Abstract
Primary malignant cardiac tumors are rare. Among these tumors, cardiac plasmacytoma is extremely rare and is the subject of few case reports. We present the case of a 73-year-old man who had isolated cardiac plasmacytoma 26 years after successful treatment of an axillary plasmacytoma. Multiple imaging methods—including echocardiography, cardiac magnetic resonance, and positron-emission tomography/computed tomography—were valuable and complementary to each other in this patient's diagnosis and optimal management. His case illustrates the use of these techniques in the successful diagnosis and treatment of a rare clinical entity, cardiac plasmacytoma.
Keywords: Antineoplastic combined chemotherapy protocols/therapeutic use, combined modality diagnosis, combined modality therapy, extramedullary plasmacytoma, heart neoplasms/diagnosis/pathology/therapy/ultrasonography, immunoglobulin light chains, immunoglobulin kappa-chains, magnetic resonance imaging/methods, plasmacytoma/diagnosis/chemotherapy/radiotherapy/ultrasonography, positron-emission tomographic/computed tomography
Newer imaging methods in cardiology have facilitated the recognition, diagnosis, and characterization of various cardiac masses. Our patient was a 73-year-old man with a history of soft-tissue plasmacytoma, who presented 26 years later with cardiac involvement. In the course of his treatment, we had the opportunity to use multiple imaging methods that proved extremely valuable in locating and characterizing this cardiac tumor.
Case Report
A 73-year-old white man was diagnosed with acute pancreatitis and gallstones in September 2010. During the preoperative evaluation for cholecystectomy, transthoracic echocardiography (TTE) revealed both a mass in the heart that involved the apical septum and a small pericardial effusion (Fig. 1).
Fig. 1.
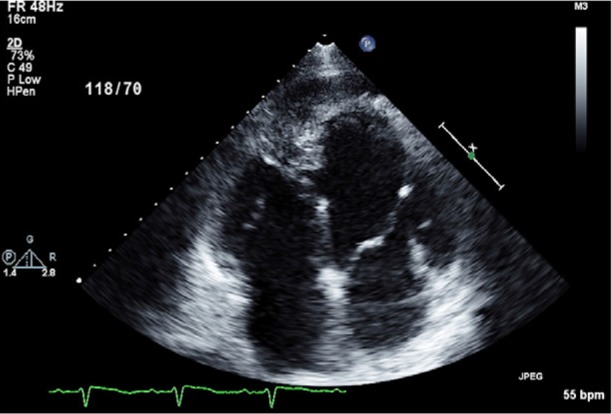
Transthoracic echocardiogram (4-chamber view) reveals, in the right ventricular apex, an echogenic mass that involves the distal interventricular septum.
Twenty-six years earlier, the patient had undergone surgical excision of a left axillary plasmacytoma; when the plasmacytoma had recurred in that same location one year later, he had been treated with excision and local radiotherapy. Twelve years before the present admission, he had experienced a myocardial infarction, for which he had undergone angioplasty and stent placement in the left anterior descending coronary artery. His risk factors included type 2 diabetes mellitus, systemic arterial hypertension, and hyperlipidemia.
After our discovery of the endocardial mass invading the interventricular septum, an endomyocardial biopsy revealed sheets of mononuclear cells with eccentric nuclei and moderately abundant cytoplasm; occasional Russell bodies were seen (Fig. 2). Immunohistochemical staining for CD138 was strongly positive in these cells. These findings suggested that the cells were plasma cells and that the mass was a plasmacytoma.
Fig. 2.
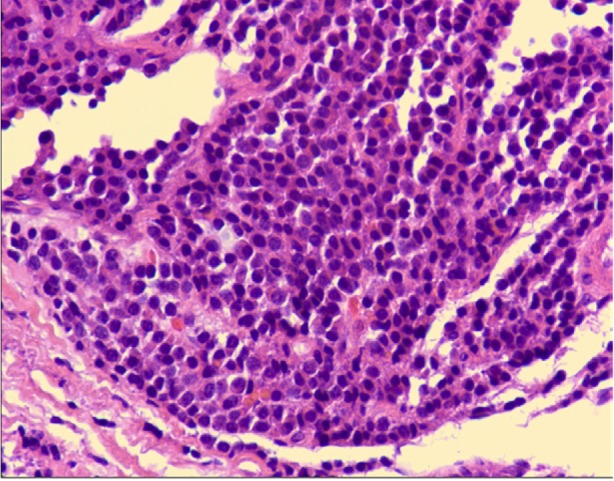
Photomicrograph shows sheets of mononuclear cells with eccentric nuclei and moderately abundant cytoplasm; occasional Russell bodies are seen (H & E, orig. ×600).
