Abstract
In this article, we present a simple, rapid prototyped polystyrene-based microfluidic device with three-dimensional (3D) interconnected microporous walls for long term perfusion cell culture. Patterned 3D interconnected microporous structures were created by a chemical treatment together with a protective mask and the native hydrophobic nature of the microporous structures were selectively made hydrophilic using oxygen plasma treatment together with a protective mask. Using this polystyrene-based cell culture microfluidic device, we successfully demonstrated the support of four days perfusion cell culture of hepatocytes (C3A cells).
I. INTRODUCTION
The ability of probing and controlling cellular behavior is essential for drug discovery and development. While the current in vitro cell culture methods are the general choices for researchers, they cannot fully replicate and predict physiologically the in vivo behavior in animal models and humans. On the other hand, microfluidics has the potential to create an interactive in vivo-like cell microenvironment because of its small sample volume, versatility in device design and comparable length scale.1,2 One of the potential applications of microfluidics is the concept of organ-on-a-chip which intends to acquire human pharmacokinetic response without the use of animal model by reconstructing the physiological relevance at the cellular/organ level.3,4 With the recent research focusing on the mimicry of in vivo-like cell microenvironment, perfusion cell culture in microfluidic devices has demonstrated to be one of the promising solutions to achieve this goal.5–15
Currently, most of the cell culture microfluidic devices are fabricated by soft lithography which is based on molding of poly(dimethylsiloxane) (PDMS).6–15 However, PDMS absorbs biomolecules non-specifically limiting its applications and compromising the accuracy of many cytotoxicity studies.16,17 On the other hand, polystyrene has been the de facto material for cell culture for decades. Therefore, polystyrene is a very desirable material for cell culture microfluidic devices and could overcome the limitation of non-specific absorption of biomolecules in PDMS-based cell culture microfluidic devices.
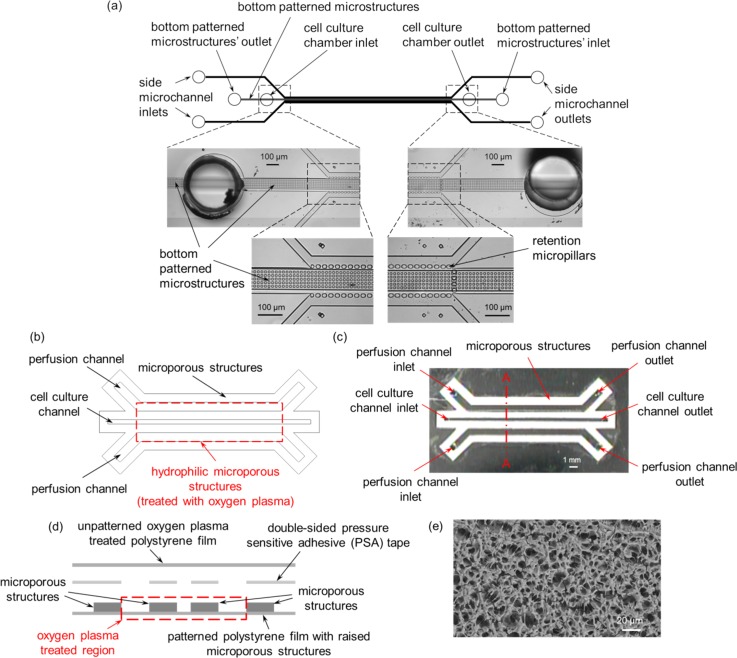
In this study, we present a simple, rapid prototyped polystyrene-based microfluidic device with three-dimensional (3D) interconnected microporous walls for long term perfusion cell culture. The polystyrene-based cell culture microfluidic device is based on our previous PDMS-based cell culture microfluidic device design (Figs. 1(a)–1(d)).11 Patterned 3D interconnected microporous structures were created by a chemical treatment together with a protective mask as previously reported (Fig. 1(e)).18 The native hydrophobic nature of the microporous structures was selectively made hydrophilic using oxygen plasma treatment together with a protective mask. Since the fabrication process only requires a desktop digital craft cutter,19 it circumvents the need for microfabrication, and hence eliminating the need for cleanroom facilities and reducing the fabrication time and cost. We used this polystyrene-based cell culture microfluidic device to evaluate the cell culture performance of C3A cells and demonstrate its potential as a perfusion-based cell culture platform technology.
