Abstract
Background:
Endometrial cancer, the most common gynecological cancer, is closely associated with endometrial hyperplasia, unopposed estrogen exposure, and genetic alterations. Phosphatase and tensin homologue (PTEN) is a tumor suppressor genes completely lost or mutated in >50% of primary endometrioid endometrial cancers. Estrogen-dependent endometrioid carcinoma is the most common type of endometrial cancer. Progesterone is a hormone that antagonizes the growth-promoting properties of estrogen in the uterus. Progestin is used as a conservative endocrine treatment of early endometrial cancer in order to preserve fertility as well as a palliative measure for advanced-stage patients. Progesterone therapy has been shown to be effective in preventing endometrial cancer as well as controlling growth of the endometrium. However, the effectiveness of progestin for women with endometrial cancer is less clear.
Methods:
In order to understand the effect of steroid hormone on endometrial cancer progression, we used a mouse endometrial cancer model with conditional loss of Pten in the mouse uterus (PRcre/+ Ptenf/f, Ptend/d). To assess the effect of steroid hormones, ovariectomized Ptenf/f and Ptend/d mice were treated with estrogen or progesterone over a period of three month.
Results:
Uterine weight gain was significantly decreased in ovariectomized PRcre/+ Ptenf/f mice compared to intact PRcre/+ Ptenf/f mice. Ovariectomized PRcre/+ Ptenf/f mice treated with P4 or vehicle also exhibited decreased uterine cancer size compared with intact PRcre/+ Ptenf/f mice. Proliferation of ovariectomized PRcre/+ Ptenf/f mice treated with P4 is highly decreased compared to other groups. The levels of stromal progesterone receptor were highly increased in ovariectomized PRcre/+ Ptenf/f mice treated with P4 which resulted in decreased epithelial proliferation.
Conclusions:
These results suggest that P4 treatment significantly reduces tumor mass but does not affect cancer progression in PRcre/+ Ptenf/f mice.
Keywords: Endometrial cancer, Progesterone, Estrogen, Progesterone receptor, PTEN
INTRODUCTION
Endometrial cancer is the most frequently diagnostic malignancy of the female reproductive tract. In the United States, approximately 49,560 cases are diagnosed and about 8,190 women die from the disease each year.1 Estrogen-dependent endometrioid carcinoma is the most common type of endometrial cancer. An increased incidence of endometrial cancer has been found in association with prolonged, unopposed estrogen exposure in postmenopausal women. Lately, progesterone therapy has been used as a process to impede the development of endometrial cancer associated with unopposed estrogen in the belief that the combined therapy will prevent the increase of estrogen-induced risk for endometrial cancer.2,3
The majority of endometrial cancers are adenocarcinomas, which originate in uterine epithelial cells. The inner layer of the human uterus, the endometrium, consists of heterogeneous cell types that undergo dynamic changes in response to the ovarian steroid hormones estrogen and progesterone to support embryo development and implantation. Estrogen stimulates the proliferation of epithelial cells in the mouse uterus. In contrast, progesterone is inhibitory to this estrogen-mediated proliferation of the luminal and glandular epithelial cells. However, progesterone, alone or in conjunction with estrogen, leads to uterine stromal cell proliferation. The ability of these steroid hormones to regulate uterine cell proliferation depends upon the ability of hormonal stimulation to regulate growth factor communication networks between the uterine stroma and epithelium. An imbalance caused by increased estrogen action and/or decreased progesterone action can result in abnormal endometrial proliferation and endometrial adenocarcinoma. Therefore, elucidating the molecular mechanisms by which the steroid hormones control uterine physiology is important to understanding the pathology of these diseases.4–6
Phosphatase and tensin homologue (PTEN) is one of the most frequently mutated tumor suppressor genes in type I endometrioid endometrial carcinomas, approximately 50–80%, and is involved in the control of cell proliferation, differentiation, and apoptosis.7,8 PTEN is completely lost or mutated in about 50% of primary endometrioid endometrial cancer and in at least 20% of endometrial hyperplasia, the precancerous lesions of the endometrium.8,9 The presence of PTEN mutations in hyperplasia suggests that PTEN inactivation may occur as an initiating event in endometrial carcinogenesis and is involved in the development of cytologic atypia in hyperplasia.