On initial evaluation, our patient had mild dyspnea on exertion. His blood pressure was 121/76; heart rate, 73 beats/min; and respiratory rate, 18 breaths/min. An electrocardiogram showed sinus rhythm, left anterior hemiblock, and inverted T waves in leads V1 through V6. A skeletal survey revealed osteolytic lesions in the calvaria and on the 2nd cervical vertebra (C2). Serum protein electrophoresis revealed a faint abnormal band, too small to quantitate; but it was identified via immunofixation as an immunoglobulin G λ band, which was most likely an oligoclonal band. No paraprotein was identified on urine protein electrophoresis, but immunofixation revealed a serum free κ light-chain band. The serum free light-chain analysis showed an elevated κ light chain of 53.8 mg/L, with an abnormal κ:λ ratio of 2.38. This finding is consistent with the cardiac biopsy report that our patient's abnormal cells expressed κ light chain.
Bone marrow biopsy specimens revealed neither morphologic nor immunophenotypic evidence of myeloma. Cytogenetic findings were of a normal male karyotype. These negative findings suggested that the patient had a rare solitary cardiac plasmacytoma.
The patient underwent further investigation with TTE, cardiac magnetic resonance (CMR), and a positron-emission tomographic/computed tomographic (PET/CT) scan to better identify the tumor's margins and the extent of cardiac infiltration (Fig. 1). The PET/CT results also further characterized the skeletal lesions at the calvaria and the C2.
A TTE showed a mass that involved the apex, filled the pericardial space, and extended even beyond. It also revealed a small congenital ventricular septal defect with left-to-right shunting. The left ventricular (LV) ejection fraction was 0.30.
Cardiac magnetic resonance from the 3-chamber view, aided by gadolinium intravenous contrast administration, clearly revealed a mass—isointense on T1 and T2 weighting—that involved the distal ventricular septum and was 5.7 × 2.2 cm in size (Fig. 3).
Fig. 3.
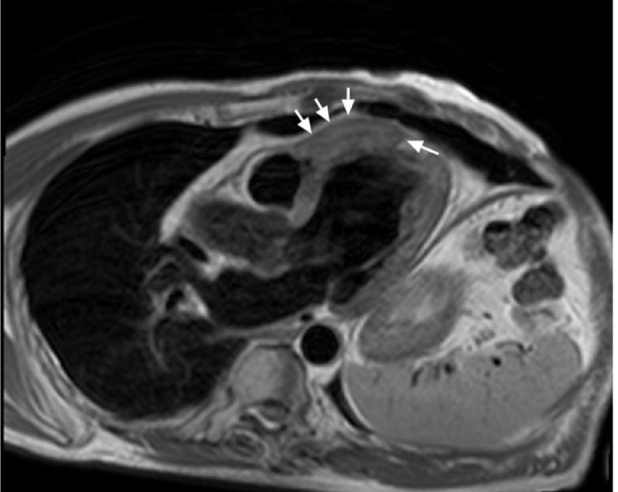
Cardiac magnetic resonance image with T2 weighting (3-chamber view) shows the 5.7 × 2.2-cm tumor (arrows).
A whole-body PET/CT scan with intravenous fluorodeoxyglucose (Fig. 4) showed an area of increased tracer uptake, which signified a tumor. The tumor involved the anterior LV and the apical intraventricular septum. It was 5.7 × 3 cm, which was similar to the CMR measurement. The maximum standardized uptake value (SUV) was 8. Focal uptake of tracer was also seen in the 4th anterior rib, just anterior to the mass. Furthermore, PET/CT scanning showed only marginal uptake in a small lesion in the right frontal bone, and no uptake in the C2 lesion.
