Abstract
O6-Methylguanine-DNA methyltransferase (MGMT) is a DNA repair protein, the loss of MGMT expression was commonly known due to hypermethylation of CpG islands in its promoter region. Overexpression of p53 protein may be associated with downregulated MGMT expression in brain tumors. The aims of this study were to investigate the role of MGMT expression loss and its correlation with p53 overexpression in endometrial cancers. MGMT and p53 expression was examined in formalin-fixed, paraffin-embedded tissues from 36 endometrial cancer cases using immnunohistochemical staining. The loss of MGMT expression was detected in 11 (30.6%) out of the 36 endometrial cancers and p53 immunoreactivity was detected in 23 (63.9%) out of the 36 endometrial cancers. Ten (90.9%) of the 11 cases with negative MGMT immunoreactivity showed positive p53 expression, so the loss of MGMT expression was significantly associated with the p53 overexpression (P=0.03). These findings suggest that the loss of MGMT expression may be one of factors capable of p53 overexpression in endometrial cancer. Further studies are needed to define the relation between MGMT and p53 for examining the mechanisms of tissue-specific MGMT expression.
Keywords: O6-Methylguanine-DNA methyltransferase (MGMT), p53, Endometrial cancer, Immunohistochemistry
INTRODUCTION
O6-Methylguanine-DNA methyltransferase (MGMT) is a DNA repair protein that protects cells against the carcinogenic and cytotoxic effects of alkylating agents.1 MGMT activity has been determined in various types of tumors, and was found to be relatively high in colon, ovary, breast and brain cancers.2,3 The lack of MGMT expression may be related to the development of gliomas,4 non-small cell lung cancers,5 and colon cancers.6,7 In the absence of MGMT activity, O6-alkylguanine mispairs with thymine during DNA replication and results in guanine-cytosine to adenine-thymine transitions.6,8 Other studies that showed loss of MGMT protein expression associated with methylation in diffuse large B cell lymphoma and colorectal and brain tumors.6,7 Aberrant methylation of 5’ cytosine residues of a guanine residue in CpG islands in the promoter regions of tumor suppressor genes is an important mechanism of gene transcriptional inactivation and has been associated with tumorigenesis. Several studies have provided evidence of the linkage between epigenetic inactivation of MGMT and the appearance of G to A transition mutations in genes in human primary tumors.6
Tumor suppressor gene p53 plays an important role in the cell cycle, DNA damage, cell death and cell differentiation, and it is commonly mutated in human tumors.9 Several studies have suggested the involvement of p53 protein in the expression of the MGMT gene. There are studies reported that MGMT gene expression was significantly lower in p53 altered tumors,4 and MGMT promoter methylation may increase the occurrence of p53 mutation in lung cancer.10 Other studies reported that wild-type p53 acts as an inhibitor of MGMT gene expression.11
To the best of our knowledge, the relationship between MGMT and p53 expression in tissues of endometrial cancer has not yet been studied in Korea. So we investigated the expression patterns of MGMT and p53 using immunohistochemistry to elucidate the tissues-specific relationship between MGMT and p53 expressions in endometrial cancers.
MATERIALS AND METHODS
1. Tissue samples
A retrospective study was carried out on 36 cases with well differentiated endometrial adenocarcinomas admitted in local hospital from 2008 to 2010 in Busan. The ages of the 36 patients ranged from 34 to 68 years (median age: 52 years). All cancer cases were obtained endometrial curettage, and histopathologically confirmed and had no preoperative chemotherapy or radiotherapy. The HE stained were reviewed in each case to confirm the original diagnosis, which was based on the FIGO classification.
2. Immunohistochemical analysis
Immunohistochemical study for MGMT and p53 was performed on the formalin-fixed, paraffin-embedded, 4 μm thick tissue section using the avidin-biotin-peroxidase complex method. Deparaffinization of all the sections was performed through a series of xylene baths, and rehydration was performed with a series of graded alcohol solutions. To enhance immunoreactivity, microwave antigen retrieval was performed at 750 W for 30 min in Tris-EDTA buffer (pH 9.0). After blocking endogenous peroxidase activity with 5% hydrogen peroxidase for 10 min, primary antibody incubation was performed for 1 hour at room temperature. The primary antibody was a mouse monoclonal antibody directed against MGMT (Lab Vision, Fremont CA, USA) and p53 (DakoCytomation, Denmark) used in a 1:100 dilution. An Envision Chem Kit (DakoCytomation, Carpinteria, CA, USA) was used for the secondary antibody at room temperature for 30 min. After washing the tissue samples in Tris-buffered saline for 10 min, 3, 3’-diaminobenzidine was used as a chromogen, and Gill’s hematoxylin counterstain was applied.