PTEN acts as a negative regulator of phosphoinositide 3-kinases (PI3K) signaling, a regulator of a number of cellular functions through the activation of AKT. Interestingly, the PTEN/PI3K/AKT signaling pathway can also be activated by estrogen, suggesting a complex interaction between these two signaling pathways. Loss of PTEN and subsequent AKT activation resulted in the activation of estrogen receptor-dependent pathways that play an important role in the tumorigenesis of endometrial cancer.8,10,11 Moreover, there are reports that steroid hormones may regulate endometrial PTEN expression helping to protect the balance between proliferative and anti-proliferative actions in normal endometrium.12
In order to understand the effect of steroid hormone on endometrial cancer, we induced endometrial cancer through conditional loss of Pten in the mouse uterus (PRcre/+ Ptenf/f, Ptend/d). To assess the effect of steroid hormone, ovariectomized Ptenf/f and PRcre/+ Ptenf/f mice were treated with vehicle or progesterone over a period of three months. Uterine weight gain was significantly decreased in ovariectomized PRcre/+ Ptenf/f mice treated with vehicle or progesterone compared to intact PRcre/+ Ptenf/f mice. Ovariectomized PRcre/+ Ptenf/f mice treated with vehicle or progesterone exhibited a decreased cancer size compared with intact PRcre/+ Ptenf/f mice. Histological analysis displayed endometrial adenocarcinoma with invasion into the myometrium in intact and ovariectomized PRcre/+ Ptenf/f mice treated with vehicle or progesterone. These results suggest that tumor mass is significantly reduced in the absence of steroid hormones but does not affect cancer progression in PRcre/+ Ptenf/f mice.
MATERIALS AND METHODS
1. Animals and tissue collection
Mice were cared for and used in the designated animal care facility according to the Michigan State University institutional guidelines. All animal procedures were approved by the Institutional Animal Care and Use Committee of Michigan State University. In order to achieve Pten ablation, mice with floxed Pten (Ptenf/f) were bred to the PRcre/+ mouse.13,14 As a result of cre recombinase insertion at the progesterone receptor (PR) locus, floxed genes are edited specifically in PR expressing cells, including all compartments of the mouse uterus.14,15 This model (PRcre/+ Ptenf/f, Ptend/d) was previously used to ablate Pten in the uterus resulting in endometrial adenocarcinoma.16
For the study of steroid hormone dependency on cancer progression in the endometrial cancer mouse model, Ptenf/f mice and PRcre/+ Ptenf/f mice were ovariectomized at two months of age. Two weeks later, ovariectomized mice were treated with one of the following: Vehicle (beeswax), Progesterone (20 mg/pellet), Estrogen (20 μg/pellet) pellets subcutaneously every month over a period of three months (progesterone treatment) or one month (estrogen treatment). Uterine tissues were collected and fixed with 4% (vol/vol) paraformaldehyde and paraffin embedded for histological analysis.
2. Immunohistochemistry
Uterine sections from paraffin-embedded tissue were cut at 5 μm and mounted on silane-coated slides, deparaffinized, and rehydrated in a graded alcohol series. Sections were preincubated with 10% normal goat serum in PBS (pH 7.5) and then incubated with primary antibody diluted in 10% normal goat serum in PBS (pH 7.5) overnight at 4°C at the following dilutions: 1:200 for anti-PR antibody (sc-7208, Santa Cruz Biotech., Santa Cruz, CA), 1:500 for anti-ERα (M-7047, DAKO Corp., Carpinteria, CA), 1:1,000 for anti-phospho-Histone H3 (06–570, Millipore, Billerica, MA) and 1:500 for anti-cleaved Caspase 3 (#9661, Cell Signaling, Danvers, MA). On the following day, sections were washed in PBS and incubated with a biotinylated secondary antibody (5 μl/ml; Vector Laboratories, Burlingame, CA) for one hour at room temperature. Immunoreactivity was detected using the Vectastain Elite DAB kit (Vector Laboratories, Burlingame, CA). The sections were counterstained with hematoxylin, dehydrated, and mounted. PR, ERα, Phospho-Histone H3 and cleaved Caspase 3 immunostained sections were counted by assessing the percentage of positive cells.
3. Statistical methods
Analyses were performed using Graphpad (San Diego, CA). Tukey’s post hoc multiple comparisons test was used to analyze the differences between multiple groups. P values lower than 0.05 were considered statistically significant.
RESULTS
1. Diminution of endometrial cancer size by progesterone treatment in PRcre/+ Ptenf/f mice
Previous studies have shown the role of mutations in PTEN and unopposed estrogen stimulation in the pathogenesis of uterine endometrioid carcinoma and recently, Pten heterozygous mice (Pten+/−) treated with estrogen resulted in the increased carcinoma incidence.17 In order to determine the role of ovarian steroid hormone in uterine specific Pten ablated mice, (PRcre/+ Ptenf/f, Ptend/d), first we confirmed the development of endometrial cancer in the PRcre/+ Ptenf/f mice. Similar to previous work,13 we also observed the development of endometrial cancer (Fig. 1B).