Fig. 4.
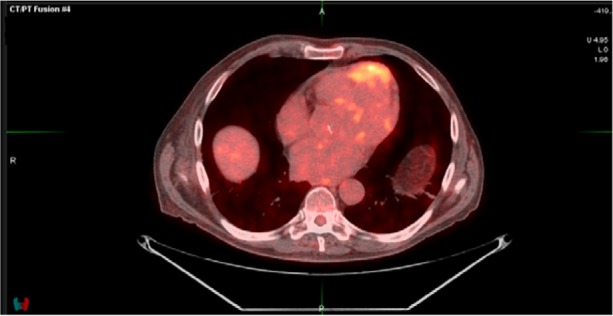
Positron-emission tomographic/computed tomographic image (axial view) reveals, in the anterior portion of the heart, a large hypermetabolic mass that displays intense uptake of fluorodeoxyglucose.
After multidisciplinary evaluation by staff members of cardiothoracic surgery, cardiology, hematology/oncology, and radiation oncology, the lesion was thought to be unresectable. We planned chemotherapy, to be followed by radiotherapy. In light of the patient's severely reduced LV ejection fraction, radiotherapy for the cardiac plasmacytoma was not considered for the early stage of treatment. Because of the size and location of the lesion, therapeutic doses could have compromised cardiac function still further. We reasoned that a favorable response to the chemotherapeutic regimen would result in a smaller cardiac target, a lower radiation dose, and possibly less toxicity.
The patient was treated with 6 cycles of cyclophosphamide, bortezomib, and dexamethasone. The patient had no exacerbation of cardiac symptoms. He developed peripheral neuropathy because of the bortezomib, which responded to gabapentin. After the completion of chemotherapy, CMR images showed a mass slightly reduced in size (Fig. 5). More specifically, the tumor was 5 × 1.2 cm on the CMR 3-chamber view and was again isointense on T1- and T2-weighted imaging and cine imaging.
Fig. 5.
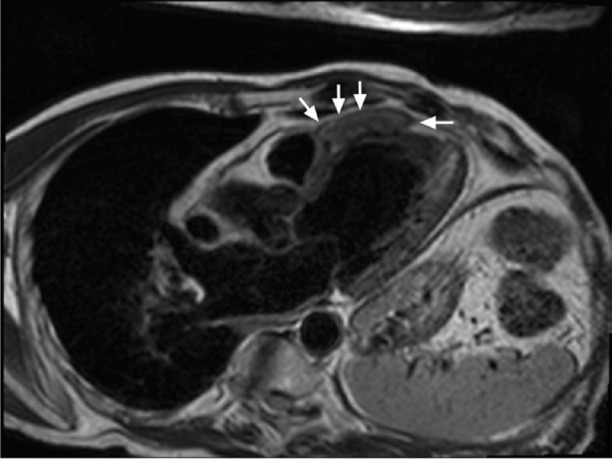
Post-chemotherapy cardiac magnetic resonance image with T2 weighting (3-chamber view) shows the slightly reduced size (arrows) of the mass (5 × 1.2 cm) before radiotherapy.
Radiotherapy consisted of a 3-dimensional technique that used 18-MV tangential beam and daily image guidance to target the CMR-defined tumor. The total radiation dose was 20 Gy, delivered in 10 fractions. The radiation treatment was well tolerated. A PET scan performed 3 months after the cessation of treatment showed a diminished area of tracer uptake in the anterior LV and the cardiac apex.
The patient remained free of chest pain, shortness of breath, and arrhythmias 6 months after completion of his treatment protocol. A PET/CT scan revealed an impressive reduction in tumor size (Fig. 6).
Fig. 6.