FIG. 1.
Schematic diagrams and images of (a) PDMS-based, and (b) and (c) polystyrene-based cell culture microfluidic devices. (d) Schematic diagram of the exploded cross-sectional view of section A–A depicted in (c). (e) Scanning electron microscope (SEM) image of the microporous structures. (a) Reproduced by permission from Goral et al., Lab Chip 10, 3380–3386 (2010).10 Copyright 2010 by The Royal Society of Chemistry.
II. EXPERIMENTAL DETAILS
A. Device fabrication and assembly
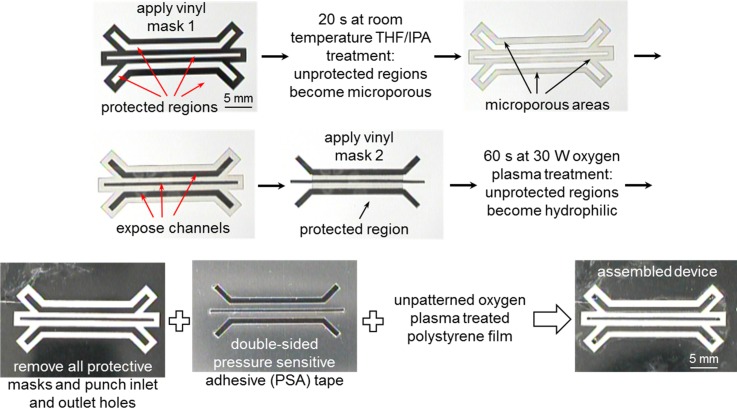
The fabrication and assembly of the polystyrene-based cell culture microfluidic devices (Figs. 1(b)–1(d)) were similar to the one described previously.18 Briefly, a first customized microfluidic device design mask was cut out from a white vinyl self-adhesive sheet (vinyl mask 1) (Item # 699009; The Paper Studio®, Oklahoma City, OK, USA) using a desktop digital craft cutter.19 The vinyl mask 1 was then adhered to one side of a 3 mil (∼75 μm) thick polystyrene film as a protective mask (Fig. 2) while another side of the polystyrene film was protected by transparent self-adhesive tapes (6200 3/400 Highland® Invisible Tape; 3M, Stationery Products Division, St. Paul, MN, USA). The masked polystyrene film was then dipped into a tetrahydrofuran (THF)/isopropanol (IPA) (Fisher Scientific, Pittsburgh, PA, USA) solvent mixture (40/60 v/v %) for 20 s at room temperature. After removing the film from the solvent mixture and blowing dry it with nitrogen gas for 2–3 min, the protective masks in the 500 μm wide perfusion and cell culture channels were removed before applying a second vinyl self-adhesive protective mask (vinyl mask 2) for the oxygen plasma treatment. Oxygen plasma treatment was performed inside an RF plasma chamber (model MPS-300; March Instruments, Inc., Concord, CA, USA) and the masked polystyrene film was exposed to oxygen plasma at 30 W for 60 s with oxygen gas flow. All the protective masks were then removed before assembling the final device.
FIG. 2.
Photographic images depicting the fabrication and assembly procedures for the polystyrene-based cell culture microfluidic device.