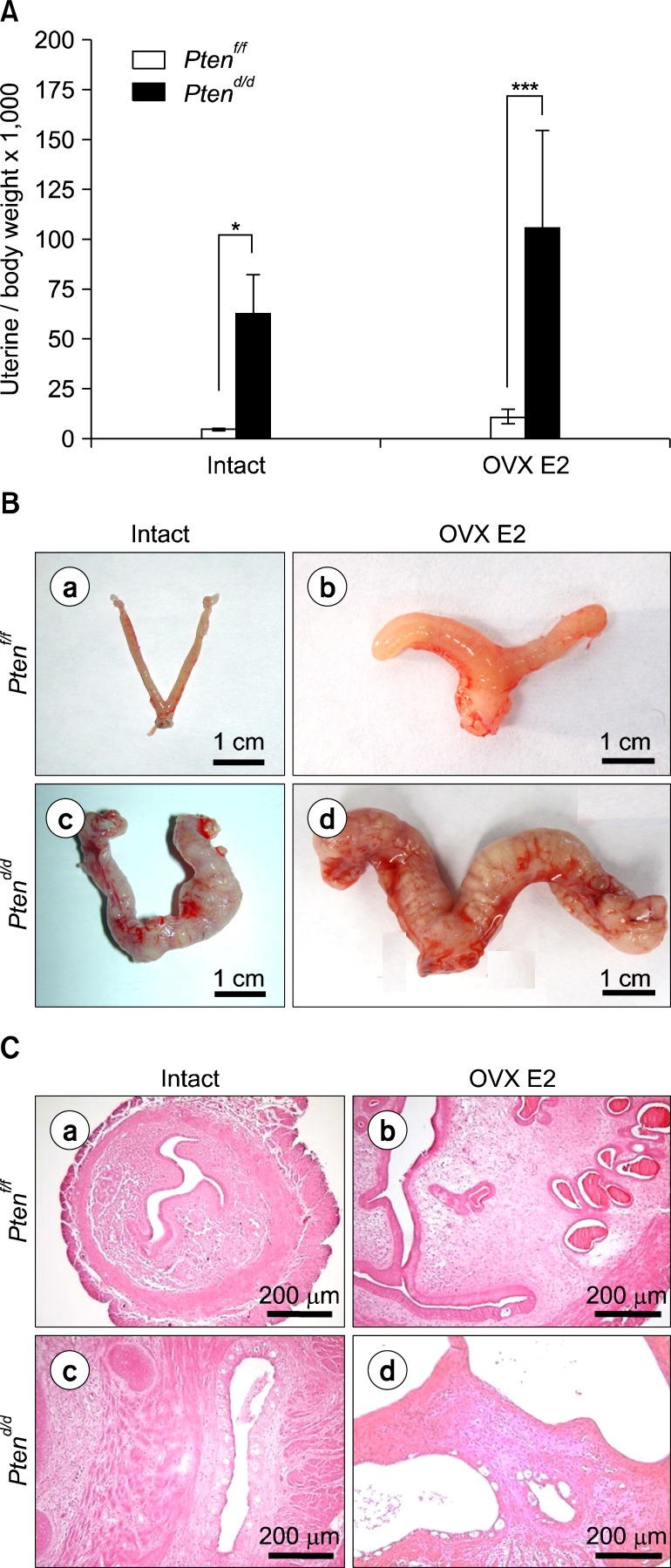
Fig. 1.
Estrogen dependent formation of endometrial cancer in PRcre+ Ptenf/f mice. (A) The ratio of uterine weight to body weight in intact and ovariectomized E2 treated PRcre+ Ptenf/f mice (OVX E2). The results represent the mean±SEM. *P< 0.05, ***P<0.001. (B) The uterine gross morphology of intact and ovariectomized E2 treated Ptenf/f mice (OVX E2) (a, b) and PRcre+ Ptenf/f mice (c, d). (C) Estrogen (E2) treatment induces endometrial cancer development in Pten ablated mice uterus. Hematoxylin and eosin staining of intact and ovariectomized E2 treated Ptenf/f mice (OVX E2) (a, b) and PRcre+ Ptenf/f mice (c, d).
To examine the effect of estrogen on PRcre/+ Ptenf/f mice, we ovariectomized Ptenf/f and PRcre/+ Ptenf/f mice at two months of age and treated with vehicle or estrogen over a period of one month, and then mice were sacrificed at 3.5 months of age (n=5 per treatment per genotype). Ovariectomized estrogen-treated PRcre/+ Ptenf/f mice showed significantly increased uterine weight and gross morphology when compared to Ptenf/f mice as observed in intact PRcre/+ Ptenf/f mice (Fig. 1A, 1B). Histological morphology showed development of endometrial cancer in intact and ovariectomized PRcre/+ Ptenf/f mice treated with estrogen. These results showed that PRcre/+ Ptenf/f mice developed severe endometrial cancer within one month of estrogen treatment compared to intact mice.
