Abstract
Background:
Intestinal mucositis is a most frequently occurring toxicity in cancer chemotherapy, and consequent malnutrition reduces tolerance to cancer therapies. Therefore it is important to lessen the severity of mucotitis and to develop complementary agents capable of reducing mucotitis-related symptoms. This study was conducted to determine 5-fluorouracil (5-FU) induced intestinal damage to understand intestinal damages due to chemotherapy and to provide information on biomarkers which can be used to screen complementary agents in future studies.
Methods:
BALB/c mice were divided into three experimental groups and subjected to the intraperitoneal injection of either 100 mg/kg or 200 mg/kg of 5-FU. The third group was used as PBS controls. Body weights and the consistency of the stools were recorded every day, and the animals were sacrificed on the 7th day post 5-FU administration. The expressions of intestinal tight junction proteins and mRNAs of tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) were determined.
Results:
The body weight of the animals treated with 5-FU was significantly decreased in a dose-dependent manner. However, mice given 100 mg/kg 5-FU rapidly recovered the original body weight. Symptom of diarrhea was also more severe in 200 mg/kg 5-FU treated group than that of the 100 mg/kg 5-FU treated animals. The expressions of occludin and claudin-1, not ZO-1 protein expressions in 200 mg/kg 5-FU treated animals were significantly reduced compared to those of the control group or 100 mg/kg 5-FU group. The expression of Nuclear factor-kappa B p65 (NF-κB p65) protein and TNF-α mRNA were significantly higher in 5-FU treated group compared to those of control group. No difference was observed with IL-1β expression.
Conclusions:
These results suggested that selected tight junction proteins and inflammatory cytokines are related to 5-FU induced mucositis, and thereby can be used as targets of developing complementary agents.
Keywords: Mucotitis, Chemotherapy, 5-Fluorouracil, Tight junction protein, Cytokines
INTRODUCTION
Gastrointestinal mucositis is a common side effect of anticancer chemotherapy that involves the small intestines. Not only does mucositis decrease the quality of life in most cancer patients because of its associated intense pain, it is also a high-risk factor for hematosepsis with neutropenia and malnutrition. This association, thus, renders mucositis a clinically important disease. Moreover, this specific side effect does not allow appropriate chemotherapy and radiotherapy, and becomes an obstacle that delays the administration of effective cancer treatment.1
5-Fluorouracil (5-FU) is an anticancer drug that is widely used in the treatment of colorectal cancer, and it possesses a chemical structure similar to that of uracil and thymine.2 A major part of it (more than 80%) is catabolized by hepatic dihydropyrimidine dehyrogenase (DPD), whereas the rest induces cell death by inhibiting RNA and DNA syntheses through fluorodeoxyuridine monophosphate (FdUMP) and fluorouridine triphosphate (FUTP).3,4 The major side effects of 5-FU include myelosuppression, diarrhea, cardiotoxicity, dermatitis, and mucositis.5,6 Of these, gastrointestinal mucositis has been reported in approximately 80% of patients who have received cancer treatment by using 5-FU.2 A previous study showed that rat animal model treated with 5-FU showed reduced gastrointestinal villi length, increased crypt depth, elevated apoptosis index, increased myeloperoxidase (MPO) activity, reduced glutathione (GSH) concentration, and increased inflammatory mediator levels.7
Anticancer drugs induce apoptosis of cancer cells as well as normal cells which are rapidly dividing cells. Normal gastrointestinal cells have short cell cycles and develop side effects such as mucositis occur due to apoptosis as induced by exposure to anticancer drugs. The small intestine, thus, is considered to be the most vulnerable to anticancer drugs, because the mucosal replacement cycle is approximately 3–4 days. Gastrointestinal mucositis leads to problems that influence the prognosis of patients, such as pain, decreased nutritional intake, increased need of intravenous nutrition, and increased risk of systemic infection. Its prevention, early detection, and treatment are the most significant because its treatment is difficult. Therefore, this study aimed to identify intestinal integrity biomarkers of gastrointestinal mucositis, which has attracted much attention as one of the most common side effects of the use of anticancer drugs. Appropriate biomarkers can be used as targets to develop complementary agents to lessen the side effects of chemotherapy and eventually improve the patients’ quality of life.