In the final device assembly, the corresponding microfluidic device design was cut out from a 1.9 mil (∼50 μm) thick double-sided pressure sensitive adhesive (PSA) tape (ARcare® 92712; Adhesive Research, Inc., Glen Rock, PA, USA). After inlet and outlet holes were punched through the patterned polystyrene film, the double-sided PSA tape with the top and bottom protective layers removed was manually aligned and sandwiched between the patterned polystyrene film and an unpatterned, oxygen plasma treated polystyrene film. Finally, a small piece of PDMS was attached to the inlet and outlet regions to create a leak free connection between the inlet and outlet tubing.
B. Flow characterization
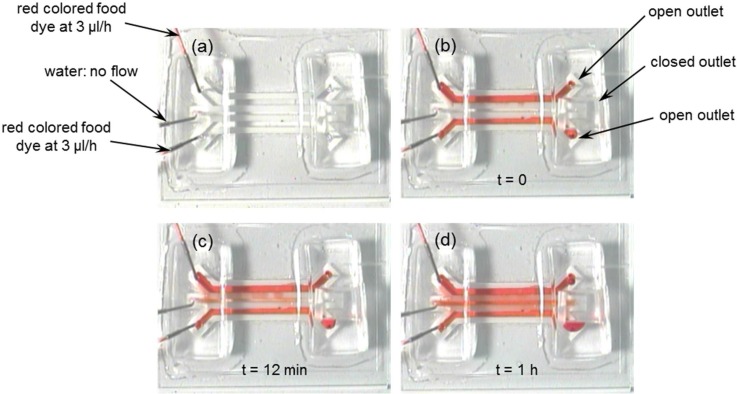
Perfusion flow characteristics between the two side perfusion channels and the middle cell culture channel of the polystyrene-based cell culture microfluidic device were experimentally characterized using a red colored food dye solution (McCormick & Co., Inc., Hunt Valley, MD, USA). Deionized water was first introduced into the middle empty cell culture channel at a flow rate of 5 μl/min using a syringe pump (Model: SP230IW; World Precision Instruments, Sarasota, FL, USA) and time was allowed for the deionized water to completely wet the oxygen plasma treated regions of the two side microporous walls before sealing the outlet of the middle cell culture channel with a piece of PDMS. Next, red colored food dye solution was introduced into the two side perfusion channels at a flow rate of 5 μl/min per inlet using the syringe pump. Once the side perfusion channels were filled with the food dye, the flow rate was changed to 3 μl/h and the flow was monitored and imaged using a CCD camera.
C. C3A cell culture
We first thawed and cultured cryopreserved C3A cells, a derivative of HepG2/C3A human hepatoblastoma cell line (CRL-10741™, American Type Culture Collection (ATCC), Manassas, VA, USA), in a sterile cell culture flask (Product # 430641, Corning Incorporated, Corning, NY, USA) in Eagle's Minimum Essential Medium (EMEM) (ATCC® No. 30–2003, ATCC) supplemented with 10% fetal bovine serum (FBS) (Catalog No. 16000-077, Invitrogen Corporation, Carlsbad, CA, USA) and 1% Penicillin-Streptomycin (Catalog No. 15140-163, Invitrogen Corporation) in a CO2 HEPA incubator (Model 3130, Forma Scientific, Inc., Marietta, OH, USA) at 37 °C, 95% humidity, and 5% CO2.
