Abstract
Carcinoid tumors are rare neuroendocrine malignancies that typically originate from the gastrointestinal tract. Patients who are diagnosed with carcinoid heart disease generally have poor prognoses because of advanced metastases during staging and few therapeutic options. We present the case of a 61-year-old woman with right-sided heart failure, secondary to carcinoid heart disease caused by a primary ovarian carcinoid tumor. After undergoing surgical resection of the left ovary and fallopian tube, the patient experienced complete resolution of her heart failure symptoms. In addition to the patient's case, we discuss the diagnosis, nature, and treatment of this rare condition.
Keywords: Carcinoid heart disease/diagnosis/etiology/pathology/radiography/surgery, heart valves/pathology, ovarian neoplasms/complications/surgery, treatment outcome, tricuspid valve insufficiency/etiology
Carcinoid tumors are rare neuroendocrine malignancies that typically arise from the gastrointestinal tract; their prevalence is 1 in 100,000 in the general population.1 Primary ovarian carcinoid tumor constitutes only 0.5% of all carcinoid tumors.2–4 In 10% of patients, carcinoid syndrome will develop when vasoactive substances released by the tumor drain into the inferior vena cava and the systemic circulation.1 These substances initiate a process of endocardial fibrosis that leads to the formation of carcinoid plaques, typically on the right-sided heart valves.1,5 These plaques can cause valvular dysfunction and right-sided heart failure.6 We present the case of a woman with right-sided heart failure secondary to carcinoid syndrome that was caused by a primary ovarian carcinoid tumor.
Case Report
In September 2011, a 61-year-old woman with hypertension, hyperlipidemia, and diabetes mellitus presented at our cardiology clinic with a 2-month history of worsening bilateral lower-extremity edema, dyspnea on exertion, nausea, abdominal bloating, loose stools, and a 20-lb weight gain. She had no history of tobacco or illicit-drug use, alcohol abuse, or anorexigen exposure. Physical examination revealed a grade 2/6 holosystolic murmur at the left sternal border, jugular venous pressure elevated to the angle of the jaw with a prominent V wave, an abdominal fluid wave, and severe lower-extremity edema. A transthoracic echocardiogram revealed normal left ventricular size and function; interventricular septal flattening during systole and diastole was consistent with right ventricular (RV) volume or pressure overload. The RV was severely enlarged, with normal systolic function. The tricuspid annulus was markedly dilated. The retracted, shortened tricuspid leaflets displayed restricted mobility and poor coaptation (Fig. 1). Severe tricuspid regurgitation was apparent upon color-flow Doppler echocardiographic analysis (Fig. 2). The patient's peak pulmonary artery pressure was mildly increased. The pulmonic valve was poorly visible and had only mild-tomoderate regurgitation. The aortic and mitral valves were normal in appearance.
Fig. 1.
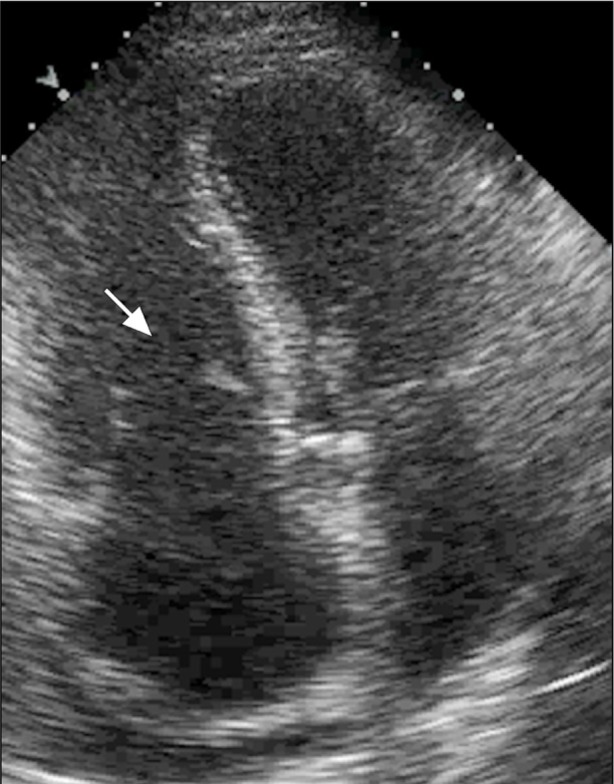
Transthoracic echocardiogram (apical 4-chamber view) shows a severely dilated right ventricle and tricuspid annulus. The tricuspid leaflets (seen during diastole, arrow) are retracted and shortened and exhibit poor coaptation.
