Most adults with Down syndrome eventually develop Alzheimer’s disease pathology. Hartley et al. examine the association between amyloid-β deposition and cognition in adults with Down syndrome without dementia. No association is seen after controlling for mental and chronological age, suggesting that many individuals can tolerate amyloid-β deposition without cognitive decline.
Keywords: Down syndrome, Alzheimer’s disease, amyloid, dementia, PiB
Abstract
Nearly all adults with Down syndrome show neuropathology of Alzheimer’s disease, including amyloid-β deposition, by their fifth decade of life. In the current study, we examined the association between brain amyloid-β deposition, assessed via in vivo assessments of neocortical Pittsburgh compound B, and scores on an extensive neuropsychological battery of measures of cognitive functioning in 63 adults (31 male, 32 female) with Down syndrome aged 30–53 years who did not exhibit symptoms of dementia. Twenty-two of the adults with Down syndrome were identified as having elevated neocortical Pittsburgh compound B retention levels. There was a significant positive correlation (r = 0.62, P < 0.0001) between age and neocortical Pittsburgh compound B retention. This robust association makes it difficult to discriminate normative age-related decline in cognitive functioning from any potential effects of amyloid-β deposition. When controlling for chronological age in addition to mental age, there were no significant differences between the adults with Down syndrome who had elevated neocortical Pittsburgh compound B retention levels and those who did not on any of the neuropsychological measures. Similarly, when examining Pittsburgh compound B as a continuous variable, after controlling for mental age and chronological age, only the Rivermead Picture Recognition score was significantly negatively associated with neocortical Pittsburgh compound B retention. Our findings indicate that many adults with Down syndrome can tolerate amyloid-β deposition without deleterious effects on cognitive functioning. However, we may have obscured true effects of amyloid-β deposition by controlling for chronological age in our analyses. Moreover, our sample included adults with Down syndrome who were most ‘resistant’ to the effects of amyloid-β deposition, as adults already exhibiting clinical symptoms of dementia symptoms were excluded from the study.
Introduction
Down syndrome is a developmental disorder most commonly involving triplication of chromosome 21 and occurring in 1:800 live births (Yang et al., 2002). Nearly all adults with Down syndrome show neuropathology of Alzheimer’s disease by their fifth decade of life (Mann and Esiri, 1989). The deposition of amyloid-β plaques is purported to be a central event in the pathogenesis of Alzheimer’s disease, often occurring decades before the appearance of dementia and other neuropathological signs of Alzheimer’s disease (Aizenstein et al., 2008). The gene for the amyloid precursor protein is located on chromosome 21, thus accounting for the overproduction of amyloid-β in adults with Down syndrome (Bush and Beail, 2004).
Early amyloid-β deposition may be related to subtle declines in episodic and/or executive functioning, called mild cognitive impairment (Petersen et al., 2001). Recently, imaging agents such as Pittsburgh compound B (PiB), have enabled in vivo assessment of amyloid-β via PET scanning (Klunk et al., 2004). Several studies examining amyloid-β deposition as a marker of mild cognitive impairment in older adults in the general population have yielded discrepant results (Jack et al., 2008; Wolk et al., 2009; Rowe et al., 2010; Nebes et al., 2013). Within the Down syndrome population, Nelson et al. (2011) found that neocortical PiB retention level was unrelated to cognitive functioning after controlling for chronological age. However, cognitive functioning was only indirectly assessed through caregiver reports of difficulties with cognitive tasks (e.g. remembering information).
In this study, we examined the association between amyloid-β deposition assessed using neocortical PiB and scores on an extensive neuropsychological battery in 63 healthy adults with Down syndrome. We hypothesized that (i) the PiB-positive group would be older than the PiB-negative group; (ii) neocortical PiB retention and chronological age would be positively correlated; (iii) the PiB-positive group would evidence slightly lower episodic memory and executive functioning versus the PiB-negative group; and (iv) PiB retention would be negatively associated with tasks involving episodic memory and executive functioning.
Materials and methods
Participants
The sample was drawn from the baseline visit of an ongoing longitudinal study examining the developmental course of amyloid-β and progression to Alzheimer’s disease in adults with Down syndrome. Between 2010 and 2013, 28 subjects (44.4%) were evaluated at the University of Pittsburgh and 35 subjects (55.6%) were seen at the University of Wisconsin-Madison. Participants were ≥30 years of age, with trisomy 21 (confirmed by genetic testing) and a mental age of ≥30 months. All participants scored in the asymptomatic range (<3 CCS score) on the Dementia Scale for Down syndrome (Gedye, 1995). None of the adults with Down syndrome were reported to be taking memory enhancement or Alzheimer’s disease medications or had a medical or psychiatric condition that would impair cognitive functioning or be contraindicated with MRI/PET scans.
Screening measures
Stanford-Binet Abbreviated Battery Intelligence questionnaire
The Stanford-Binet, 5th edition was used to obtain an estimate of intellectual functioning or mental age. The tool has strong reliability and validity (Roid, 2003) and has been used with adults with Down syndrome (Couzens et al., 2011).
Dementia Scale for Down Syndrome
Caregivers were interviewed using the Dementia Scale for Down Syndrome, a 60-item measure of dementia in adults with Down syndrome. The tool has good specificity and sensitivity (Gedye, 1995).
Vineland Adaptive Behaviour Scales
The Vineland Adaptive Behaviour Scales, 2nd Edition was completed by caregivers; the total score was used to assess adaptive functioning. The tool has strong psychometric properties (Sparrow et al., 2005).
Short form of the Severe Impairment Battery
The 26-item short form of the Severe Impairment Battery was used to assess cognitive functioning. This tool was developed for adults with dementia and has been shown to be valid and reliable (Saxton et al., 2005).