Before seeding C3A cells in the middle cell culture channel, the polystyrene-based cell culture microfluidic device, tubing (Tygon S-54-HL, Saint-Gobain Performance Plastics, Akron, OH, USA) and syringes (500 μl, gas tight 1700 series Hamilton, Reno, NV, USA) were sterilized with 70% ethanol. The middle cell culture channel was first manually filled with 70% ethanol using a syringe and time was allowed for the ethanol to completely wet the microporous structures. Then, the two side perfusion channels were filled with 70% ethanol and the device was incubated with 70% ethanol for at least 5 min. Next, the device was dried by vacuum pumping and was put inside an oven at 37 °C for overnight to ensure that all traces of ethanol remaining inside the microporous structures were dried out. Once the device was completely dried, the middle cell culture channel was primed with medium at 5 μl/min and time was allowed for the medium to completely wet the oxygen plasma treated regions of the two side microporous walls. This was followed by priming the two side perfusion channels with the medium at 5 μl/min. A 200 μl droplet of C3A cell suspension (1 × 107 cells/ml) was pipetted into the middle cell culture channel outlet and cells were pulled into the middle cell culture channel by withdrawing the syringe connected to the inlet of the middle cell culture channel at a flow rate of 5 μl/min. Cell seeding was monitored under a bright field microscope and the syringe pump was stopped when the desired amount of cells were loaded inside the middle cell culture channel. The inlet and outlet of the middle cell culture channel were sealed separately with a piece of PDMS. Next, the device was placed inside a 37 °C cell culture incubator so that cells could attach to the middle cell culture channel bottom surface. After 2 h of cell seeding, perfusion of cell culture media was started by pumping with two syringes connected to the two side perfusion channels at a flow rate of 3 μl/h. As a control, C3A cells were also cultured in static condition in the device where all of the inlets and outlets were separately sealed with a piece of PDMS after cell seeding.
D. LIVE/DEAD® viability/cytotoxicity assay kit
The LIVE/DEAD® Viability/Cytotoxicity Assay Kit for mammalian cells (Molecular Probes, Inc., Eugene, OR, USA) was used to determine the viability (live and dead) of the C3A cells after cell culture. A fluorescent dye mixture solution containing 2 μl of ethidium homodimer-1 and 4 μl of calcein in 2 ml of phosphate buffer saline (PBS) solution was first introduced into the middle cell culture channel at a flow rate of 1 μl/min for 15 min and then cells were allowed to incubate with the mixture at 37 °C for 30 min. After incubation, the cells inside the middle cell culture channel were rinsed with PBS at a flow rate of 1 μl/min for another 15 min. Fluorescent images of viable cells were captured using a Zeiss Axiovert 200 inverted fluorescence microscope equipped with an epifluorescence condenser and a camera system (CarlZeiss MicroImaging, Inc., Thornwood, NY, USA).
III. RESULTS AND DISCUSSION
A. Device fabrication and assembly
In most PDMS-based perfusion cell culture microfluidic devices, evenly spaced barriers such as micropillars or slits were incorporated into the devices to separate the cell culture and perfusion channels.10–15 These barriers prevented cells from escaping from the cell culture channels and into the perfusion channels while allowing cell culture media to be perfused from the perfusion channels and into the cell culture channels. The shape, size, and spacing of the barriers had to be optimized depending on the cell types and device design. Although simulation models could be used to help optimize the perfusion flow characteristics between the cell culture and perfusion channels, a final device optimization might still be required by performing the actual cell loading, seeding, and culture experiments in order to evaluate the effectiveness of the barriers. Also, in order to fabricate the precise shape, size, and spacing of the barriers, conventional photolithography using cleanroom microfabrication techniques was required resulting in higher cost and longer lead time until a device was optimized and functional. As a result, other than the PDMS material compatibility concerns, it would be very attractive to have alternative ways to replace the barriers and fabricate the perfusion cell culture microfluidic devices.