Supplemental motion image (1.5MB, m1v) is available for Figure 1.
Fig. 2.
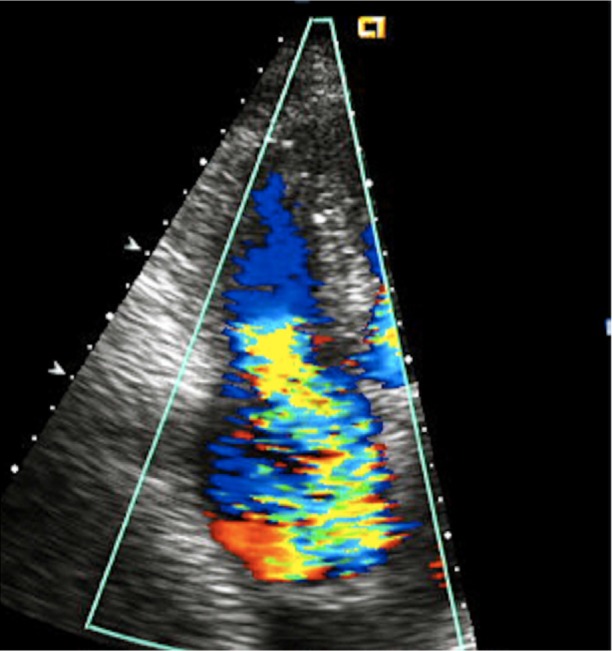
Transthoracic echocardiogram (apical 4-chamber view) shows severe tricuspid regurgitation upon color-flow Doppler analysis of the right atrium.
Supplemental motion image (1.5MB, m1v) is available for Figure 2.
A computed tomographic scan of the abdomen and pelvis was performed to evaluate the patient's gastrointestinal symptoms. It revealed a 6 × 6 × 7-cm left pelvic mass with abdominal and pelvic ascites (Fig. 3). Ultrasonographic images of the pelvis showed a complex, solid left adnexal mass. On the basis of these studies, the patient was referred for an exploratory laparotomy, bilateral salpingooopherectomy, and omentectomy. Resection of the left ovary and fallopian tube revealed a 164-g ovoid mass, 7.8 × 5.9 × 4.7 cm in size (Fig. 4). The tumor cells stained strongly positive for chromogranin and synaptophysin, and the final pathologic diagnosis was low-grade epithelial carcinoid neoplasm (Fig. 5). On the basis of the echocardiographic and pathologic findings, the diagnosis was carcinoid heart disease caused by a primary ovarian carcinoid tumor.
Fig. 3.