Cognitive functioning
Verbal learning and memory
The Cued Recall Test (Zimmerli and Devenny, 1995) assesses verbal learning and memory. The Free Recall Score is the number of objects correctly recalled in free recall trials. The Total Score is the number of objects correctly recalled in free recall and cued recall trials. The Intrusion Score is the number of incorrect responses in the cued recall trials.
The Story Recall Logical Memory I and Logical Memory II subtests of the Wechsler Memory Scale, 4th Edition (Wechsler, 2003) assess immediate and delayed recall of verbal information. The Initial Attempt score is the amount of information recalled across two trials. The Initial–Delayed score is the amount of information retained after a 10-min delay. The tool has been used in adults with Down syndrome and is sensitive to mild memory decline (Brugge et al., 1994).
Visual memory
The Visual Memory subtests of the Rivermead Behavioural Memory Test for Children (Wilson et al., 1991) assess everyday memory abilities and have been used with adults with Down syndrome (Hon et al., 1998).
Attention and processing speed
The Wechsler Intelligence Scales for Children-Revised (Wechsler, 1974) Digits Forward assesses short-term auditory memory and has been used with adults with Down syndrome and dementia (Devenny et al., 2005).
The Corsi Block Tapping Test-Forward assesses visuospatial memory and is a valid measure in individuals with Down syndrome (Schapiro et al., 1992). The number of items in each correct sequence was used.
The NEPSY Visual Attention subtest assesses visual attention (Visual Attention Accuracy) and processing speed (Visual Attention Time) and has been used as a measure of treatment change in medication trials for adults with Down syndrome (Heller et al., 2006).
Executive and working memory
The Wechsler Intelligence Scale for Children, 4th Edition Digit Span Backwards assesses the ability to manipulate verbal information while in working memory. It is sensitive to age-associated differences in adults with intellectual disability (Numminen et al., 2002). The Backward Corsi Span assesses visuospatial short-term working memory and has been used in adults with Down syndrome (Devenny et al., 2005). For both tests, total number of correct items in each sequence was used. The Stroop Dog and Cat Task, modified by Ball et al. (2008), assesses executive functioning. The Errors Score is the number of errors made during the switch trial. The Time score is switch trial time minus initial trial time. Performance on this task has been found to be related to memory changes in adults with Down syndrome (Nash and Snowling, 2008).
Visuospatial construction
The Wechsler Intelligence Scale for Children, 4th Edition Block Design subtest and Haxby extension (Haxby, 1989) assess visualspatial construction and have been shown to differentiate non-demented from demented adults with Down syndrome (Schapiro et al., 1992). The Developmental Test of Visual-Motor Integration, 5th Edition (Beery et al., 2004) assesses visual-motor integration skills. The Purdue Pegboard (Vega, 1969) measures fine motor speed and central executive processing functioning. The Total Hands Score (right and left hand) and Both Hands Score (both hands together) were included in analyses.
Language
The NEPSY-2nd Edition (Korkman et al., 2007) Word Generation Semantic Fluency subtest assesses verbal fluency and is sensitive to language declines in an adult with Down syndrome and dementia (Devenny et al., 2005). The Expressive-One Word Picture Vocabulary Test (Brownell, 2000) assesses expressive language and has been commonly used in individuals with mild to moderate intellectual disability (Ypsilanti et al., 2005). Finally, the Peabody Picture Vocabulary Test-Revised (Dunn and Dunn, 1981) measures receptive language.
Image acquisition and analysis
Magnetic resonance imaging
Structural T1-weighted 3 T MRI scans were acquired using GE Medical Systems (Wisconsin) and Siemens Magnetom Trio (Pittsburgh) MRI scanners to acquire high resolution volumetric spoiled gradient or MPRAGE sequence, respectively. MRI data were used for PET-MRI registration, brain region definition and magnetic resonance-guided correction of the PET data for atrophy-related CSF dilution.
Pittsburgh compound B-positron emission tomography imaging
The 11C-PiB was synthesized at high specific activity (>2000 mCi/µmol), in batches in excess of 40 mCi. Up to 15 mCi of radiotracer was administered through an intravenous catheter by slow bolus injection (20–30 s). After a 35-min uptake period, the subject was positioned in the PET scanner for a 30-min time series acquisition (from 40–70 min post-injection), followed by a 6–10 min windowed transmission scan to correct for attenuation of the annihilation radiation. PET data were acquired on Siemens ECAT EXACT HR + PET scanners. Time series PET data were reconstructed using filtered back-projection, corrected for photon attenuation, deadtime, normalization, scatter, and radioactive decay.
Image processing and analysis
PET-MRI registration was performed using automated methods (Minoshima, et al., 1993). Images were re-oriented along the anterior–posterior commissure (AC-PC line) and interframe motion was corrected for on a frame-by-frame basis. Regions of interest were defined on the magnetic resonance images and transferred to the PET data for sampling over single and multiple transverse planes using a technique with high rater reliability (Rosario et al., 2011). Regions of interest for this report included frontal cortex, anterior cingulate gyrus, parietal cortex, lateral temporal cortex, precuneus cortex, and anterior ventral striatum; subcortical white matter and cerebellum was used as reference regions. PiB retention was assessed using the standardized uptake value ratio determined over the 50–70 min post-injection interval. An average global standardized uptake value ratio retention (Global 6) measure was computed across the regions of interest. The Global 6 region of interest is largely reflective of cortical retention, but also includes striatal retention that previously was noted in some adults with Down syndrome (Handen et al., 2012). A two-component magnetic resonance-based CSF correction was applied to the PET binding measures to correct for the dilutional effect of expanded CSF spaces accompanying normal ageing and disease-related cerebral atrophy (Meltzer et al., 1999). See Handen et al. (2012) for further detail on processing and analysis.