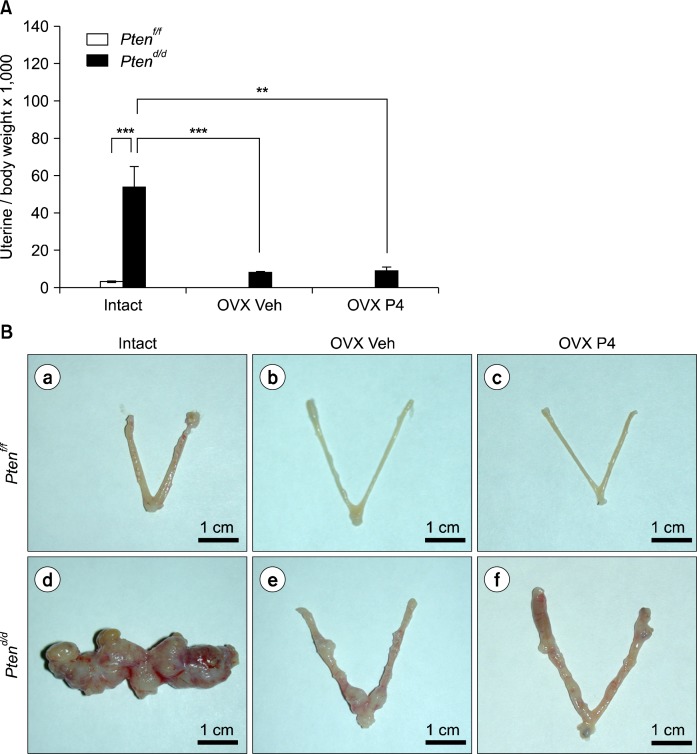
Progesterone hormone therapy has been shown to slow cancer cell growth for up to 30% of women who had advanced endometrial cancer.3,18 Next, we examined the effect of progesterone on endometrial cancer development when Pten is mutated. Ptenf/f and PRcre/+ Ptenf/f mice were overiectomized at two month of age and treated with vehicle or progesterone over a period of three months (n=5 per treatment per genotype). Mice were sacrificed at 5.5 months of age. Uterine weight, gross and histological morphology was examined (Fig. 2). Both ovariectomized vehicle and progesterone treated PRcre/+ Ptenf/f mice showed significantly decreased uterine weight when compared to intact PRcre/+ Ptenf/f mice (Fig. 2A). Gross morphology of ovariectomized vehicle and P4 treated PRcre/+ Ptenf/f mice displayed dramatically reduced development of endometrial cancer compared to intact PRcre/+ Ptenf/f mice (Fig. 2B). Histological analysis showed endometrial adenocarcinoma with invasion into the myometrium in all groups indicating that endometrial cancer progression is not affected. Interestingly, reduction of endometrial cancer size was observed in ovariectomized vehicle and progesterone treated PRcre/+ Ptenf/f mice (Fig. 2C). These results suggest that tumor mass is significantly reduced in the absence of estrogen but does not affect cancer metastasis in the Pten ablated mouse uterus.
Fig. 2.
Ovariectomized vehicle (OVX Veh) and P4 treated PRcre+ Ptenf/f mice (OVX P4) show a decrease uterine weight compared with intact. (A) The ratio of uterine weight to body weight in intact, ovariectomized vehicle (OVX Veh) and P4 treated PRcre+ Ptenf/f mice (OVX P4). The results represent the mean±SEM. **P<0.01, ***P<0.001. (B) The uterine gross morphology of intact, ovariectomized vehicle (OVX Veh) and P4 treated PRcre+ Ptenf/f mice (OVX P4) (a–c) and PRcre+ Ptenf/f mice (d–f). (C) Hematoxylin and eosin staining of intact, ovariectomized vehicle (OVX Veh) and P4 treated PRcre+ Ptenf/f mice (OVX P4) (a–c) and PRcre+ Ptenf/f mice (d, e and f for low magnification; g, h and i for high magnification).
2. Decreased proliferation in ovariectomized PRcre/+ Ptenf/f mice after P4 treatment
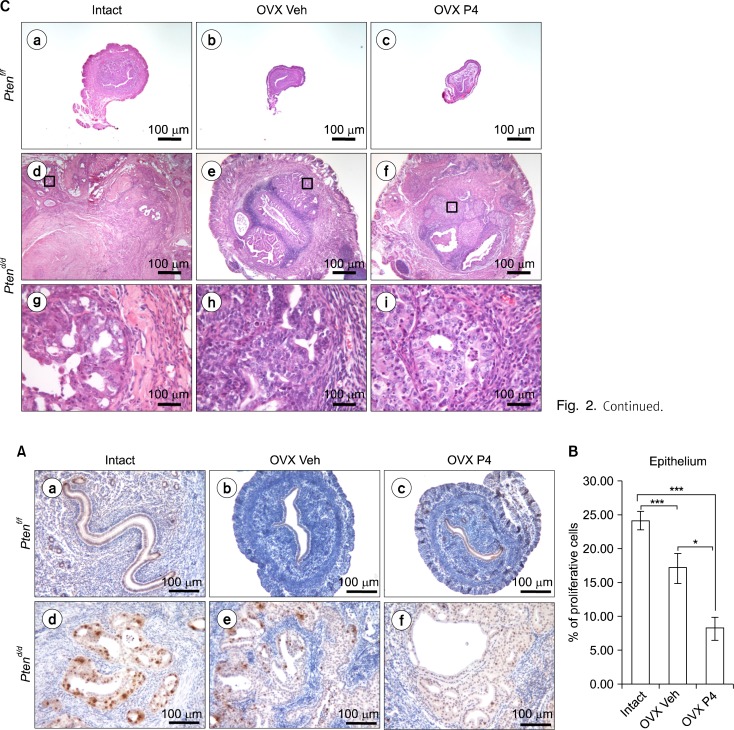
To address whether reduction of tumor mass in PRcre/+ Ptenf/f mice is caused by an alteration in endometrial uterine cell proliferation and/or apoptosis, we performed immunohistochemical analysis for phosphohistone H3, a mitotic marker in the endometrium of intact, ovariectomized vehicle or progesterone treated PRcre/+ Ptenf/f mice. Phosphohistone H3 immunostaining was significantly decreased in the endometrial epithelium of ovariectomized progesterone treated PRcre/+ Ptenf/f mice compared with other groups, but there are no changes in stromal cells (Fig. 3A, 3B). These results suggest that progesterone suppresses proliferation of uterine epithelial cells in PRcre/+ Ptenf/f mice contributing to reduced tumor mass.