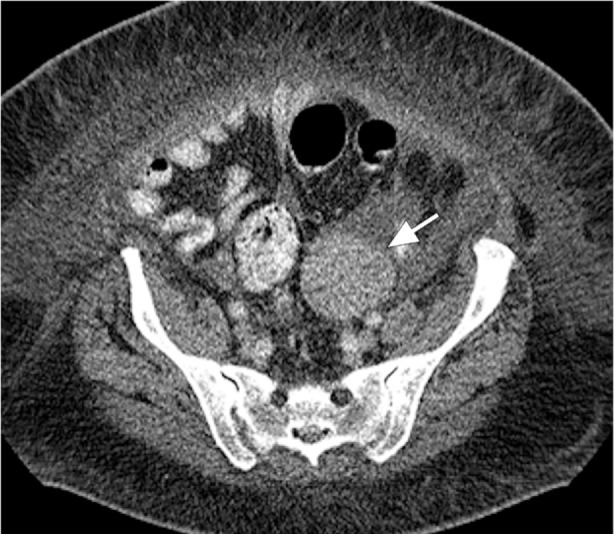
Computed tomogram of the abdomen and pelvis shows the ovarian tumor, a 6 × 6-cm left adnexal mass (arrow), medial to the left iliac process, with well-defined margins. The homogeneous mass displays hypercontrast enhancement without fat or calcification. No invasion of surrounding structures or abnormal lymph node in the pelvis suggests any metastatic disease.
Fig. 4.
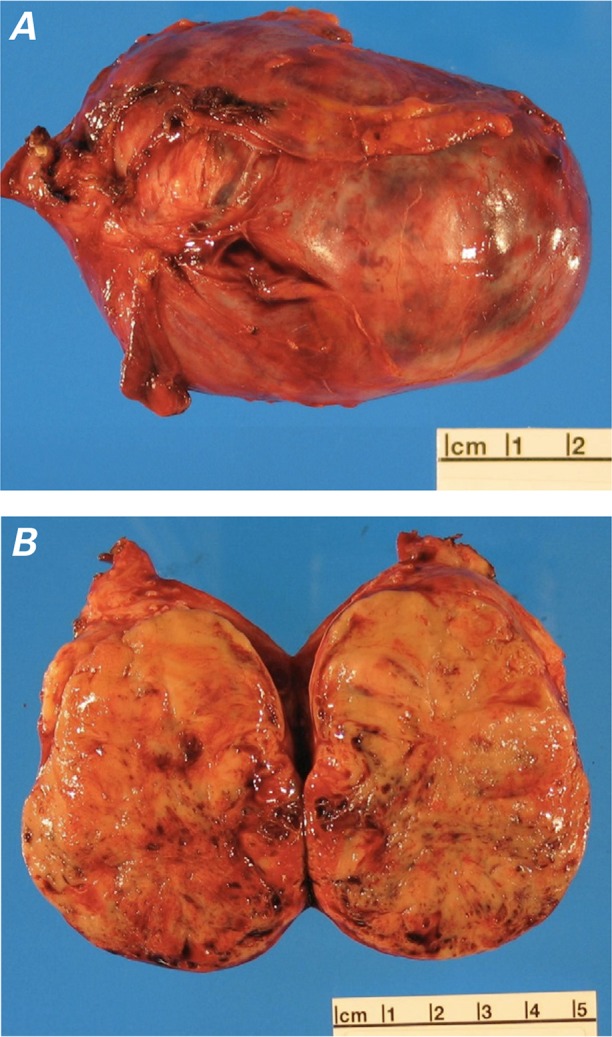
Photographs of the excised mass. A) The left ovarian mass weighed 164 g, was 7.8 × 5.9 × 4.7 cm in size, and had erythematous serosal surfaces with minimal fibrous adhesions and no gross evidence of surface involvement. B) The cut surface of the mass had solid, pale brown parenchyma with scattered foci of hemorrhage.
Fig. 5.