MATERIALS AND METHODS
1. Experimental animals
Nine, white, 6-week-old BALB/c male mice, obtained from the Central Laboratory Animals, Seoul, Korea, were utilized in this study. After 1 week of acclimatization, the mice were randomly classified into 3 groups, with 3 mice in each group. Phosphate-buffered saline (PBS) solution was administered to the control group, and 100 mg/kg and 200 mg/kg of 5-FU dissolved in PBS were injected intraperitoneally to the 2 experimental groups, respectively (Fig. 1). The mice were kept in a room maintained at a temperature of 24±2°C, relative humidity of 60±5%, and a dark cycle of 12 h. Weight and diarrhea scores were measured daily in all mice starting from the day of 5-FU administration.
Fig. 1.
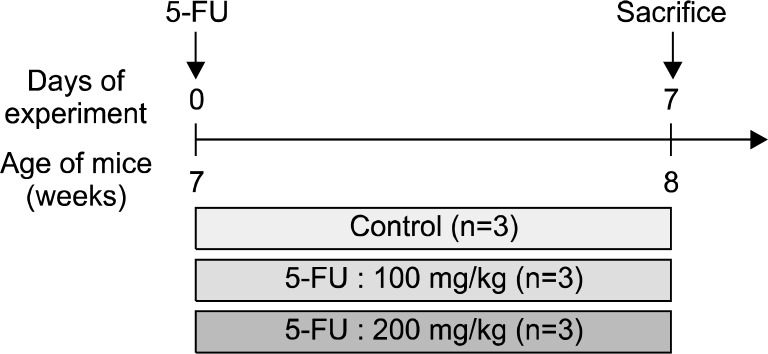
Experimental design. BALB/c mice were divided into 3 experimental groups and subjected to either 100 mg/kg or 200 mg/kg of 5-FU injection. Animals were sacrificed on the 7th day post 5-FU administration.
2. Sample collection and pretreatment
Once the treatment period was completed, the mice were fasted for 12 h and anesthetized using Zoletil solution diluted in a saline solution, followed by longitudinal laparotomy. Liver and spleen were isolated and washed in PBS. After pat drying the tissues, their weights were measured and stored at −80°C. The small and large intestines were separated and their lengths were measured. Mucous were scraped and stored at −80°C. Tight junction (TJ) protein expression and cytokine secretion were also measured.
3. Diarrhea assessment
The presence or absence and degree of diarrhea were determined based on the standards established for the diarrhea assessment.8 The diarrhea score was assessed daily for each animal by using a 4-point scale with the following standards: 0=normal (normal stool or absent); 1=slight (slightly wet and soft stool); 2=moderate (wet and unformed stool with moderate perianal staining of the coat); and 3=severe (watery stool with severe perianal staining of the coat). The calculated average values were utilized in the study.
4. Protein expression by western blot analysis
Small intestine tissues were homogenized using the protein extraction reagent PRO-PREPTM (Intronbio, Sungnam, South Korea), and were subjected to centrifugation (13,000 rpm, 10 min, 4°C) after keeping on ice for 20 min. The extracted proteins were quantified using a Protein Assay kit (Bio-Rad, CA, USA), and 50 μg of the proteins were added to each sample. The proteins were then separated using SDS-PAGE and were transferred onto a PVDF membrane (Koma Biotechnology, Seoul, South Korea). The transferred membrane was blocked using 2% skim milk to inhibit non-specific proteins, and primary antibodies were added. Occludin (dilution 1:200 Invitrogen, Carlsbad, CA, USA), β-actin (dilution 1:4,000; Sigma, Saint Louis, MO, USA), and claudin-1 (1:250, Invitrogen, Carlsbad, CA, USA), ZO-1 (1:50: invitrogen, Carlsbad, CA, USA), NF-κB p65 (1:1,000; Cell Signaling, Danver, MA, USA), were used as antibodies. After incubating with the primary antibodies, the membranes were washed with PBS/Tween-20 (PBST) three times and were then incubated with secondary antibodies. Each protein band was then confirmed and quantified using an enhanced chemiluminescence (ECL) system (Amersham, Arlington Heights, IL, USA). The intensities of the bands were measured using LAS3000 (Fujifilm, Tokyo, Japan).