Subcortical volumes were calculated for the hippocampus as well as for the striatum (consisting of the right and left caudate and putamen) from structural T1 magnetic resonance images using the FMRIB software library FIRST (Patenaude et al., 2011) processing stream. Quality was assessed visually by overlaying FIRST-generated subcortical regions on the magnetic resonance image. Of the 63 total scans, nine failed the FIRST processing or were deemed as having motion artefact significant enough to impact volumetric measures, and were not included in analyses. Intracranial volumes were calculated from T2 magnetic resonance images, which were processed through SPM8’s unified segmentation method (Ashburner and Friston, 2005). The resulting grey matter, white matter, and CSF probability maps were summed and binarized at a manually chosen threshold between 0.1 and 0.3. Two additional scans did not have T2 MRI acquisitions and were not included in analyses. The subcortical volumes (mm3) of the remaining 52 scans (17 PiB-positive, 35 PiB-negative) were normalized for intersubject variation in head size by dividing by the intracranial volumes (cm3), after Jack et al. (1997).
Determination of Pittsburgh compound B status
Cut-offs for PiB-positivity were determined using the sparse k-means clustering with resampling as described previously (Cohen et al., 2013). Participants with PiB retention values exceeding the cut-off point in one (or more) of the brain regions encompassing the Global 6 were defined as PiB-positive. The cut-off points in the present sample were: frontal cortex = 1.71, anterior cingulate gyrus = 1.78, parietal cortex = 1.63, lateral temporal cortex = 1.50, precuneus cortex = 1.73, and anterior ventral striatum = 1.48. This is comparable with procedures used to determined PiB status in previous studies (Jack et al., 2008; Wolk et al., 2009; Rowe et al., 2010).
Procedure
Day 1: After receipt of informed consent, participants were administered the neuropsychological battery and caregivers were interviewed to complete the Dementia Scale for Down Syndrome and Vineland Adaptive Behaviour Scales, 2nd Edition. Day 2: Participants completed the MRI/PET scans. Day 2 was performed within 5 months of Day 1 [mean = 21.34, standard deviation (SD) = 9.23].
Data analysis plan
Distributions of variables and histograms of the residuals were reviewed; data were found to be normally distributed without skew. An alpha level of P ≤ 0.05 was used to judge statistical significance for all analyses. Two participants were unable to complete select measures in the neuropsychological battery, due to an inability to understand and/or comply with task instructions. Using a two-sided alpha level of 0.05 and power of 0.80, our sample size (n = 63) allows for the detection of moderate to large effects (cohen’s D ≥ 0.76).
Independent samples t-tests and chi-square statistics and Fisher’s exact and Freeman-Halton probabilities were used to identify potential differences in socio-demographic characteristics (age, sex, race/ethnicity, employment status, mental age, adaptive behaviour, medication) and APOE status between the PiB-positive and PiB-negative groups. Pearson correlations, one-way ANOVA and t-tests examined the association between neocortical PiB as a continuous variable and socio-demographic characteristics. Using PiB as a dichotomous variable, one-way analysis of covariance (ANCOVAs) examined potential differences in cognitive functioning in the two groups. Mental age was included in analyses to control for differences in intellectual level. Using neocortical PiB as a continuous variable, multiple linear regressions examined the association between PiB retention and cognitive functioning after controlling for mental age. To understand effects of amyloid-β deposition on cognition separate from normative age-related declines, we re-ran ANOVAs and multiple linear regression analyses controlling for chronological age and mental age.
Finally, to determine if findings were affected by regional atrophy, we conducted independent sample t-tests to compare the anterior ventral striatum volume, the region with the earliest and highest levels of amyloid-β in our sample, between the PiB-positive and PiB-negative groups, using hippocampus volume as a control. The above described ANOVAs and multiple linear regression analyses were then re-run controlling for anterior ventral striatum volume.
Results
Socio-demographic characteristics and Pittsburgh compound B
Of the 63 adults evaluated, 22 (35%) were identified as PiB-positive. Nearly all (n = 21; 95%) of the PiB-positive subjects were above threshold in the anterior ventral striatum. This was the only region above the threshold for 33% (n = 7) of these 21 subjects. Of the remaining PiB-positive subjects, 59.1% (n = 13) were above threshold in anterior cingulate, 54.5% (n = 12) were above threshold in the frontal cortex, 54.5% (n = 12) were above threshold in the lateral temporal cortex, 36.4% (n = 8) were above threshold in the parietal cortex, and 54.5% (n = 12) were above threshold in the precuneus cortex.
Table 1 displays the socio-demographic characteristics and APOE status of the two groups. As hypothesized, the PiB-positive group was significantly older than the PiB-negative group [t(62) = −7.50, P < 0.001]. Independent sample t-tests and chi-square statistics indicated no significant difference between the groups for any other socio-demographic variables.
Table 1.
