll Surg. 2004;198(5):842–3. doi: 10.1016/j.jamcollsurg.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38(3):261–2. [PMC free article] [PubMed] [Google Scholar]
- 13.Obrenovic-Kircanski B, Mikic A, Parapid B, Djukic P, Kanjuh V, Milic N et al. A 30-year-single-center experience in atrial myxomas: from presentation to treatment and prognosis. Thorac Cardiovasc Surg. 2013;61(6):530–6. doi: 10.1055/s-0032-1322545. [DOI] [PubMed] [Google Scholar]
- 14.Yuan SM, Shinfeld A, Lavee J, Kuperstein R, Haizler R, Raanani E. Imaging morphology of cardiac tumours. Cardiol J. 2009;16(1):26–35. [PubMed] [Google Scholar]
- 15.Acikel S, Dogan M, Akdemir R, Kilic H, Yesilay AB, Cagirci G. Multisided cardiac hemangiomas mimicking biatrial thrombus: atypically located cardiac hemangiomas of left atrial appendage and right atrium. J Am Soc Echocardiogr. 2009;22(4):434.e7–9. doi: 10.1016/j.echo.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Aal AK, Gaddikeri S, Saddekni S, Oser RF, Underwood E, Wei S. Primary leiomyosarcoma of the inferior vena cava invading the right atrium: a technique for intraluminal biopsy through a transvenous approach. Vasc Endovascular Surg. 2011;45(8):743–6. doi: 10.1177/1538574411418009. [DOI] [PubMed] [Google Scholar]
- 17.Worley MJ, Jr, Aelion A, Caputo TA, Kent KC, Salemi A, Krieger KH et al. Intravenous leiomyomatosis with intracardiac extension: a single-institution experience. Am J Obstet Gynecol. 2009;201(6):574.e1–5. doi: 10.1016/j.ajog.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu CK, Luo JL, Yang CY, Huang YT, Wu XM, Cheng CL et al. Intravenous leiomyomatosis with intracardiac extension. Intern Med. 2009;48(12):997–1001. doi: 10.2169/internalmedicine.48.1780. [DOI] [PubMed] [Google Scholar]