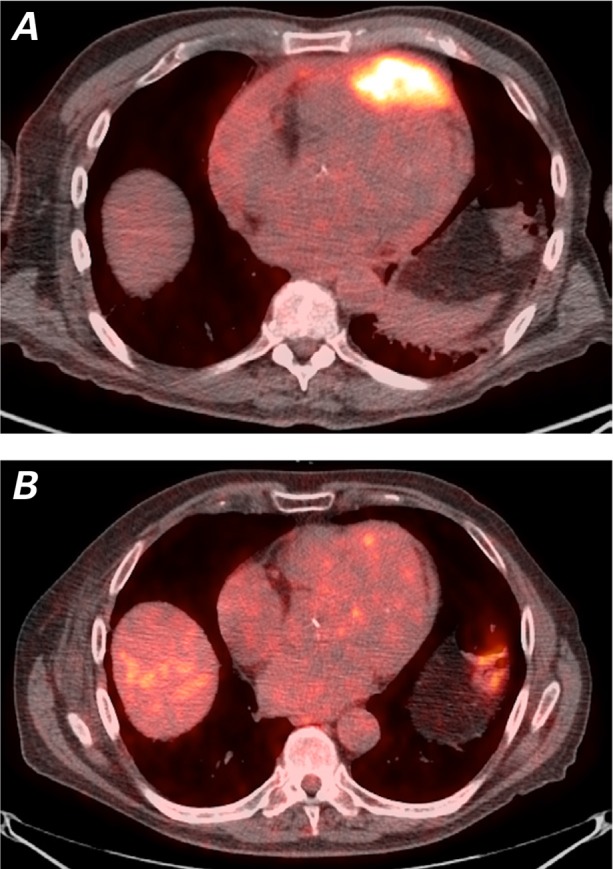
Positron-emission tomographic/computed tomographic images (axial views) show A) the large mass in the anterior portion of the heart at the time of presentation and B) substantial reduction of tumor size post-radiotherapy. Almost no tracer uptake is visible at the site of the previously existing hypermetabolic mass.
Discussion
Malignant cardiac tumors are rare entities. Most affect the myocardium through direct invasion, or by venous, arterial, or lymphatic metastases.1,2 Most primary tumors of the heart are benign, and most of the benign cardiac tumors are myxomas.3 Primary malignant tumors are very rare and are usually sarcomas.
Cardiac plasmacytoma is an extremely uncommon malignant heart tumor. Plasmacytoma can manifest itself either as a solitary plasma-cell tumor (primary) or as part of multiple-myeloma disease (secondary).4,5 Cardiac involvement can occur in either event. Both conditions are rare entities; only a few cases have been reported.6–14 Primary plasmacytoma can occur as bone plasmacytoma or as extramedullary plasmacytoma.
Our patient had a cardiac plasmacytoma confirmed by cardiac biopsy. Transthoracic echocardiography, possessed of high temporal and spatial resolution, identified a cardiac mass that involved the apex and the pericardial space. Cardiac magnetic resonance clearly revealed the tumor's margins and size. The mass was isointense on T1- and T2-weighted imaging. On CMR, gadolinium administered intravenously further characterized the mass as a vascular structure. Cardiac magnetic resonance offers a wide variety of tools to localize, characterize, and evaluate cardiac masses and their physiologic effects. It provides excellent soft-tissue characterization.15 In addition, PET/CT scanning both shows the mass and identifies contiguous bone involvement.16
Summary. Mutimodality imaging can identify and characterize cardiac tumors in patients with cardiac masses. Visualization by means of echocardiography, MRI, and PET/CT enable evaluation of the extent of tumor involvement in the heart and in structures adjacent to the heart. Once the location and extent of these tumors have been determined, these techniques offer information that can be crucial to treatment and follow-up. Our patient's case illustrates the use of these techniques in the successful diagnosis and treatment of a rare clinical entity, cardiac plasmacytoma.
Addendum
In March 2014, new lymph nodes were discovered in the mediastinum of our patient, along with a retrocardiac mass. Upon biopsy, this area had a microscopic appearance similar to that of the prior biopsy samples. The problem has since been resolved through radiotherapy and chemotherapy.
Footnotes
From: Departments of Hematology and Medical Oncology (Dr. Heffner), Radiation Oncology and Winship Cancer Institute (Dr. Rossi), and Medicine, Division of Cardiology (Drs. Clements and Vrettou), Emory University School of Medicine, Atlanta, Georgia 30322
Dr. Vrettou is now at Attikon University Hospital, Athens, Greece.