In our polystyrene-based cell culture microfluidic device, since the microporous structures were highly interconnected and raised above the unpatterned surface of the rest of the flat part of the polystyrene film,18 the microporous structures were used as both hydrophobic (untreated) and hydrophilic (oxygen plasma treated with a protective mask) channel side walls. The hydrophobic microporous channel side walls served as a liquid barrier to prevent liquid from leaking through the channels. On the other hand, the hydrophilic microporous channel side walls served as a cell barrier to prevent cells from escaping from the cell culture channels and into the perfusion channels while allowing cell culture media to be perfused from the perfusion channels and into the cell culture channel. Due to the highly interconnected 3D open porous structures, the hydrophilic microporous channel side walls provided an effective universal cell barrier for all cell types with efficient liquid perfusion. Also, conventional photolithography using cleanroom microfabrication techniques and soft lithography were not required since the microporous structures were created by a chemical treatment together with a protective mask. The native hydrophobic nature of the microporous structures were selectively made hydrophilic using oxygen plasma treatment together with a protective mask. The complete fabrication process from a device design concept to a working device can be completed in less than an hour in a regular laboratory setting without the need for expensive equipment. Also, in our previous study, we have already demonstrated that the polystyrene film and the double-sided PSA tape are both biocompatible for cell culture applications.19
B. Flow characterization
Time lapse images of red colored food dye solution showed good perfusion performance of the polystyrene-based cell culture microfluidic device between the two side perfusion channels and the middle cell culture channel (Fig. 3). Red colored food dye solution was completely perfused into the middle cell culture channel within an hour. Also, it matched with our previous study that oxygen plasma treatment could facilitate the perfusion of fluid across the oxygen plasma treated microporous structures.18
FIG. 3.
Time lapse photographic images during the perfusion flow characterization experiment.
C. C3A cell culture
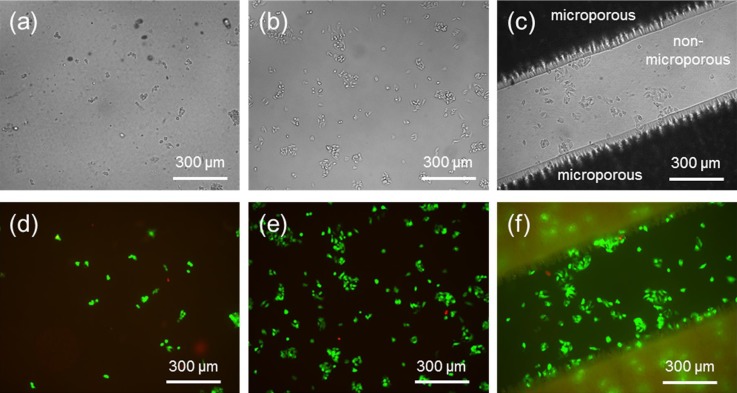
Before starting perfusion cell culture in the polystyrene-based cell culture microfluidic device, we first performed a series of static cell culture experiments using only the THF/IPA patterned, oxygen plasma treated polystyrene film (with and without microporous structures) to confirm its cell culture compatibility. We compared its cell culture performance with an unpatterned, non-oxygen plasma treated polystyrene film and a standard tissue culture treated microplate. After one day of cell culture and rinsing with PBS, bright field imaging was used to inspect cell morphology (Figs. 4(a)–4(c)) while live/dead cell staining was used to assess the viability of C3A cells (Figs. 4(d)–4(f)).
FIG. 4.
(a)–(c) Bright field images of static one day C3A cell culture on (a) an unpatterned, non-oxygen plasma treated polystyrene film, (b) a standard tissue culture treated microplate, and (c) a THF/IPA patterned, oxygen plasma treated polystyrene film (with and without microporous structures). (d)–(f) Fluorescent images of live (green)/dead (red) cell staining on respective substrates shown in (a)–(c). Initial cell seeding density was 2 × 106 cells/ml for all three cases.
The one day cell culture results confirmed that oxygen plasma treatment ensured and improved cell attachment and viability. The number of living cells on the non-oxygen plasma treated polystyrene film was much lower than the others while the number of living cells on the THF/IPA patterned, oxygen plasma treated polystyrene film (with and without microporous structures) was comparable to the standard tissue culture treated microplate. The cell culture results also confirmed that the THF/IPA patterning method did not have any negative effects on cell culture and C3A cells grew healthily on both the THF/IPA patterned (microporous) and unpatterned (flat, non-microporous) surfaces. In addition, microporous structures could easily be patterned in the middle cell culture channel to provide a 3D bottom cell culture surfaces that could potentially improve cell culture performance.