Participant characteristics
| PiB-positive (n = 22) | PiB-negative (n = 41) | t-test/chi-square | P-value | |
|---|---|---|---|---|
| Male, % (n) | 59.1 (13) | 43.9 (18) | 1.32 | 0.25 |
| Age, mean (SD) | 44.3 (3.8) | 34.5 (5.2) | −7.5 | <0.001 |
| Race/ethnicity, % (n) | 0.58 | |||
| White | 95.5 (21) | 97.6 (40) | ||
| Asian | 0 (0) | 0 (0) | ||
| American Indian Alaska Native | 4.5 (1) | 0 (0) | ||
| More than one race | 0 (0) | 2.4 (1) | ||
| APOE statusa, % (n) | ||||
| E2/E2 | 4.8 (1) | 0.0 (0) | * | 0.78 |
| E2/E3 | 23.8 (5) | 25.0 (10) | ||
| E3/E3 | 61.9 (13) | 65.0 (26) | ||
| E3/E4 | 9.5 (2) | 7.5 (3) | ||
| E4/E4 | 0.0 (0) | 2.5 (1) | ||
| Current residence, % (n) | * | 0.22 | ||
| With family | 59.1 (13) | 63.4 (26) | ||
| Group home | 22.7 (5) | 9.8 (4) | ||
| Supported apartment | 9.1 (2) | 17.1 (7) | ||
| Independently | 9.1 (2) | 7.3 (3) | ||
| Other | 0 (0) | 2.4 (1) | ||
| Employment, % (n) | * | 0.65 | ||
| Full or part time with pay | 22.7 (5) | 36.6 (15) | ||
| Full or part time with support | 18.2 (4) | 17.1 (7) | ||
| Supported workshop | 36.4 (8) | 22.0 (9) | ||
| Volunteer | 13.6 (3) | 9.8 (4) | ||
| Day treatment program or not employed | 9.1 (2) | 14.6 (6) | ||
| Mental age equivalent in years, mean (SD) | 5.7 (1.5) | 5.6 (1.2) | −0.07 | 0.94 |
| PPVT age equivalent in years, mean (SD) | 7.9 (2.3) | 8.4 (3.7) | 0.77 | 0.45 |
| Medication, % (n) | ||||
| Hypothyroidism | 45.5 (10) | 58.5 (24) | 0.99 | 0.32 |
| Hypertension | 0.0 (0) | 7.3 (3) | * | 0.55 |
| Antipsychotic | 27.3 (3) | 6.3 (2) | * | 0.10 |
| Antidepressant/anti-anxiety | 27.3 (3) | 25.0 (8) | * | 1.0 |
| Mood/behaviour stabilizer | 0.0 (0) | 3.1 (1) | * | 1.0 |
| Narcotic pain reliever | 9.1 (1) | 0.0 (0) | * | 1.0 |
| Cholesterol | 27.3 (3) | 3.1 (1) | * | 0.046 |
aApoE status was not obtained for two subjects.
*No chi-square statistics is reported as Fisher's exact test was used to calculate probability due to sparsity.
PPVT = Peabody Picture Vocabulary Test.
Pearson correlations and Student’s t-statistic or F-statistic results examined the association between neocortical PiB retention as a continuous variable and the socio-demographic characteristics of chronological age, mental age, race/ethnicity, residence, employment, Peabody Picture Vocabulary Test age equivalent and Vineland Composite. There was a significant positive correlation (r = 0.62, P < 0.0001) between age and neocortical PiB retention, but no significant correlation with any other variables.
Cognitive functioning and Pittsburgh compound B controlling for mental age
Using neocortical PiB as a dichotomous variable, one-way ANOVAs compared cognitive functioning in the PiB-positive versus PiB-negative groups after controlling for mental age (Table 2). There was a significant difference between the two groups in the Cued Recall total (t(1) = 2.18, p = .03), Expressive One Word (t(1) = −2.33, p = .02) and Rivermead Picture Recognition score (t(1) = −2.73, p = .01). On all of these measures, the PiB-positive group performed significantly poorer than the PiB-negative group.
Table 2.
One-way ANOVA comparing scores on neuropsychological measures between the PiB-positive versus PiB-negative group
| Neuropsychiatric measure | Unadjusted |
Adjusted by mental age |
Adjusted by chronological age and mental age |
|||||
|---|---|---|---|---|---|---|---|---|
| PiB-positive mean (SD) | PiB-negative mean (SD) | PiB-positive mean (SD) | PiB-negative mean (SD) | P-value | PiB-positive mean (SD) | PiB-negative mean (SD) | P-value | |
| Vineland | 180.2 (53.5) | 185.6 (46.1) | 179.9 (8.4) | 185.8 (6.2) | 0.57 | 184.6 (10.7) | 183.2 (7.1) | 0.92 |
| Free Recall Total | 14.2 (5.5) | 16.9 (6.4) | 14.0 (1.2) | 17.0 (0.9) | 0.05 | 14.9 (1.5) | 16.6 (1.0) | 0.42 |
| Free and Cued Recall Total | 30.7 (6.3) | 33.2 (5.8) | 30.6 (1.3) | 33.2 (0.9) | 0.10 | 31.4 (1.7) | 32.8 (1.1) | 0.54 |
| Cued Recall Intrusion | 4.1 (5.3) | 1.9 (2.9) | 4.2 (0.9) | 1.8 (0.6) | 0.03 | 3.5 (1.1) | 2.2 (0.7) | 0.40 |
| Block Design Total | 26.6 (8.1) | 28.2 (9.8) | 26.6 (1.7) | 28.3 (1.2) | 0.42 | 29.5 (2.0) | 26.7 (1.3) | 0.31 |
| Severe Impairment Battery | 46.0 (4.0) | 47.1 (3.6) | 46.0 (0.5) | 47.1 (0.4) | 0.10 | 46.4 (0.7) | 46.9 (0.4) | 0.59 |
| Visual Attention Accuracy Time | 94.2 (47.5) | 77.0 (35.4) | 94.5 (7.2) | 76.8 (5.3) | 0.05 | 88.3 (9.1) | 80.1 (6.1) | 0.51 |
| Visual Attention Accuracy | 16.0 (8.3) | 18.3 (2.8) | 15.9 (1.1) | 18.3 (0.8) | 0.08 | 16.9 (1.4) | 17.8 (0.9) | 0.63 |
| Verbal Fluency Raw | 23.5 (11.0) | 24.6 (9.4) | 23.5 (1.7) | 24.7 (1.3) | 0.58 | 22.8 (2.2) | 25.0 (1.5) | 0.46 |
| Verbal Fluency Repetitions | 3.2 (3.4) | 2.0 (2.1) | 3.2 (0.6) | 2.0 (0.4) | 0.08 | 3.3 (0.7) | 2.0 (0.5) | 0.19 |
| Purdue Pegboard – Single hands | 13.9 (3.3) | 15.2 (3.6) | 13.9 (0.7) | 15.2 (0.5) | 0.14 | 14.6 (0.9) | 14.8 (0.6) | 0.85 |