References
- 1.Lymburner RM. Tumours of the heart: histopathological and clinical study. Can Med Assoc J. 1934;39(4):368–73. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC403303/pdf/canmedaj00142-0016.pdf [cited 2014 Jul 23] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruce CJ. Cardiac tumours: diagnosis and management. Heart. 2011;97(2):151–60. doi: 10.1136/hrt.2009.186320. [DOI] [PubMed] [Google Scholar]
- 3.Burke A, Jeudy J, Jr, Virmani R. Cardiac tumours: an update: cardiac tumours. Heart. 2008;94(1):117–23. doi: 10.1136/hrt.2005.078576. [DOI] [PubMed] [Google Scholar]
- 4.Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000;96(6):2037–44. [PubMed] [Google Scholar]
- 5.Serefhanoglu S, Sayinalp N, Haznedaroglu IC, Goker H, Cetiner D, Aksu S et al. Extramedullary plasmacytomas of the thyroid and pericardium as initial presentation of multiple myeloma. Ann Hematol. 2008;87(10):853–4. doi: 10.1007/s00277-008-0484-x. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson K, McElwain TJ, Mackay AM. Myeloma of the heart. Br Heart J. 1974;36(3):309–12. doi: 10.1136/hrt.36.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owens P, Morgan-Hughes G, Kelly S, Ring N, Marshall AJ. Myeloma and a mass in the heart. J R Soc Med. 2003;96(6):288–9. doi: 10.1258/jrsm.96.6.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulus A, Swaika A, Miller KC, Spangenthal EJ, Guo R, Vonfricken K et al. Clinical relapse in a patient with multiple myeloma presenting as an atrial plasmacytoma. J Clin Oncol. 2011;29(3):e47–9. doi: 10.1200/JCO.2010.30.3370. [DOI] [PubMed] [Google Scholar]
- 9.Wong B, Teo K, Taylor D, Nguyen GK. Cardiac plasmacytoma. Cardiovasc Pathol. 2004;13(1):49–53. doi: 10.1016/S1054-8807(03)00095-4. [DOI] [PubMed] [Google Scholar]
- 10.Wan X, Tarantolo S, Orton DF, Greiner TC. Primary extramedullary plasmacytoma in the atria of the heart. Cardiovasc Pathol. 2001;10(3):137–9. doi: 10.1016/s1054-8807(01)00066-7. [DOI] [PubMed] [Google Scholar]
- 11.Torstveit JR, Bennett WA, Hinchcliffe WA, Cornell WP. Primary plasmacytoma of the atrium. Report of a case with successful surgical management. J Thorac Cardiovasc Surg. 1977;74(4):563–6. [PubMed] [Google Scholar]
- 12.Chim CS, Loong F, Ma ES, Cheung W, Chan RH, Ooi GC. Plasma cell problems: case 2. Extramedullary cardiac plasmacytoma presenting with cardiac tamponade. J Clin Oncol. 2005;23(13):3140–3. doi: 10.1200/JCO.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 13.Santana O, Vivas PH, Ramos A, Safirstein S, Agatston AS. Multiple myeloma involving the pericardium associated with cardiac tamponade and constrictive pericarditis. Am Heart J. 1993;126(3 Pt 1):737–40. doi: 10.1016/0002-8703(93)90437-e. [DOI] [PubMed] [Google Scholar]
- 14.Tak T, Rashtian M, De T, ar M, Chandraratna PA, Gill P. An unusual case of metastatic intracardiac plasmacytoma. Can J Cardiol. 1994;10(8):857–60. [PubMed] [Google Scholar]
- 15.O'Donnell DH, Abbara S, Chaithiraphan V, Yared K, Killeen RP, Cury RC, Dodd JD. Cardiac tumors: optimal cardiac MR sequences and spectrum of imaging appearances. AJR Am J Roentgenol. 2009;193(2):377–87. doi: 10.2214/AJR.08.1895. [DOI] [PubMed] [Google Scholar]
- 16.Okwuosa TM, Williams KA. “Mass-ive” infarction: case report and review of myocardial metastatic malignancies. J Nucl Cardiol. 2008;15(5):719–26. doi: 10.1016/j.nuclcard.2008.06.017. [DOI] [PubMed] [Google Scholar]