Fig. 3.
The regulation of proliferation and apoptosis in Pten ablated mice uterus after P4 treatment. (A) Immunostaining for phospho-histone H3 in intact, ovariectomized vehicle (OVX Veh) and P4 treated PRcre+ Ptenf/f mice (OVX P4) (a–c) and PRcre+ Ptenf/f mice (d–f). (B) Quantification of phospho-histone H3 positive cells in epithelial cells. The results represent the mean±SEM. *P< 0.05, ***P<0.001.
3. An increase of stromal PR expression in ovariectomized PRcre/+ Ptenf/f mice after P4 treatment
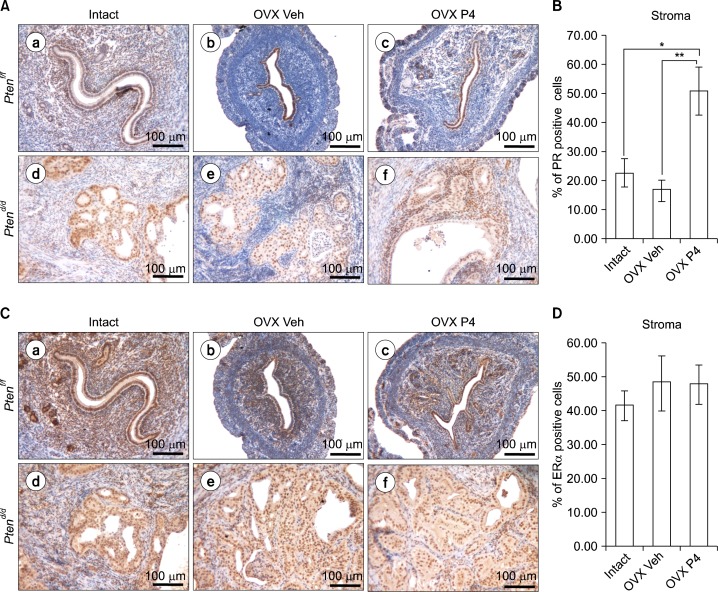
Expression of PR and ERα have been studied as prognostic factors for endometrial carcinoma.19–23 We assessed the expression of PR and ERα by immunohistochemistry in intact, ovariectomized vehicle or progesterone treated PRcre/+ Ptenf/f mice. Immunostaining of PR revealed highly increased stormal PR expression in the endometrium of ovariectomized progesterone treated PRcre/+ Ptenf/f mice, whereas intact and ovariectomized vehicle treated PRcre/+ Ptenf/f mice showed that a decrease of stromal PR expression (Fig. 4A, 4B). The expression level of ERα was not significantly changed between any groups (Fig. 4C, 4D). This data suggest that progesterone treatment leads to an increase in stromal PR expression in ovariectomized PRcre/+ Ptenf/f mice.
Fig. 4.
The expression of PR is recovered in PRcre+ Ptenf/f mice after P4 treatment. (A) Immunohistochemical analysis of PR in the uteri of intact, ovariectomized vehicle (OVX Veh) and P4 treated PRcre+ Ptenf/f mice (OVX P4) (a–c) and PRcre+ Ptenf/f mice (d–f). (B) Quantification of PR positive cells in stromal cells. The results represent the mean±SEM. *P<0.05, **P<0.01. (C) Immunohistochemical analysis of ERα in the uteri of intact, ovariectomized vehicle (OVX Veh) and P4 treated PRcre+ Ptenf/f mice (OVX P4) (a–c) and PRcre+ Ptenf/f mice (d–f). (D) Quantification of PR positive cells in stromal cells.