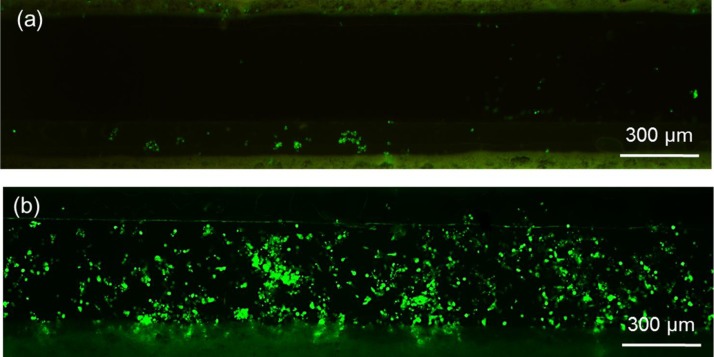
Live/dead cell staining after four days C3A cell culture in both static and perfused polystyrene-based cell culture microfluidic devices revealed the significance of perfusing media into the middle cell culture channel. The fluorescent images taken after the cell staining clearly indicated that cell viability was much better with the perfusion cell culture compared with the static cell culture (Fig. 5). These results demonstrate the microfluidic device's ability to enable long term cell culture by perfusing media into the cell culture channel.
FIG. 5.
Live (green)/dead (red) cell staining after four days (a) static and (b) perfusion cell culture in the polystyrene-based cell culture microfluidic device.
IV. CONCLUSIONS
We demonstrated the support of four days cell culture of C3A cells in a polystyrene-based cell culture microfluidic device. The microporous structures of the polystyrene-based cell culture microfluidic device can be selectively oxygen plasma treated to allow the control of hydrophilicity and hence the perfusion of media into the cell culture channel. The untreated hydrophobic nature of the microporous structures was used as a liquid barrier to prevent cells and media from leaking through the structures. Because our previous study demonstrated the acidification of water by carbon dioxide (CO2) gas with a microfluidic device fabricated with the microporous structures,18 we are currently exploring the potential of using the polystyrene-based cell culture microfluidic device to study the effect of gas diffusion (e.g., cigarette smoke or nitric oxide (NO)) or both gas diffusion and liquid perfusion on cell culture performance.
References
- 1.Chan C. Y., Huang P.-H., Guo F., Ding X., Kapur V., Mai J. D., Yuen P. K., and Huang T. J., “ Accelerating drug discovery via organs-on-chips,” Lab Chip 13, 4697–4710 (2013). 10.1039/c3lc90115g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zervantonakis I. K., Kothapalli C. R., Chung S., Sudo R., and Kamm R. D., “ Microfluidic devices for studying heterotypic cell-cell interactions and tissue specimen cultures under controlled microenvironments,” Biomicrofluidics 5, 013406 (2011). 10.1063/1.3553237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker M., “ A living system on a chip,” Nature 471, 661–665 (2011). 10.1038/471661a [DOI] [PubMed] [Google Scholar]
- 4.Huh D., Torisawa Y.-S., Hamilton G. A., Kim H. J., and Ingber D. E., “ Microengineered physiological biomimicry: Organs-on-Chips,” Lab Chip 12, 2156–2164 (2012). 10.1039/c2lc40089h [DOI] [PubMed] [Google Scholar]
- 5.Huh D., Hamilton G. A., and Ingber D. E., “ From 3D cell culture to organs-on-chips,” Trends Cell Biol. 21, 745–754 (2011). 10.1016/j.tcb.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S.-Y. C., Hung P. J., and Lee P. J., “ Microfluidic array for three-dimensional perfusion culture of human mammary epithelial cells,” Biomed. Microdevices 13, 753–758 (2011). 10.1007/s10544-011-9545-3 [DOI] [PubMed] [Google Scholar]