| Purdue Pegboard Both hands | 4.7 (1.9) | 5.7 (1.9) | 4.7 (0.4) | 5.7 (0.3) | 0.05 | 5.3 (0.5) | 5.4 (0.3) | 0.94 |
| Story Recall Initial | 1.9 (2.1) | 2.4 (2.1) | 1.9 (0.3) | 2.4 (0.3) | 0.20 | 1.9 (0.4) | 2.4 (0.3) | 0.38 |
| Story Recall Initial – Delayed | 3.5 (3.2) | 3.2 (2.8) | 3.5 (0.5) | 3.2 (0.4) | 0.63 | 3.5 (0.7) | 3.2 (0.4) | 0.76 |
| Expressive One Word | 66.1 (22.5) | 77.4 (25.8) | 65.9 (4.0) | 77.5 (2.9) | 0.02 | 67.8 (5.1) | 76.5 (3.4) | 0.21 |
| PPVT age equivalent | 93.7 (27.6) | 100.6 (44.2) | 93.4 (6.2) | 100.8 (4.6) | 0.34 | 101.2 (7.8) | 96.6 (5.2) | 0.66 |
| Rivermead Picture Recognition | 4.4 (3.5) | 6.5 (3.2) | 4.3 (0.6) | 6.5 (0.5) | 0.01 | 5.5 (0.8) | 5.9 (0.5) | 0.70 |
| VMI age equivalent | 17.1 (2.9) | 16.9 (3.0) | 17.1 (0.5) | 16.9 (0.4) | 0.79 | 17.8 (0.7) | 16.6 (0.4) | 0.18 |
| Cat and Dog Switch trial errors | 3.6 (5.3) | 1.7 (3.8) | 3.6 (0.8) | 1.7 (0.6) | 0.07 | 1.9 (1.0) | 2.6 (0.7) | 0.61 |
| Cat and Dog Switch time | 11.7 (10.3) | 9.4 (8.3) | 11.7 (1.8) | 9.4 (1.4) | 0.32 | 13.3 (2.3) | 8.5 (1.6) | 0.14 |
| Corsi Total Forward | 12.7 (7.0) | 12.0 (8.6) | 12.7 (1.6) | 12.1 (1.2) | 0.76 | 14.9 (2.0) | 10.9 (1.3) | 0.15 |
| Corsi Total Backward | 3.1 (3.6) | 4.2 (4.4) | 3.1 (0.8) | 4.2 (0.6) | 0.29 | 4.6 (1.0) | 3.4 (0.6) | 0.35 |
| Digit Span Total Forward | 11.0 (6.0) | 11.7 (7.2) | 10.9 (1.2) | 11.8 (0.9) | 0.59 | 10.9 (1.6) | 11.8 (1.1) | 0.69 |
| Digit Span Total Backward | 4.7 (5.0) | 5.2 (5.1) | 4.6 (0.9) | 5.2 (0.6) | 0.62 | 5.1 (1.1) | 4.9 (0.7) | 0.89 |
PPVT = Peabody Picture Vocabulary Test; VMI = Developmental Test of Visual-Motor Integration-5th Edition.
Multiple linear regression models controlling for mental age examined the association between neocortical PiB retention as a continuous variable and performance on the neuropsychological battery (Table 3). There were significant associations between neocortical PiB retention and measures of verbal memory (Free Recall Total [b = −4.70, SE = 2.01, p = .02] and Free and Cued Recall Total [b = −4.96, SE = 2.09, p = .02]), visual memory (Rivermead Picture Recognition [b = −3.98, SE = 1.02, p < .01]), visuospatial construction (Purdue Pegboard Total Single Hands [b = −1.30, SE = 0.63, p = .04], attention (Visual Attention Time [b = 26.71, SE = 12.10, p = .03], and executive functions (Cued Recall Total Intrusion [b = 4.14, SE = 1.40, p < .01] and Stroop Cat and Dog Switch Trial Errors [b = 4.29, SE = 1.33, p < .01], indicating that greater PiB retention was associated with poorer performance.
Table 3.
Regressions of cognitive functioning measures on PiB Level controlling for mental age and then mental age and chronological age
| Controlling for mental age |
Controlling for mental age and chronological age |
|||||
|---|---|---|---|---|---|---|
| Estimate for PiB | Standard error | Pr > |t| | Estimate for PiB | Standard error | Pr > |t| | |
| Vineland | −11.00 | 14.17 | 0.44 | −4.83 | 18.04 | 0.79 |
| Free Recall Total Score | −4.70 | 2.01 | 0.02 | −3.40 | 2.55 | 0.19 |
| Free and Cued Recall Total Score | −4.96 | 2.09 | 0.02 | −4.32 | 2.68 | 0.11 |
| Cued Recall Intrusion | 4.14 | 1.40 | 0.00 | 3.50 | 1.79 | 0.06 |
| Block Design Total | −2.19 | 2.79 | 0.44 | 2.52 | 3.42 | 0.46 |
| Severe Impairment Battery Total Score | −1.26 | 0.89 | 0.16 | −0.42 | 1.13 | 0.71 |
| Visual Attention Time (seconds) | 26.71 | 12.10 | 0.03 | 16.92 | 15.30 | 0.27 |
| Visual Attention Accuracy | −3.39 | 1.86 | 0.07 | −1.74 | 2.34 | 0.46 |
| Verbal Fluency Raw Score | −2.42 | 2.95 | 0.42 | −3.82 | 3.75 | 0.31 |
| Verbal Fluency Number of repetitions | 0.52 | 0.96 | 0.59 | −0.25 | 1.22 | 0.84 |
| Purdue Pegboard Total both single hands | −1.30 | 0.63 | 0.04 | −0.32 | 0.77 | 0.68 |
| Purdue Pegboard Both hands | −1.98 | 1.17 | 0.10 | −0.93 | 1.48 | 0.53 |
| Story Recall Initial attempt total | −0.93 | 0.58 | 0.12 | −0.94 | 0.74 | 0.21 |
| Story Recall Initial – Delayed | 0.38 | 0.89 | 0.67 | 0.28 | 1.14 | 0.81 |
| Expressive One Word | −10.66 | 6.93 | 0.13 | −3.51 | 8.71 | 0.69 |
| PPVT Age equivalent | −10.89 | 10.55 | 0.31 | 1.56 | 13.21 | 0.91 |
| Rivermead Picture Recognition | −3.98 | 1.02 | 0.00 | −2.62 | 1.27 | 0.04 |
| VMI age equivalent | −0.08 | 0.90 | 0.93 | 0.73 | 1.13 | 0.52 |
| Cat and Dog Switch trial errors | 4.29 | 1.33 | 0.00 | 2.51 | 1.64 | 0.13 |
| Cat and Dog Switch time (switch – naming) | 0.97 | 3.15 | 0.76 | 1.88 | 4.01 | 0.64 |
| Corsi Total Forward | 0.10 | 2.72 | 0.97 | 2.93 | 3.41 | 0.39 |
| Corsi Total Backward | −0.64 | 1.37 | 0.64 | 2.28 | 1.63 | 0.17 |
| Digit Span Total Forward | 0.89 | 2.12 | 0.68 | 2.16 | 2.69 | 0.42 |
| Digit Span Total Backward | 1.06 | 1.49 | 0.48 | 2.96 | 1.86 | 0.12 |
PPVT = Peabody Picture Vocabulary Test; VMI = Developmental Test of Visual-Motor Integration-5th Edition.