3. Interpretation of immunohistochemical analysis
All the slides were evaluated without knowledge of any of the clinicopathologic data. Immunoreactivity for MGMT expression was defined by presence of nuclear and cytoplasmic staining and that for p53 expression was defined by presence of nuclear staining. The percentage scoring of the immunoreactive tumor cells was categorized into four groups: 0 (0%), 1 (1–10%), 2 (11–50%), and 3 (>50%). The staining intensity was also categorized into four groups: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). A final score was obtained for each case by multiplying the percentage and the intensity score. Finally, tumors with multiplied score exceeding 3 (i.e., tumors with a moderate and strong intensity of >10% of the tumor cells) was recorded as positive immunoreactivity to MGMT and p53; all the other scores were considered negative.
4. Statistical analysis
Statistical analysis was performed using SPSS for Windows Standard version 19.0 (SPSS, Chicago, IL, USA). Fisher exact test was performed to assess the relationship between the expression patterns of MGMT and p53. A P value less than 0.05 was considered to be statistically significant.
RESULTS
The loss of MGMT expression was detected in 11 (30.6%) out of the 36 endometrial cancers, and p53 immunoreactivity was detected in 23 (63.9%) out of the 36 endometrial cancers. Ten (90.9%) of the 11 cases with negative MGMT immunoreactivity showed positive p53 expression, whereas 1 (9.1%) of the 11 cases with negative MGMT immunoreactivity showed negative p53 expression. So the loss of MGMT expression was significantly associated with the p53 overexpression (P=0.03) (Table 1, Fig. 1).
Table 1.
Relationship between O6-Methylguanine-DNA methyltransferase (MGMT) and p53 expressions in 36 endometrial cancers
| Expression | MGMT protein
|
P | |
|---|---|---|---|
| Negative N=11 (%) | Positive N=25 (%) | ||
| p53 protein | 0.03 | ||
| Negative (N=13) | 1 (9.1) | 12 (48.0) | |
| Positive (N=23) | 10 (90.9) | 13 (52.0) | |
Fig. 1.
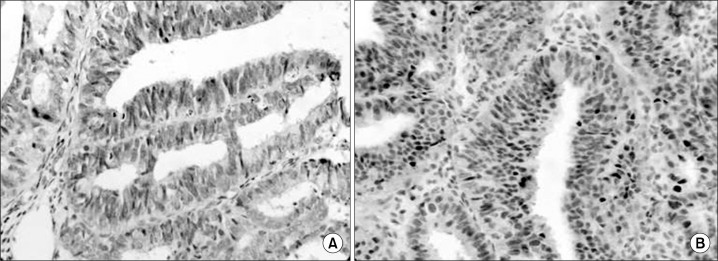
Immunohistochemical findings of O6-Methylguanine-DNA methyltransferase (MGMT) and p53 in endometrial cancer tissues; MGMT showed a negative nuclear and cytoplasmic immunoreactivity (A, ×400), p53 showed a positive nuclear immunoreactivity (B, ×400).
DISCUSSION
Loss of MGMT expression has been reported to have a significantly role in carcinogenesis in various organs.4 In this study, the loss of MGMT expression was detected in 11 (30.6%) out of the 36 endometrial cancers. There are studies that a lack of MGMT expression may be related to guanine to adenine mutation, and may be related to the development of gliomas,4 non-small cell lung cancers,5 and colonic cancers.6,7 However, other studies have been reported that the lack of MGMT expression in not commonly due to mutation, deletion, or rearrangement of the MGTM gene.12 Hypermethylation of CpG islands in its promoter region is the most important mechanism.13 Esteller et al. reported that the MGMT gene is epigenetically inactivated by promoter hypermethylation in many primary tumor types, and a direct relationship between MGMT aberrant methylation and G:C to A:T transition mutations of p53 in colorectal tumors.6,7 Methylated MGMT gene promoter has been associated with loss or decrease of MGMT expression in tumor tissues of various organs, including lung tumors.5,7 In lung carcinoma, 25% of non-small cell lung carcinoma and 37% of adenocarcinoma have been reported to show loss of MGMT expression.5
p53 is the most commonly mutated gene in human cancer with transition mutations being the main type of p53 mutation observed.9 Several studies that MGMT inactivation by hypermethylation has been reported to be associated with a shift from the G:C to A:T mutation in the p53 gene,10 which is known to be one of the most important tumor suppressor genes in human. Myong reported that MGMT loss associated with p53 overexpression in lung cancers, especially adenocarcinomas.14 Also, Osanai et al. reported that expression of p53 may be associated with the regulation of MGMT expression in breast tumors, and that MGMT immunonegativity and p53 immunopositivity may be strong predictors of breast cancer survival.15 However, other studies that wild-type p53 is accompanied by lower MGMT protein expression.11 In this studies that 10 (90.9%) of the 11 cases with negative MGMT immunoreactivity showed positive p53 expression, whereas 1 (9.1%) of the 11 cases with negative MGMT immunoreactivity showed negative p53 expression. So the loss of MGMT expression was significantly associated with the p53 overexpression (p=0.03). Rolhion et al. reported that MGMT gene expression was significantly lower in p53 mutated tumors, because mutations of p53 in human cancer are common and occur in all malignant cell types, the relationship observed between MGMT expression and p53 is highly relevant.4
Recently, the genetic and epigenetic pathways are not isolated, but rather a complex network of cross-talk between genetic and epigenetic factors may exist. The results of this study because it is limited to immunohistochemical test, further studies are needed to promoter methylation.