- 7.Kim M. S., Yeon J. H., and Park J.-K., “ A microfluidic platform for 3-dimensional cell culture and cell-based assays,” Biomed. Microdevices 9, 25–34 (2007). 10.1007/s10544-006-9016-4 [DOI] [PubMed] [Google Scholar]
- 8.Hung P. J., Lee P. J., Sabounchi P., Aghdam N., Lin R., and Lee L. P., “ A novel high aspect ratio microfluidic design to provide a stable and uniform microenvironment for cell growth in a high throughput mammalian cell culture array,” Lab Chip 5, 44–48 (2005). 10.1039/b410743h [DOI] [PubMed] [Google Scholar]
- 9.Choudhury D., Mo X., Iliescu C., Tan L. L., Tong W. H., and Yu H., “ Exploitation of physical and chemical constraints for three-dimensional microtissue construction in microfluidics,” Biomicrofluidics 5, 022203 (2011). 10.1063/1.3593407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakao Y., Kimura H., Sakai Y., and Fujii T., “ Bile canaliculi formation by aligning rat primary hepatocytes in a microfluidic device,” Biomicrofluidics 5, 022212 (2011). 10.1063/1.3580753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goral V. N., Hsieh Y. C., Petzold O. N., Clark J. S., Yuen P. K., and Faris R. A., “ Perfusion-based microfluidic device for three-dimensional dynamic primary human hepatocyte cell culture in the absence of biological or synthetic matrices or coagulants,” Lab Chip 10, 3380–3386 (2010). 10.1039/c0lc00135j [DOI] [PubMed] [Google Scholar]
- 12.Toh Y. C., Lim T. C., Tai D., Xiao G. F., van Noort D., and Yu H., “ A microfluidic 3D hepatocyte chip for drug toxicity testing,” Lab Chip 9, 2026–2035 (2009). 10.1039/b900912d [DOI] [PubMed] [Google Scholar]
- 13.Ong S.-M., Zhang C., Toh Y.-C., Kim S. H., Foo H. L., Tan C. H., van Noort D., Park S., and Yu H., “ A gel-free 3D microfluidic cell culture system,” Biomaterials 29, 3237–3244 (2008). 10.1016/j.biomaterials.2008.04.022 [DOI] [PubMed] [Google Scholar]
- 14.Toh Y.-C., Zhang C., Zhang J., Khong Y. M., Chang S., Samper V. D., van Noort D., Hutmacher D. W., and Yu H., “ A novel 3D mammalian cell perfusion-culture system in microfluidic channels,” Lab Chip 7, 302–309 (2007). 10.1039/b614872g [DOI] [PubMed] [Google Scholar]
- 15.Lee P. J., Hung P. J., and Lee L. P., “ An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture,” Biotechnol. Bioeng. 97, 1340–1346 (2007). 10.1002/bit.21360 [DOI] [PubMed] [Google Scholar]
- 16.Wong I. and Ho C. M., “ Surface molecular property modifications for poly(dimethylsiloxane) (PDMS) based microfluidic devices,” Microfluid. Nanofluid. 7, 291–306 (2009). 10.1007/s10404-009-0443-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthier E., Young E. W. K., and Beebe D., “ Engineers are from PDMS-land, biologists are from polystyrenia,” Lab Chip 12, 1224–1237 (2012). 10.1039/c2lc20982a [DOI] [PubMed] [Google Scholar]
- 18.Yuen P. K. and DeRosa M. E., “ Flexible microfluidic devices with three-dimensional interconnected microporous walls for gas and liquid applications,” Lab Chip 11, 3249–3255 (2011). 10.1039/c1lc20157c [DOI] [PubMed] [Google Scholar]
- 19.Yuen P. K. and Goral V. N., “ Low-cost rapid prototyping of flexible microfluidic devices using a desktop digital craft cutter,” Lab Chip 10, 384–387 (2010). 10.1039/b918089c [DOI] [PubMed] [Google Scholar]