5. RNA isolation and real-time quantitative PCR analysis
The levels of mRNA expression of TNF-α and IL-1β in the small intestines were analyzed using real-time quantitative polymerase chain reaction (qPCR). Trizol (Invitrogen, Carlsbad, CA, USA) was used for RNA extraction. After the tissues were homogenized in 1 ml of Trizol, 200 μl chloroform was added to a DEPC-treated tube and was shaken for a few seconds. The samples were then centrifuged at 13,500 rpm at 4°C for 15 min. The same amount of isopropanol as the RNA supernatant was added, and the mixture was centrifuged. The samples were then washed with 70% ethanol and solubilized in DEPC-water. Total RNA (1 μg) of synthesized cDNA from a cDNA synthesis kit (Genepole, Gwangmyeong, Korea), an oligo (DT) primer mix, and a random hexane primer mix were added to 1 μg RNA. Real-time PCR was conducted using Quanti-Mix SYBR Kit (Genepole, Gwangmyeong, South Korea) by using a 7500 Fast Real Time PCR system (Applied Bio-systems, Foster City, CA, USA). TNF-α, IL-1β, and β-actin were synthesized by Bioneer (Daejeon, Korea), and their sequences are presented in Table 1. Relative quantification values and 2−ΔΔCt were calibrated using β-actin, which is a housekeeping gene. Each experiment was performed in triplicate.
Table 1.
Gene-specific primers used for RT-PCR
| Gene | Direction | Sequence |
|---|---|---|
| TNF-α | Forward | ACG TGG AAC TGG CAG AAG AG |
| Reverse | CTC CTC CAC TTG GTG GTT TG | |
| IL-1β | Forward | CTC CAT GAG CTT TGT ACA AGG |
| Reverse | TGC TGA TGT ACC AGT TGG GG | |
| α-Actin | Forward | CAT GGA TGA CGA TAT CGC T |
| Reverse | CAT GAG GTA GTC TGT CAG GT |
6. Statistical analysis
For the results of all experimental analyses, means and standard deviation in each group were calculated using a SAS program. After performing one-way ANOVA, statistical significance of the means in each group was tested using Duncan’s multiple range test, at a significance level of α= 0.05.
RESULTS
1. Body weight changes and main organ weight
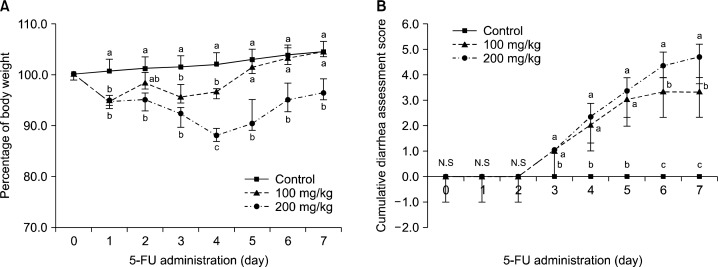
Body weight changes in the experimental animals are shown in Fig. 2. Compared to the control group, the extent of body weight reduction in the experimental groups manifested significant differences from Day 1 after the 5-FU administration (Fig. 2A, P<0.05). Significantly higher reduction in the body weight was observed in the group administered with 200 mg/kg of 5-FU compared to the 100 mg/kg group (Fig. 2A). The weights of the liver and kidney and the lengths of the small and large intestines are presented in Table 2. The weight of the spleen significantly decreased in the 5-FU treated experimental groups (P<0.05). No significant differences in the weight of the liver and lengths of the small and large intestines were observed.
Fig. 2.
Effects of 5-FU on the daily body weight changes and diarrhea assessment scores. Animals treated with 5-FU or its vehicle were monitored during the 7-day experimental period. Scores were recorded as 0, 1, 2, or 3 where 0 is normal stool (normal stool or absent), 1 is slight stool (slightly wet and soft stool), 2 is moderate stool (wet and unformed stool with moderate perianal staining of the coat) and 3 is severe stool (watery stool with severe perianal staining of the coat). The results are shown represent the means±S.D (n=3). Means with the different letters (a–c) at each point are significantly different from each other (P<0.05) by Duncan’s multiple-range test.