DISCUSSION
Estrogen-dependent endometrioid carcinoma is the most common type of gynecological cancer.6,24 Numerous studies support that prolonged, unopposed estrogen exposure has been associated with increased incidence of endometrial cancer3,25 and aberrant activation of the PTEN/PI3K/AKT signaling pathways.26 The PTEN/PI3K/AKT signaling pathway can be activated by estrogen.27 We used a PRcre/+ Ptenf/f (Ptend/d) mouse model16,28 to examine the effect of ovarian steroid hormone in tumorigenesis of endometrial cancer. To understand the effect of estrogen on the endometrial tumorigenesis, PRcre/+ Ptenf/f mice were treated with estrogen for 90 days. Estrogen treatment induced more severe endometrial tumorigenesis compared to control group, thereby indicating its important role in cancer development (Fig. 1).
To examine dependency of ovarian steroid hormones on the endometrial tumorigenesis of Pten mutation, PRcre/+ Ptenf/f mice were ovariectomized to remove the endogenous steroid hormone. Ovariectomized PRcre/+ Ptenf/f mice showed significantly decreased uterine weight when compared to intact PRcre/+ Ptenf/f mice (Fig. 2). The development of endometrial cancer in PRcre/+ Ptenf/f mice is independent of ovarian steroid hormone. Mice with a single deleted Pten allele (Pten+/−) also developed complex atypical hyperplasia and ∼20% developed endometrial cancer.17,29 These results suggest that the development of endometrial cancer in Pten mutation is independent of steroid hormones.
Progesterone is a hormone that antagonizes the growth-promoting properties of estrogen in the uterus.6,30 Progestin has been used in the conservative endocrine treatment to early endometrial cancer patients in order to preserve their fertility, as well as in palliative treatment to advanced-stage patients.31–34 Progesterone therapy prevents the development of endometrial cancer associated with unopposed estrogen by blocking estrogen actions as well as in palliative treatment to advanced-stage patients who are poor surgical candidates.31,33,34 However, more than 30% of patients with progestin treatment did not respond to progestin due to de novo or acquired progestin resistance.33,35–38 The mechanism of progestin resistance is still unknown. P4 treatment showed reduction of endometrial cancer size (Fig. 2B) but did not suppress metastasis of endometrial adenocarcinoma into the myometrium (Fig. 2C). These results suggest that tumor mass is significantly reduced in the absence of estrogen but does not affect cancer metastasis in the Pten ablated mouse uterus.
Estrogen stimulates proliferation of epithelial cells in the mouse uterus.6,30 In contrast, progesterone inhibits the estrogen-mediated proliferation of the luminal and glandular epithelial cells.5,30,39 It is reported that endometrial cancer is an estrogen-dependent disease and that progesterone therapy has been used successfully to slow the growth of endometrial tumors in women by its inhibitory effects on estrogen action.31–34,40 Reduction of tumor mass was found in ovariectomized vehicle and progesterone treated PRcre/+ Ptenf/f mice. Interestingly, endometrial cancer progression is not affected in ovariectomized vehicle and progesterone treated PRcre/+ Ptenf/f mice (Fig. 2). This indicates that the progression of endometrial cancer can be associated with Pten mutation and the correlation between the expression of PTEN and clinical status of endometrial cancer patients should be evaluated. Even though ovariectomized vehicle and progesterone treated PRcre/+ Ptenf/f mice exhibited endometrial cancer progression, we observed an alteration in endometrial uterine cell proliferation. Proliferation is significantly decreased in ovariectomized vehicle and progesterone treated PRcre/+ Ptenf/f mice compared to intact PRcre/+ Ptenf/f in epithelial (Fig. 3). Despite clinical use of progesterone, the antitumor mechanisms for progesterone therapy is still unknown. Our results showed that progesterone can induce decreased cellular proliferation during endometrial tumorigenesis.
Expression of PR and ERα have been reported as prognostic factors for endometrial carcinoma.19–23 Decreased expression of PTEN gene and expression of PR and ERα occurs in the majority of patients with endometrial cancer.41 Expression of progesterone receptor (PR) is positively correlated with a good prognosis and response to progesterone treatment.42 However, more than 30% of patients with progesterone treatment did not respond to progesterone due to de novo or acquired progesterone resistance.33,35–38 In this study, we addressed that the expression of stromal PR was significantly increased in ovariectomized progesterone treated PRcre/+ Ptenf/f mice, whereas highly decreased in intact and ovariectomized vehicle treated PRcre/+ Ptenf/f mice. The level of ERα was not changed among the groups (Fig. 3). It is studied that progesterone inhibits the proliferation of endometrial epithelial cells via PR in stromal cells by blocking the production of mitogenic mediators in the stroma.43,44 This suggest that the induction of stromal PR by P4 treatment in ovariectomized PRcre/+ Ptenf/f mice may play a role in the reduction of tumor mass via regulating epithelial cell proliferation.