Cognitive functioning and Pittsburgh compound B controlling for mental age and chronological age
To disentangle the effects of amyloid-β deposition on cognitive functioning from normative age-related declines, one-way ANOVAs and multiple linear regression analyses controlling for both chronological age and mental age were re-run (Tables 2 and 3). After controlling for chronological age, there was no longer a significant difference between the two groups on any neuropsychological measures. In the multiple linear regression models, only the negative association between neocortical PiB retention and the Rivermead Picture Recognition score (b = −2.62, SE = 1.27, p = .04) remained significant. Thus, PiB group status did not influence between-person differences in cognitive functioning independent of chronological age. To illustrate this point, Fig. 1 displays the association between the Free and Cued Recall Total Score and chronological age for the PiB-positive versus PiB-negative groups.
Figure 1.
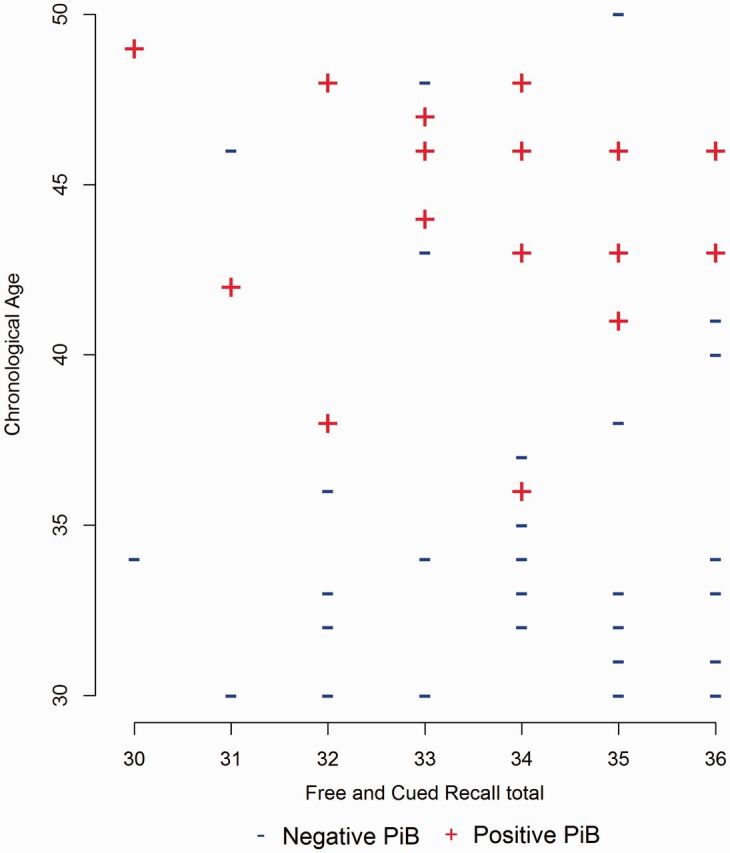
Free and Cued Recall Total score and chronological age for PiB-positive versus PiB-negative groups.
Cognitive functioning and Pittsburgh compound B controlling for regional atrophy
To investigate if any of the results were influenced by regional atrophy, we examined volume in the anterior ventral striatum in the PiB-positive versus PiB-negative groups, the area with the earliest and highest levels of amyloid-β, and the hippocampus. As shown in Table 4, independent sample t-tests indicated that there were not significance differences between the PiB-positive and PiB-negative groups for the right (t(50) = 0.02, P = .86), left (t(50) = 0.78, P = .44), and total hippocampal (t(50) = 0.32, P = .75) volumes. However, the PiB-positive group had significantly lower left (t(49.14) = 2.68, P = .01), right (t(48.41) = 2.81, P <.01), and total anterior ventral striatum (t (49.46) = 2.99, P < .01) volumes than the PiB-negative group. The above ANOVAs and multiple linear regression analyses were re-run controlling for mental age, chronological age, and total anterior ventral striatum volume. The pattern of findings remained the same.
Table 4.