Table 2.
Tissue weights and intestinal length in mice injected with PBS or 5-FU
| Group | Liver (g/100 g) | Spleen (g/100 g) | Small intestine (cm) | Large intestine (cm) |
|---|---|---|---|---|
| Control | 4.6±0.15a | 0.41±0.04a | 34.8±0.76a | 7.97±1.10a |
| 5-FU 100 mg/kg | 5.17±0.59a | 0.31±0.02b | 36.20±2.97a | 6.77±0.25a |
| 5-FU 200 mg/kg | 5.16±0.15a | 0.32±0.03b | 34.53±3.90a | 6.77±0.60a |
Means with different letters (a, b) within a column are significantly different from each other at p<0.05 as determined by Duncan’s multiple-range test.
2. Diarrhea assessment
The most common symptom of gastrointestinal mucositis is diarrhea. Treatment with 5-FU significant increased the cumulative diarrhea score from Day 3 after the 5-FU administration (Fig. 2B, P<0.05). Significant differences were also observed between the 100 mg/kg and 200 mg/kg groups (P<0.05) from Day 6 after the 5-FU administration (Fig. 2B, P<0.05).
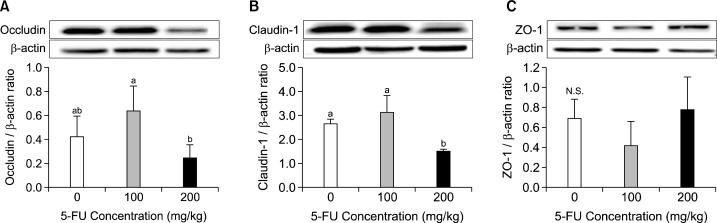
3. Claudin-1, occludin, and ZO-1 expressions of the mucous of the small intestine
The protein expression levels of claudin-1, occludin, and zonula occludens-1 (ZO-1) involved in gastrointestinal permeability were measured. The results revealed that claudin-1 expression in the mucous of the small intestine significantly decreased in the 200 mg/kg 5-FU group (Fig. 3, P< 0.05), whereas those of occludin and ZO-1 did not show significant differences.
Fig. 3.
Effects of 5-FU on the mucous occludin (A), claudin-1 (B), and ZO-1 (C) protein expression. Mice were given intraperitonealy injection of 5-FU. After 7 days, tight junction protein expressions of occludin (A), claudin-1 (B), and ZO-1 (C) in the small intestine mucosa were determined. Densitometry results after normalized by β-actin and are shown as mean±S.D. Means with the different letters (a, b) are significantly different from each other (P<0.05) by Duncan’s multiple-range test.
4. NF-κB p65 protein expression in the mucous of the small intestine
NF-κB is a critical transcription factor in inflammatory responses. NF-κB protein expression in the mucous of the small intestine was significantly higher in both the groups compared to that in the control (Fig. 4, P<0.005). The expression level was slightly lower in the group in which high concentration of 5-FU was used compared to that in the low concentration (Fig. 4, P<0.05).
Fig. 4.
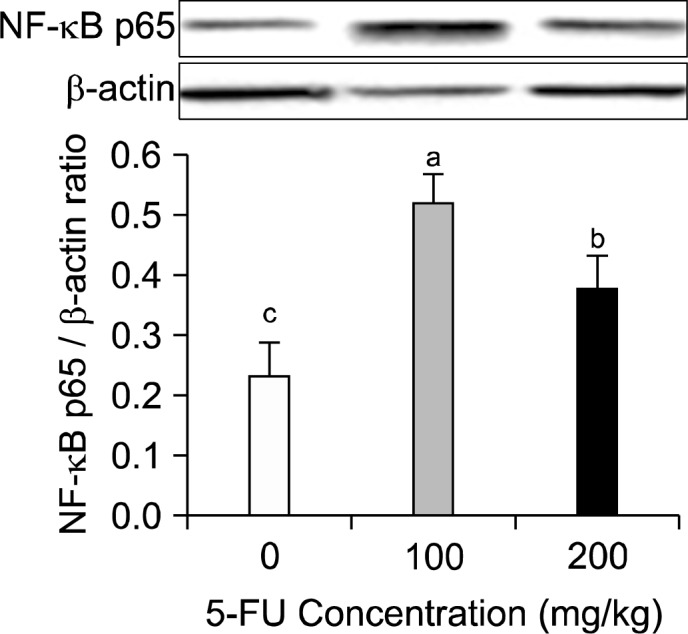
Effect of 5-FU on intestinal mucous NF-κB expression. Mice were given intraperitonealy injection of 5-FU. After 7 days, protein expression of NF-κB in the small intestine mucosa was determined. Densitometry results after normalized by β-actin and are shown as mean±S.D. Means with the different letters (a–c) are significantly different from each other (P< 0.05) by Duncan’s multiple-range test.