In conclusion, our results show that the steroid hormones intervene in the endometrial tumorigenesis of PRcre/+ Ptenf/f mice. PTEN/PI3K/AKT signal pathway is aberrantly regulated in a wide range of human tumors making them excellent candidates for selective anticancer therapies. However, the relationship between PTEN/PI3K/AKT and steroid hormone signaling pathways is not well known. Based on the extensive crosstalk between PTEN/PI3K/AKT and steroid hormone signaling, drug combination approaches targeting both pathways would seem to be a rational clinical strategy to improve the efficacy of endocrine therapies. This analysis will identify potential therapeutic targets for the treatment of endometrial cancer. Our study will allow the development of future PTEN/PI3K/AKT and steroid hormone signaling- targeted therapeutic tools to attenuate the progress of endometrial cancer.
Acknowledgments
This work was supported by American Cancer Society Research Scholar Grant RSG-12-084-01-TBG (to J.W.J.).
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Jick SS. Combined estrogen and progesterone use and endometrial cancer. Epidemiology. 1993;4:384. doi: 10.1097/00001648-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Jick SS, Walker AM, Jick H. Estrogens, progesterone, and endometrial cancer. Epidemiology. 1993;4:20–4. doi: 10.1097/00001648-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- 5.Martin L, Das RM, Finn CA. The inhibition by progesterone of uterine epithelial proliferation in the mouse. J Endocrinol. 1973;57:549–54. doi: 10.1677/joe.0.0570549. [DOI] [PubMed] [Google Scholar]
- 6.Martin L, Finn CA, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol. 1973;56:133–44. doi: 10.1677/joe.0.0560133. [DOI] [PubMed] [Google Scholar]
- 7.Hale GE, Hughes CL, Cline JM. Endometrial cancer: hormonal factors, the perimenopausal “window of risk”, and isoflavones. J Clin Endocrinol Metab. 2002;87:3–15. doi: 10.1210/jcem.87.1.8132. [DOI] [PubMed] [Google Scholar]
- 8.Sun H, Enomoto T, Fujita M, Wada H, Yoshino K, Ozaki K, et al. Mutational analysis of the PTEN gene in endometrial carcinoma and hyperplasia. Am J Clin Pathol. 2001;115:32–8. doi: 10.1309/7JX6-B9U9-3P0R-EQNY. [DOI] [PubMed] [Google Scholar]
- 9.Levine RL, Cargile CB, Blazes MS, van Rees B, Kurman RJ, Ellenson LH. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res. 1998;58:3254–8. [PubMed] [Google Scholar]
- 10.Maxwell GL, Risinger JI, Gumbs C, Shaw H, Bentley RC, Barrett JC, et al. Mutation of the PTEN tumor suppressor gene in endometrial hyperplasias. Cancer Res. 1998;58:2500–3. [PubMed] [Google Scholar]
- 11.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 12.Guzeloglu-Kayisli O, Kayisli UA, Al-Rejjal R, Zheng W, Luleci G, Arici A. Regulation of PTEN (phosphatase and tensin homolog deleted on chromosome 10) expression by estradiol and progesterone in human endometrium. J Clin Endocrinol Metab. 2003;88:5017–26. doi: 10.1210/jc.2003-030414. [DOI] [PubMed] [Google Scholar]
- 13.Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, et al. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008;68:5619–27. doi: 10.1158/0008-5472.CAN-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 15.Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32:148–9. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 16.Kim TH, Franco HL, Jung SY, Qin J, Broaddus RR, Lydon JP, et al. The synergistic effect of Mig-6 and Pten ablation on endometrial cancer development and progression. Oncogene. 2010;29:3770–80. doi: 10.1038/onc.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi A, Wang H, Jiang G, Douglas W, Chan JS, Korach KS, et al. Endometrial tumorigenesis in Pten(+/−) mice is independent of coexistence of estrogen and estrogen receptor alpha. Am J Pathol. 2012;180:2536–47. doi: 10.1016/j.ajpath.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piver MS, Recio FO, Baker TR, Hempling RE. A prospective trial of progesterone therapy for malignant peritoneal cytology in patients with endometrial carcinoma. Gynecol Oncol. 1992;47:373–6. doi: 10.1016/0090-8258(92)90142-6. [DOI] [PubMed] [Google Scholar]
- 19.Carcangiu ML, Chambers JT, Voynick IM, Pirro M, Schwartz PE. Immunohistochemical evaluation of estrogen and progesterone receptor content in 183 patients with endometrial carcinoma. Part I: Clinical and histologic correlations. Am J Clin Pathol. 1990;94:247–54. doi: 10.1093/ajcp/94.3.247. [DOI] [PubMed] [Google Scholar]
- 20.Kleine W, Maier T, Geyer H, Pfleiderer A. Estrogen and progesterone receptors in endometrial cancer and their prognostic relevance. Gynecol Oncol. 1990;38:59–65. doi: 10.1016/0090-8258(90)90012-a. [DOI] [PubMed] [Google Scholar]