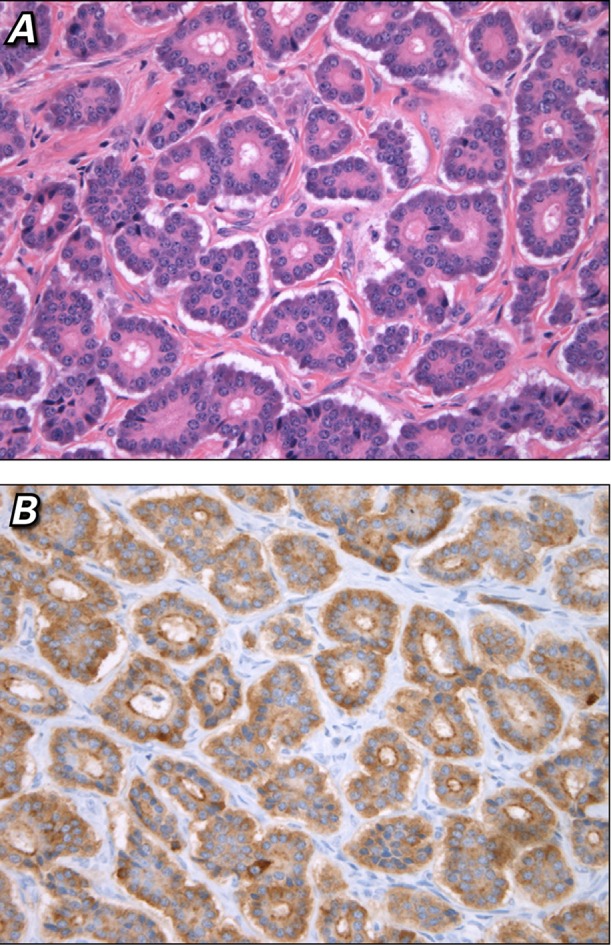
Photomicrographs. A) The mass was composed of a neoplastic population of small, bland epithelial cells with round nuclei, finely granular chromatin, and small nucleoli, which primarily formed an insular architectural pattern with nests of cells admixed with small acini (H & E, orig. ×400). B) The neoplastic cells uniformly expressed chromogranin and synaptophysin (immunohistochemical stain, orig. ×400).
After tumor resection, the patient's symptoms of right-sided heart failure completely resolved: she was in New York Heart Association (NYHA) functional class I, with normal RV systolic function. A postoperative transthoracic echocardiogram showed persistent, severe tricuspid regurgitation with a severely enlarged RV and normal systolic function. The patient was closely monitored by her oncologist for more than one year. She continued to have negative carcinoid tumor markers and no evidence of an intestinal tumor on serial computed tomograms of the abdomen and pelvis. As of April 2013, she was asymptomatic; however, symptoms of heart failure might recur.
Discussion
This patient's symptoms of facial flushing, abdominal bloating, and diarrhea are characteristic of carcinoid syndrome and are thought to be caused by tumor secretion of bioactive substances, including histamine, bradykinins, prostaglandins, tachykinins, and serotonin.7 The classic echocardiographic findings of carcinoid heart disease included short, retracted, and thickened tricuspid valve leaflets and severe tricuspid regurgitation.5
Patients with carcinoid heart disease typically have a poor prognosis, because they present after the tumor has metastasized to the liver from the gastrointestinal tract. Treatment options include surgery, chemotherapy, or both; however, these yield limited benefit in this population.7 Our patient had no evidence of metastatic disease but developed carcinoid heart disease from an ovarian tumor that secreted vasoactive substances directly into the venous system, bypassing the portal circulation. In these rare cases, tumor resection can be curative; however, valvular dysfunction often persists, necessitating valve replacement.8
The optimal timing of valve replacement in relation to the severity of valvular dysfunction and heart failure is unclear. Patients undergoing valve replacement for carcinoid heart disease reportedly have worse clinical outcomes when they present with NYHA class III/IV symptoms and moderate-to-severe RV enlargement on echocardiography.8,9 Waiting for heart failure and ventricular remodeling to progress might substantially increase the patient's perioperative risk.8,9 To date, only a few case reports have described successful valve replacement surgery in a patient with primary ovarian carcinoid and right-sided heart failure due to tricuspid regurgitation.10–12
This case classically illustrates the presentation of right-sided heart failure caused by carcinoid syndrome and affirms the diagnostic importance of echocardiography in carcinoid heart disease. Our patient experienced complete resolution of her heart failure after surgical resection of the tumor and without replacement of the culprit tricuspid valve. We are aware of only one other case report of a patient with severe tricuspid regurgitation who had a similar clinical response.13