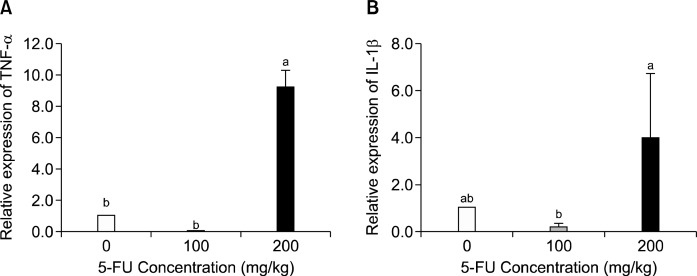
5. TNF-α and IL-1β mRNA expressions in the mucous of the small intestine
The levels of mRNA expression of TNF-α and IL-1β, which are pro-inflammatory cytokines, were analyzed in the mucous of the small intestine. TNF-α showed a 98% increased expression in the group treated with 200 mg/kg 5-FU compared to the control group (Fig. 5, P<0.005). IL-1β showed a 32% increased expression in the group administered 200 mg/kg of 5-FU, although there was not statistically significant (Fig. 5).
Fig. 5.
Effect of 5-FU on mucous TNF-α (A), IL-1β (B) mRNA expression. Mice were given intraperitoneal injection of 5-FU. After 7 days, mRNA expression of TNF-α and IL-1β in the small intestine mucosa were determined. The expression of the β-actin gene was used to normalize the PCR reactions. The results are shown as means±S.D (n=3). Means with the different letters (a, b) are significantly different from each other (P<0.05) by Duncan’s multiple-range test.
DISCUSSION
5-FU, an antimetabolite, is one of the anticancer drugs widely utilized in the treatment of cancers such as colorectal cancer and breast cancer. It is known to have concomitant side effects including myelosuppression, nausea, vomiting, abdominal pain, and mucositis.9 This study aimed to provide information on intestinal damage biomarkers in 5-FU treated mice, which can be used as intervention targets of complementary agents to relieve mucositis derived side effects.
Previous studies have shown decreased body weight in experimental animals after 5-FU administration.10,11 The present study also confirmed that the body weight of mice significantly decreased from Day 1 after 5-FU administration. However, diarrhea was observed at Day 5 after 5-FU administration. This indicates that 5-FU administration acutely affects the body weight of the animals possibly due to the activation of inflammatory responses followed by gastrointestinal malfunction. The etiology of mucositis involves the production of reactive oxygen species (ROS) and the amplification of inflammatory signals induced by anticancer drugs or radiation.12 These causative factors are recognized to play a major role in mucositis, while no effective means to prevent or treat mucotitis has been suggested. ROS activates transcription factors that result in a series of phenomena defined as acute tissue reactions that exert direct damages on various cells, tissues, and blood vessels regardless of the cause.2 Among these, NF-κ B is a transcription factor that plays a key role in the production of pro-inflammatory cytokines such as TNF-α and IL-1β, which lead to tissue damages and cell death and resulting in mucositis. Determination of the NF-κB protein expression in mucous in the small intestine revealed that both the 5-FU-treatment groups (100 mg/kg and 200 mg/kg) showed significantly higher expression levels compared to that of the control group, and this is in agreement with the results of previous studies.13,14 However, there was no dose-dependent increase in the expression of total NF-κB expression. Although the present study did not measure nuclear translocation of NF-kB as a measure of its activation, a previous study showed that increases in LPS-induced expression of cytosolic or nuclear NF-κB p65 were similar suggesting total NF-kB expression may reflect nuclear translocation.15 We believed that our results reflect the influence of “overflow,” due to the accumulated damages in tissues. On the other hand, the mRNA expression level of TNF-α in the mucous of the small intestine significantly increased in the high-concentration group (200 mg/kg) compared to that in the control group. This result is consistent with that of other studies, which showed that the level of pro-inflammatory cytokine expression increases with the severity of mucositis.16,17 However, there was no induction of TNF-α or IL-1β at 100 mg. It could have been more convincing to show the increased expression of pro-inflammatory cytokines. However, the expression of these cytokines are also regulated by other signals generated by physiological stimuli, and may provide possible explanation. A recent study reported that the expression of NF-κB and pro-inflammatory cytokines are not always unidirectional in their report.18