- 21.Iwai K, Fukuda K, Hachisuga T, Mori M, Uchiyama M, Iwasaka T, et al. Prognostic significance of progesterone receptor immunohistochemistry for lymph node metastases in endometrial carcinoma. Gynecol Oncol. 1999;72:351–9. doi: 10.1006/gyno.1998.5286. [DOI] [PubMed] [Google Scholar]
- 22.Nyholm HC, Nielsen AL, Lyndrup J, Dreisler A, Thorpe SM. Estrogen and progesterone receptors in endometrial carcinoma: comparison of immunohistochemical and biochemical analysis. Int J Gynecol Pathol. 1993;12:246–52. doi: 10.1097/00004347-199307000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda K, Mori M, Uchiyama M, Iwai K, Iwasaka T, Sugimori H. Prognostic significance of progesterone receptor immunohistochemistry in endometrial carcinoma. Gynecol Oncol. 1998;69:220–5. doi: 10.1006/gyno.1998.5023. [DOI] [PubMed] [Google Scholar]
- 24.Deligdisch L, Holinka CF. Endometrial carcinoma: two diseases? Cancer Detect Prev. 1987;10:237–46. [PubMed] [Google Scholar]
- 25.Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975;293:1167–70. doi: 10.1056/NEJM197512042932303. [DOI] [PubMed] [Google Scholar]
- 26.Di Cristofano A, Ellenson LH. Endometrial Carcinoma. Annu Rev Pathol. 2007;2:57–85. doi: 10.1146/annurev.pathol.2.010506.091905. [DOI] [PubMed] [Google Scholar]
- 27.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–46. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- 28.Kim TH, Lee DK, Cho SN, Orvis GD, Behringer RR, Lydon JP, et al. Critical tumor suppressor function mediated by epithelial Mig-6 in endometrial cancer. Cancer Res. 2013;73:5090–9. doi: 10.1158/0008-5472.CAN-13-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fyles A, Wood G, Li M, Manoukian AS, Gowing K, Khokha R, et al. Neither ovariectomy nor progestin treatment prevents endometrial neoplasia in pten+/− mice. Gynecol Oncol. 2008;108:395–401. doi: 10.1016/j.ygyno.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Huet-Hudson YM, Andrews GK, Dey SK. Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the peri-implantation period. Endocrinology. 1989;125:1683–90. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- 31.Benshushan A. Endometrial adenocarcinoma in young patients: evaluation and fertility-preserving treatment. Eur J Obstet Gynecol Reprod Biol. 2004;117:132–7. doi: 10.1016/j.ejogrb.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Minaguchi T, Nakagawa S, Takazawa Y, Nei T, Horie K, Fujiwara T, et al. Combined phospho-Akt and PTEN expressions associated with post-treatment hysterectomy after conservative progestin therapy in complex atypical hyperplasia and stage Ia, G1 adenocarcinoma of the endometrium. Cancer Lett. 2007;248:112–22. doi: 10.1016/j.canlet.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Hahn HS, Yoon SG, Hong JS, Hong SR, Park SJ, Lim JY, et al. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int J Gynecol Cancer. 2009;19:1068–73. doi: 10.1111/IGC.0b013e3181aae1fb. [DOI] [PubMed] [Google Scholar]
- 34.Yamazawa K, Hirai M, Fujito A, Nishi H, Terauchi F, Ishikura H, et al. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod. 2007;22:1953–8. doi: 10.1093/humrep/dem088. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol. 2004;95:133–8. doi: 10.1016/j.ygyno.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 36.Hoekstra AV, Kim JJ, Keh P, Schink JC. Absence of progesterone receptors in a failed case of fertility-sparing treatment in early endometrial cancer: a case report. J Reprod Med. 2008;53:869–73. [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med. 2010;28:81–90. doi: 10.1055/s-0029-1242998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaku T, Yoshikawa H, Tsuda H, Sakamoto A, Fukunaga M, Kuwabara Y, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett. 2001;167:39–48. doi: 10.1016/s0304-3835(01)00462-1. [DOI] [PubMed] [Google Scholar]
- 39.Paria BC, Huet-Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci U S A. 1993;90:10159–62. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaunitz AM. Injectable depot medroxyprogesterone acetate contraception: an update for U.S. clinicians. Int J Fertil Womens Med. 1998;43:73–83. [PubMed] [Google Scholar]
- 41.Samulak D, Grosman-Dziewiszek P, Michalska MM, Mojs E, Samulak K, Romanowicz H, et al. Evaluation of Expression of the PTEN Gene, Oestrogen and Progesterone Receptors as Diagnostic and Predictive Factors in Endometrial Cancer. Pathol Oncol Res. 2013 Sep 13; doi: 10.1007/s12253-013-9684-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrlich CE, Young PC, Stehman FB, Sutton GP, Alford WM. Steroid receptors and clinical outcome in patients with adenocarcinoma of the endometrium. Am J Obstet Gynecol. 1988;158:796–807. doi: 10.1016/0002-9378(88)90075-0. [DOI] [PubMed] [Google Scholar]
- 43.Kurita T, Young P, Brody JR, Lydon JP, O’Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4708–13. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–6. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]