Footnotes
From: Department of Medicine, Division of Cardiology (Drs. Goldman and Yang) and Department of Pathology (Dr. Adamson), University of Washington, Seattle, Washington 98195
References
- 1.Bhattacharyya S, Davar J, Dreyfus G, Caplin ME. Carcinoid heart disease. Circulation. 2007;116(24):2860–5. doi: 10.1161/CIRCULATIONAHA.107.701367. [DOI] [PubMed] [Google Scholar]
- 2.Nyktari E, Patrianakos AP, Parthenakis FI, Koutsopoulos A, Vardas PE. Carcinoid heart disease in a patient with primary ovarian carcinoid tumor. Hellenic J Cardiol. 2007;48(4):234–5. [PubMed] [Google Scholar]
- 3.Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79(4):813–29. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Shin HW, Kim H, Yoon HJ, Park HS, Cho YK, Nam CW et al. Ovarian tumor-associated carcinoid heart disease presenting as severe tricuspid regurgitation. J Cardiovasc Ultrasound. 2011;19(1):45–9. doi: 10.4250/jcu.2011.19.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellikka PA, Tajik AJ, Khandheria BK, Seward JB, Callahan JA, Pitot HC, Kvols LK. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993;87(4):1188–96. doi: 10.1161/01.cir.87.4.1188. [DOI] [PubMed] [Google Scholar]
- 6.Zuetenhorst JM, Bonfrer JM, Korse CM, Bakker R, van T, interen H, Taal BG. Carcinoid heart disease: the role of urinary 5-hydroxyindoleacetic acid excretion and plasma levels of atrial natriuretic peptide, transforming growth factor-beta and fibroblast growth factor. Cancer. 2003;97(7):1609–15. doi: 10.1002/cncr.11226. [DOI] [PubMed] [Google Scholar]
- 7.Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128(6):1717–51. doi: 10.1053/j.gastro.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Moller JE, Pellikka PA, Bernheim AM, Schaff HV, Rubin J, Connolly HM. Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation. 2005;112(21):3320–7. doi: 10.1161/CIRCULATIONAHA.105.553750. [DOI] [PubMed] [Google Scholar]
- 9.Connolly HM, Schaff HV, Mullany CJ, Abel MD, Pellikka PA. Carcinoid heart disease: impact of pulmonary valve replacement in right ventricular function and remodeling. Circulation. 2002;106(12 Suppl 1):I51–I56. [PubMed] [Google Scholar]
- 10.Takahashi H, Okada K, Asano M, Matsumori M, Morimoto Y, Okita Y. Bioprosthetic pulmonary and tricuspid valve replacement in carcinoid heart disease from ovarian primary cancer. Circ J. 2009;73(8):1554–6. doi: 10.1253/circj.cj-08-0561. [DOI] [PubMed] [Google Scholar]
- 11.Bonaros N, Muller S, Bonattie J, Kafka R, Tzankov A, Bale R, Bartel T. Primary ovarian carcinoid heart disease curatively treated with a two-stage procedure. Thorac Cardiovasc Surg. 2007;55(7):467–9. doi: 10.1055/s-2006-955908. [DOI] [PubMed] [Google Scholar]
- 12.Sworn MJ, Edlin GP, McGill DA, Mousley JS, Monro JL. Tricuspid valve replacement in carcinoid syndrome due to ovarian primary. Br Med J. 1980;280(6207):85–6. doi: 10.1136/bmj.280.6207.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez O, Alberca MT, Santonja C. Echocardiographic diagnosis of carcinoid disease: exceptional evolution after ovarian tumor resection without cardiac surgery. Eur J Echocardiogr. 2007;8(6):497–500. doi: 10.1016/j.euje.2006.07.003. [DOI] [PubMed] [Google Scholar]