Intestinal epithelium possesses highly dynamic structure and acts as selective barrier between outside environment and underlying tissue. TJ proteins act as gate keepers to prohibit the passage of noxious molecules, while allowing the exchange of ions, solutes and water. TJ proteins include claudins, occulindin and ZO-1. TJ barrier disruption leads to the increased permeability of luminal bacteria and proinflammatory molecules to activate inflammatory responses and tissue damages. However, a limited number of in vivo studies have provided evidences on the association between TJ protein losses in mucositis. The present study showed that the expressions of claudin-1 and occludin, which constitute a part of the TJ protein, were significantly reduced compared to that in the control group when 200 mg/kg 5-FU was administered for 7 days. This indicates that 5-FU treatment possibly increases intestinal permeability to a significant degree not only disrupting the basic functions of cell membrane but also playing a critical role in the pathogenesis systemic inflammation. The increased permeability is shown to result in the inflow of endotoxins, various pathogenic microorganisms, and varied high molecular substances.19 A previous study suggested that occludin knockout mice exhibited not only chronic inflammation and hyperplasia of the gastric epithelium but abnormal changes in testicule and salivary gland suggesting more complex role of occludin.20 A previous study suggested that hydrogen peroxide induced TJ disruption is mediated by a tyrosine kinase dependent mechanism.21 Claudin is a protein structurally discriminated from occuldin. Claudins are suggested as the backbone of TJ proteins, and the claudin knockout mice revealed abnormal barrier formation and paracellular permselectivity.22 Our results suggest that 5-FU treatment significantly reduces intestinal membrane TJ protein expression possibly exhibiting stuructural and functional defects. In the case of ZO-1, which is one of the TJ proteins, no significant intergroup differences were observed. Such discrepancies in the TJ proteins may be attributable to their different interactions with other membrane compartments including actin. Although ZO-1 proteins are known to mediate early TJ protein assembly, functional role of ZO-1 has not been clearly demonstrated. Also, a recent report showed that normal TJ structures were formed with ZO-1 deficient cells suggesting further studies on the role of ZO-1 in TJ proteins.23 Since our results indicate claudins and occuldin expressions are dramatically reduced by 5-FU treatment, it is presumable that severe functional defects are induced by 5-FU treatment and therefore appropriate interventions towards there TJ proteins may alleviate chemotherapy-induced mucositis.
In conclusion, 5-FU treated animals revealed significant body weight loss in a dose-dependent manner. Clear and significant TJ protein losses are observed suggesting appropriate intervention towards these targets may alleviate chemotherapy-associated mucositis.
Acknowledgments
This research was supported by High Value-added Food Technology Development Program, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea (312006-3).