Hippocampus and anterior ventral striatum Volume for PiB-positive and PiB-negative groups
| PiB-positive (n = 17) |
PiB-negative (n = 35) |
||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t-value | df | Pr > |t| | |
| Right hippocampus | 2.4993 | 0.3142 | 2.4822 | 0.3292 | −0.18 | 50 | 0.8594 |
| Left hippocampus | 2.2867 | 0.3106 | 2.3613 | 0.3318 | 0.78 | 50 | 0.4417 |
| Total hippocampus | 4.7860 | 0.6036 | 4.8435 | 0.6118 | 0.32 | 50 | 0.7509 |
| Right striatum | 6.2403 | 0.3318 | 6.5888 | 0.6059 | 2.68 | 49.14 | 0.0101 |
| Left striatum | 6.2159 | 0.2989 | 6.5367 | 0.5202 | 2.81 | 48.409 | 0.0070 |
| Total striatum | 12.456 | 0.5603 | 13.125 | 1.0517 | 2.99 | 49.458 | 0.0043 |
Measures have been normalized by dividing by the intercranial volume (cm3).
Discussion
The present study builds on the small body of research examining in vivo assessments of amyloid-β deposition via PiB retention in adults with Down syndrome to understand the early developmental course of Alzheimer’s disease. Findings indicate that many adults with Down syndrome can tolerate amyloid-β deposition without deleterious effects on cognition. As a dichotomous variable, subtle differences between the PiB-positive and PiB-negative groups were found on measures of verbal and visual episodic memory as well as on a measure of expressive vocabulary, when controlling for mental age. However, these differences became non-significant when chronological age was also controlled for. As a continuous variable, neocortical PiB retention was significantly associated with lower performance on measures of verbal and visual episodic memory and executive functioning when mental age was included as a covariate. However, only visual episodic memory (Rivermead Picture Recognition) remained at a significant level when chronological age was included in models.
Chronological age was associated with neocortical PiB retention, which was expected and is in line with previous work (Jack et al., 2008; Wolk et al., 2009; Rowe et al., 2010; Nelson et al., 2011). Adults with Down syndrome who were PiB-positive were, on average, 10 years older than those in the PiB-negative group. In our regression models, chronological age accounted for 39% of the variability in PiB retention. This robust association makes it difficult to discriminate normative age-related declines in cognitive functioning from any potential effects of amyloid-β deposition; thus, we may have obscured true effects of amyloid-β deposition by controlling for chronological age in our analyses. It is also likely that our sample included adults with Down syndrome who were most ‘resistant’ to the effects of amyloid-β deposition, as adults already exhibiting clinical symptoms of dementia symptoms were excluded. However, within-person analyses may reveal associations between amyloid-β and cognitive declines over time. Our sample was also limited to adults with Down syndrome with a mental age ≥30 months, which restricted variability in cognitive functioning, and potentially made it more difficult to detect subtle associations. Finally, the sample may also not have been large enough to detect subtle differences in cognitive functioning related to amyloid-β.
In the present study, we examined neocortial PiB standardized uptake value ratio in six regions of interest, which is consistent with previous work (Wolk et al., 2009; Rowe et al., 2010; Nelson et al., 2011). In the study sample, amyloid-β deposition first occurred in the striatal region. Therefore, we re-ran analyses using striatal PiB retention as opposed to neocortical PiB retention. The pattern of findings remained the same; after controlling for mental age and chronological age, striatal PiB retention (as a dichotomous and as a continuous variable) was not significantly related to cognitive functioning. Analyses were also re-run controlling for anterior ventral striatum volume to ensure that regional atrophy was not obscuring effects of amyloid-β; the pattern of findings remained the same. We limited our follow-up investigation to the anterior ventral striatum, as this was the region with the earliest and highest amyloid-β levels. However, future studies should include examination of the impact of atrophy in other brain regions as well as an investigation of the impact of regional differences in amyloid-β deposition on specific domains of cognitive functioning. In conclusion, although adults with Down syndrome are at high risk for developing the neuropathology of Alzheimer’s disease, our findings indicate that many adults with Down syndrome can tolerate amyloid without deleterious effects on cognitive functioning.
Acknowledgements
We would like to thank the psychologists, project managers, and statisticians who make this research possible. We would also like to thank the adults with Down syndrome and their families for their time and commitment to this research.
Glossary
Abbreviation
- PiB
Pittsburgh compound B
Funding
This research is funded by the National Institute of Aging (R01 AG031110 to B.H. and B.C.) and the National Institute on Child Health and Human Development (P30 HD03352 to M.M.).