These findings suggest that the loss of MGMT expression may be one of factors capable of p53 overexpression in endometrial cancer, but some follow-up is needed since this study was limited to 36 cases of well differentiated endometrial adenocarcinoma. Further studies are needed to define the relation between MGMT and p53 for examining the mechanisms of tissue-specific MGMT expression.
Acknowledgments
This paper was supported by RESEARCH FUND offered from Catholic University of Pusan in 2011.
REFERENCES
- 1.Pegg AE. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990;50:6119–29. [PubMed] [Google Scholar]
- 2.Citron M, Decker R, Chen S, Schneider S, Graver M, Kleynerman L, et al. O6-methylguanine-DNA methyltransferase in human normal and tumour tissue from brain, lung, and ovary. Cancer Res. 1991;51:4131–4. [PubMed] [Google Scholar]
- 3.Preuss I, Haas S, Eichhorn U, Eberhagen I, Kaufmann M, Beck T, et al. Activity of the DNA repair protein O6-methylguanine-DNA methyltransferase in human tumour and corresponding normal tissue. Cancer Detect Prev. 1996;20:130–6. [PubMed] [Google Scholar]
- 4.Rolhion C, Penault-Llorca F, Kemeny JL, Kwiatkowski F, Lemaire JJ, Chollet P. O6-methylguanine-DNA methyltransferase (MGMT) expression in human glioblastomas in relation to patient characteristics and p53 accumulation. Int J Cancer. 1999;84:416–20. doi: 10.1002/(sici)1097-0215(19990820)84:4<416::aid-ijc15>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Mattern J, Koomägi R, Volm M. Smoking-related increase of O6-methylguanine-DNA methyltransferase expression in human lung carcinomas. Carcinogenesis. 1998;19:1247–50. doi: 10.1093/carcin/19.7.1247. [DOI] [PubMed] [Google Scholar]
- 6.Esteller M, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA, Watkins DN, et al. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is associated with G to A mutations in K-ras in colorectal tumorigenesis. Cancer Res. 2000;60:2368–71. [PubMed] [Google Scholar]
- 7.Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59:793–7. [PubMed] [Google Scholar]
- 8.Zaidi NH, Pretlow TP, O’Riordan MA, Dumenco LL, Allay E, Gerson SL. Transgenic expression of human MGMT protects against azoxymethane-induced aberrant crypt foci and G to A mutations in the K-ras oncogene of mouse colon. Carcinogenesis. 1995;16:451–6. doi: 10.1093/carcin/16.3.451. [DOI] [PubMed] [Google Scholar]
- 9.Pfeifer GP. Involvement of DNA damage and repair in mutational spectra. Mutat Res. 2000;450:1–3. doi: 10.1016/s0027-5107(00)00012-9. [DOI] [PubMed] [Google Scholar]
- 10.Wu JY, Wang J, Lai JC, Cheng YW, Yeh KT, Wu TC, et al. Association of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation with p53 mutation occurrence in non-small cell lung cancer with different histology, gender, and smoking status. Ann Surg Oncol. 2008;15:3272–7. doi: 10.1245/s10434-008-0078-9. [DOI] [PubMed] [Google Scholar]
- 11.Yuan Q, Matsumoto K, Nakabeppu Y, Iwaki T. A comparative immunohistochemistry of O6-methylguanine-DNA methyltransferase and p53 in diffusely infiltrating astrocytomas. Neuropathology. 2003;23:203–9. doi: 10.1046/j.1440-1789.2003.00504.x. [DOI] [PubMed] [Google Scholar]
- 12.Fornace AJ, Jr, Papathanasiou MA, Hollander MC, Yarosh DB. Expression of the O6-methylguanine-DNA methyltransferase gene MGMT in MER+ and MER-human tumor cells. Cancer Res. 1990;50:7908–11. [PubMed] [Google Scholar]
- 13.Qian XC, Brent TP. Methylation hot spots in the 5’ flanking region denote silencing of the O6-methylguanine-DNA methyltransferase gene. Cancer Res. 1997;57:3672–7. 1; [PubMed] [Google Scholar]
- 14.Myong NH. Role of loss of O6-methylguanine dna methyltransferase (MGMT) expression in non-small cell lung carcinomas (NSCLCs): with reference to the relationship with p53 overexpression. Cancer Res Treat. 2010;42:95–100. doi: 10.4143/crt.2010.42.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osanai T, Takagi Y, Toriya Y, Nakagawa T, Aruga T, Iida S, et al. Inverse correlation between the expression of O6-methylguanine-DNA methyl transferase (MGMT) and p53 in breast cancer. Jpn J Clin Oncol. 2005;35:121–5. doi: 10.1093/jjco/hyi036. [DOI] [PubMed] [Google Scholar]