REFERENCES
- 1.Keefe DM, Cummins AG, Dale BM, Kotasek D, Robb TA, Sage RE. Effect of high-dose chemotherapy on intestinal permeability in humans. Clin Sci (Lond) 1997;92:385–9. doi: 10.1042/cs0920385. [DOI] [PubMed] [Google Scholar]
- 2.Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- 3.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 4.Zhang N, Yin Y, Xu S J,, Chen WS. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13:1551–69. doi: 10.3390/molecules13081551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sausville EA, Longo DL, Braunwald E, Fauci AS, Kasper DL, Hauser SL, et al. Harrison's Principles of Internal Medicine. New York: The McGraw-Hill Companies; 2001. Principles of cancer treatment. [Google Scholar]
- 6.Gradishar WJ, Vokes EE. 5-Fluorouracil cardiotoxicity: a critical review. Ann Oncol. 1990;1:409–14. doi: 10.1093/oxfordjournals.annonc.a057793. [DOI] [PubMed] [Google Scholar]
- 7.Soares PM, Mota JM, Gomes AS, Oliveira RB, Assreuy AM, Brito GA, et al. Gastrointestinal dysmotility in 5-fluorouracil-induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemother Pharmacol. 2008;63:91–8. doi: 10.1007/s00280-008-0715-9. [DOI] [PubMed] [Google Scholar]
- 8.Kurita A, Kado S, Kaneda N, Onoue M, Hashimoto S, Yokokura T. Modified irinotecan hydrochloride (CPT-11) administration schedule improves induction of delayed-onset diarrhea in rats. Cancer Chemother Pharmacol. 2000;46:211–20. doi: 10.1007/s002800000151. [DOI] [PubMed] [Google Scholar]
- 9.Bellm LA, Epstein JB, Rose-Ped A, Martin P, Fuchs HJ. Patient reports of complications of bone marrow transplantation. Support Care Cancer. 2000;8:33–9. doi: 10.1007/s005209900095. [DOI] [PubMed] [Google Scholar]
- 10.Han X, Wu Z, Di J, Pan Y, Zhang H, Du Y, et al. CXCL9 attenuated chemotherapy-induced intestinal mucositis by inhibiting proliferation and reducing apoptosis. Biomed Pharmacother. 2011;65:547–54. doi: 10.1016/j.biopha.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Huang TY, Chu HC, Lin YL, Ho WH, Hou HS, Chao YC, et al. Minocycline attenuates 5-fluorouracil-induced small intestinal mucositis in mouse model. Biochem Biophys Res Commun. 2009;389:634–9. doi: 10.1016/j.bbrc.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–84. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 13.Chang CT, Ho TY, Lin H, Liang JA, Huang HC, Li CC, et al. 5-Fluorouracil induced intestinal mucositis via nuclear factor-κB activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS One. 2012;7:e31808. doi: 10.1371/journal.pone.0031808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM. Serum levels of NFkappaB and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol Ther. 2008;7:1139–45. doi: 10.4161/cbt.7.7.6207. [DOI] [PubMed] [Google Scholar]
- 15.Oh YC, Cho WK, Jeong YH, Im GY, Lee KJ, Yang HJ, et al. Anti-inflammatory effect of Sosihotang via inhibition of nuclear factor-κB and mitogen-activated protein kinases signaling pathways in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Food Chem Toxicol. 2013;53:343–51. doi: 10.1016/j.fct.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Curra M, Martins MA, Lauxen IS, Pellicioli AC, Sant'Ana Filho M, Pavesi VC, et al. Effect of topical chamomile on immunohistochemical levels of IL-1β and TNF-α in 5-fluorouracil-induced oral mucositis in hamsters. Cancer Chemother Pharmacol. 2013;71:293–9. doi: 10.1007/s00280-012-2013-9. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe S, Suemaru K, Takechi K, Kaji H, Imai K, Araki H. Oral mucosal adhesive films containing royal jelly accelerate recovery from 5-Fluorouracil-induced oral mucositis. J Pharmacol Sci. 2013;121:110–8. doi: 10.1254/jphs.12181fp. [DOI] [PubMed] [Google Scholar]
- 18.Kolgazi M, Uslu U, Yuksel M, Velioglu-Ogunc A, Ercan F, Alican I. The role of cholinergic anti-inflammatory pathway in acetic acid-induced colonic inflammation in the rat. Chem Biol Interact. 2013;205:72–80. doi: 10.1016/j.cbi.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18:479–97. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 20.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–42. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, et al. Phosphorylation of Y398 and Y402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem. 2009;284:1559–69. doi: 10.1074/jbc.M804783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, et al. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem. 2004;279:44785–94. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]