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–17. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ball SL, Holland AJ, Treppner P, Watson PC, Huppert FA. Executive dysfunction and its association with personality and behaviour changes in the development of Alzheimer's disease in adults with Down syndrome and mild to moderate learning disabilities. Brit J Clin Psychol. 2008;47:1–29. doi: 10.1348/014466507X230967. [DOI] [PubMed] [Google Scholar]
- Beery KE, Buktenica NA, Beery NA. The Beery-Buktenica developmental test of visual-motor integration. 5th edn. Bloomington, MN: Pearson; 2004. [Google Scholar]
- Brownell R. The expressive one-word vocabulary test. East Moline, IL: LinguiSystems, Inc.; 2000. [Google Scholar]
- Brugge KL, Nichols SL, Salmon DP, Hill LR, Delis DC, Aaron L, et al. Cognitive impairment in adults with Down's syndrome: similarities to early cognitive changes in Alzheimer's disease. Neurology. 1994;44:232–8. doi: 10.1212/wnl.44.2.232. [DOI] [PubMed] [Google Scholar]
- Cohen AD, Mowrey W, Weissfeld LA, Aizenstein HJ, McDade E, Mountz JM, et al. Classification of amyloid-positivity in controls: comparison of visual read and quantitative approaches. Neuroimage. 2013;71:207–15. doi: 10.1016/j.neuroimage.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzens D, Cuskelly M, Haynes M. Cognitive development and down syndrome: age-related change on the Stanford-Binet Test (fourth edition) Am J Intell Dev Disabil. 2011;116:181–204. doi: 10.1352/1944-7558-116.3.181. [DOI] [PubMed] [Google Scholar]
- Devenny DA, Wegiel J, Schupt N, Jenkins E, Zigman W, Krinsky-McHale SJ, et al. Dementia of the Alzheimer's Type and accelerated aging in down syndrome. Sci Aging Knowl Environ. 2005;14:1–14. doi: 10.1126/sageke.2005.14.dn1. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody picture vocabulary test-revised. Circle Pines, MN: American Guidance Service; 1981. [Google Scholar]
- Gedye A. Dementia scale for Down's syndrome: manual. Vancouver, BC: Gedye Research and Consulting; 1995. [Google Scholar]
- Handen BL, Cohen AD, Channamalappa U, Bulova P, Cannon SQ, Cohen WI, et al. Imaging brain amyloid in nondemented young adults with Down syndrome using Pittsburgh compound B. Alzheimer Dement. 2012;8:496–501. doi: 10.1016/j.jalz.2011.09.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV. Neuropsychological evaluation of adults with Down's syndrome: patterns of selective impairment in non-demented old adults. J Ment Defic Res. 1989;33:193–210. doi: 10.1111/j.1365-2788.1989.tb01467.x. [DOI] [PubMed] [Google Scholar]
- Heller JH, Spiridigliozzi GA, Crissman BG, Sullivan JA, Eells RL, Li JS, et al. Safety and efficacy of rivastigmine in adolescents with Down syndrome: a preliminary 20-week, open-label study. J Child Adol Psychop. 2006;16:755–65. doi: 10.1089/cap.2006.16.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon J, Huppert FA, Holland AJ, Watson P. The value of the Rivermead Behavioural Memory Test (children's version) in an epidemiological study of older adults with Down syndrome. Brit J Clin Psychol. 1998;37:15–29. doi: 10.1111/j.2044-8260.1998.tb01276.x. [DOI] [PubMed] [Google Scholar]
- Jack CR, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–79. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's Disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY-II. San Antonio, TX: Harcourt Assessment Inc.; 2007. [Google Scholar]
- Mann DM, Esiri MM. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down's syndrome. J Neurol Sci. 1989;89:169–79. doi: 10.1016/0022-510x(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Kinahan PE, Greer PJ, Nichols TE, Comtat C, Cantwell MN, et al. Comparative evaluation of MR-based partial-volume correction schemes for PET. J Nucl Med. 1999;40:2053–65. [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA, et al. Automated detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med. 1993;34:322–9. [PubMed] [Google Scholar]
- Nash HM, Snowling MJ. Semantic and phonological fluency in children with Down syndrome: atypical organization of language or less efficient retrieval strategies? Cogn Neuropsychol. 2008;25:690–703. doi: 10.1080/02643290802274064. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Snitz BE, Cohen AD, Aizenstein HJ, Saxton JA, Halligan EM, et al. Cognitive aging in persons with minimal amyloid-beta and white matter hyperintensities. Neuropsychologia. 2013;51:2202–9. doi: 10.1016/j.neuropsychologia.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LD, Siddarth P, Kepe V, Scheihel KE, Huang SC, Barrio JR, et al. Positron emission tomography of brain Œ≤-amyloid and tau levels in adults with Down syndrome. Arch Neurol. 2011;68:768–74. doi: 10.1001/archneurol.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numminen H, Service E, Ruoppila I. Working memory, intelligence and knowledge base in adult persons with intellectual disability. Res Dev Disabil. 2002;23:105–18. doi: 10.1016/s0891-4222(02)00089-6. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–22. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Roid GH. Stanford-Binet intelligence scales. 5th edn. Itasca, IL: Riverside; 2003. [Google Scholar]
- Rosario BL, Weissfeld LA, Laymon C, Mathis CA, Klunk WE, Berginc M, et al. Inter-rater reliability of manual and automated region-of-interest delineation for PiB PET. Neuroimage. 2011;55:933–41. doi: 10.1016/j.neuroimage.2010.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–83. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Saxton J, Kastango KB, Hugonot-Diener L, Boller F, Verny M, Sarles CE, et al. Development of a short form of the severe impairment battery. Am J Geriat Psychiat. 2005;13:999–1005. doi: 10.1176/appi.ajgp.13.11.999. [DOI] [PubMed] [Google Scholar]
- Schapiro MB, Azari NP, Grady CL, Haxby JV, Horwitz B. Down syndrome: differentiating mental retardation and dementia with brain imaging techniques. In: Nadel L, Epstein CJ, editors. Down syndrome and Alzheimer Disease. New York, NY: Wiley-Liss; 1992. pp. 103–22. [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland adaptive behavior scales. 2nd edn. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Vega A. Use of Purdue pegboard and finger tapping performance as a rapid screening test for brain damage. J Clin Psychol. 1969;25:255–8. doi: 10.1002/1097-4679(196907)25:3<255::aid-jclp2270250306>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler intelligence scale for children-revised. New York: Psychological Corporation; 1974. [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Fourth Edition: Administration and scoring manual. San Antonio: The Psychological Corporation; 2003. [Google Scholar]
- Wilson B, Ivani-Chalian C, Aldrich F. Rivermead behavioral memory test for children. Bury St Edmunds, U.K.: Thames Valley Test Co.; 1991. [Google Scholar]
- Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–68. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359:1019–25. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
- Ypsilanti A, Grouios G, Alevriadou A, Tsapkini K. Expressive and receptive vocabulary in children with Williams and Down syndromes. J Intellect Disabil Res. 2005;49:353–64. doi: 10.1111/j.1365-2788.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- Zimmerli E, Devenny DA. Cued recall as a screen for dementia in the MR population. Paper presented at the Gatlinburg conference on research and theory in mental retardation and developmental disabilities, 1995 March 11-14; Gatlinburg, TN. [Google Scholar]


