Abstract
Gastric cancer, as well as inflammation, caused by Helicobacter pylori, activates the production of chemokines by activation of redox-sensitive transcription factor NF-κB in gastric epithelial cells. Mitogen-activated protein kinases including extracellular signal-regulated kinase (ERK) and p38 kinase (p38) are activated by Helicobacter pylori, which may regulate NF-κB activation in the infected cells. However the mechanisms how ERK and p38 induce NF-κB activation have not been investigated. Present study aims to investigate the role of ERK and p38 on the activation of NF-κB in Helicobacter pylori-infected AGS cells. Western blot analysis was performed for determining the levels of IκB, p105, p50 and p65 in gastric epithelial cells infected with Helicobacter pylori and treated with ERK inhibitor U0126 and p38 inhibitor SB203580. Helicobacter pylori induced the degradation of IκBα and upregulation of p105, p50 and p65 in the infected cells. U0126 inhibited the degradation of IκBα while SB203580 suppressed expression of p105, p50 and p65 in Helicobacter pylori-infected cells. ERK and p38 differentially activate NF-κB; ERK induces degradation of IκBα while p38 upregulates the expression of p50 and p65, subunits of NF-κB in Helicobacter pylori-infected gastric epithelial AGS cells.
Keywords: ERK, p38, NF-κB, Helicobacter pylori
INTRODUCTION
Helicobacter pylori (H. pylori) has been considered as a major etiologic agent causing chronic gastritis, along with other features, including lymphoid follicles or lymphoid aggregates, surface epithelial degradation with mucous depletion, and intestinal metaplasia.1 One of the potential toxic factors involving H. pylori-induced gastric injury is oxygen radicals, which are released from activated neutrophils since H. pylori exhibits chemotactic activity for neutrophils.2 Thus, neutrophil infiltration of the gastric epithelium was the initial pathological abnormality described in H. pylori gastritis and remains a hallmark of active infection. Chemoattractant cytokine (chemokine) response is particularly important in the early stages of H. pylori-induced inflammation. Chemokines modulate leukocyte adhesion and activate signal transduction cascades, leading to novel gene expression programs. Theses also mediate other leukocyte function necessary for leukocytes to leave the circulation and infiltrate tissues. Thus, increase of chemokine production and release is an important mechanism for leukocyte recruitment in response to injury or infection.
NF-κB is a member of the Rel family including p50 (NF-κB1), p52 (NF-κB2), Rel A (p65), c-Rel, Rel B, and Drosophila morphogen dorsal gene product.3 In resting cells, NF-κB is localized in the cytoplasm as a hetero- or homodimer, which are non-covalently associated with cytoplasmic inhibitory proteins, including IκBα. Upon stimulation by a variety of pathogenic inducers such as viruses, mitogens, bacteria, agents providing oxygen radicals and inflammatory cytokines, the NF-κB complex migrates into the nucleus and binds DNA recognition sites in the regulatory regions of the target genes.4 Previously we found that H. pylori increased lipid peroxidation, an indicative of oxidative damage, and induced the activation of two species of NF-κB dimers (a p50/p65 heterodimer and a p50 homodimer) in gastric epithelial cells.5,6 Pyrrolidine dithiocarbamate (PDTC), a proven free radical scavenger and NF-κB inhibitor, potentially inhibits NF-κB interaction with its upstream regulatory binding site thereby preventing NF-κB-mediated transcriptional activation in H. pylori-infected gastric epithelial cells.7 In addition, p105, the precursor of p50 subunit of NF-κB is processed by an ATP-dependent process that requires proteasomes and ubiquitin conjugation. The C-terminal region of p105 is rapidly degraded, leaving the N-terminal p50 domain.8
Mitogen-activated protein kinases (MAPKs) comprise an important group of serine and threonine signaling kinases that transduce a varity of extracellular stimuli through a cascade of protein phosphorylations, leading to activation of transcription factors. Among MAPK, extracellular signal-regulated kinase (ERK) pathway is linked to cellular proliferation and differentiation as well as proinflammatory cellular response. Previously we showed the activation of ERK and p38 in H. pylori-infected gastric epithelial cells, which is upstream signaling for NF-κB activation in gastric epithelial AGS cells.9 However, ERK and p38 may differentially activate NF-κB in gastric epithelial AGS cells infected with H. pylori-infected .In this study, we examined the role of ERK and p38 on the activation of NF-κB in by H. pylori-infected AGS cells, by determining the levels of IκB, p105, p50 and p65 in gastric epithelial cells infected by H. pylori and treated with ERK inhibitor U0126 and p38 inhibitor SB203580.
MATERIALS AND METHODS
1. H. pylori strain
H. pylori strains NCTC 11637 was obtained from the National Collection of Type Cultures (NCTC; Colindale, United Kingdom). H. pylori was grown on chocolate agar plates (Becton Dickinson Microbiology Systems, Cockeysville, Maryland) for 24 h in a microaerobic and humidified atmosphere at 37°C.
2. Cell culture and H. pylori infection
Human gastric cancer AGS cells (gastric adenocarcinoma, ATCC CRL 1739) were obtained from the American Type Culture Collection (Rockville, Maryland) and grown in RPMI-1640 medium (pH 7.4; Sigma, St. Louis, Missouri) media with 10% fetal bovine serum, 4 mM glutamine (GIBCO-BRL, Grand Island, New York) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). The cells were seeded in 12-well cell culture plates at 105 cells per well in a volume of 1 ml and cultured to reach 80% confluency. Prior to stimulation, each well was washed twice with 1 ml of fresh cell culture medium containing no antibiotics. Bacterial cells were harvested, washed with phosphate buffered saline (PBS), and then resuspended in antibiotic-free cell culture medium. The bacterial cells were added to the cultured cells at a bacterium/cell ratio of 500:1 in a 1 ml volume. A ratio of bacterium/cell was adapted from previous studies.5,6 An ERK inhibitor U0126 (Catalog # 9903, Cell Signaling Technology, Inc., Beverly, MA, USA) and a p38 inhibitor SB203580 (Catalog # 559389, Calbiochem Biochemicals, San diego, CA, USA) were dissolved in dimethylsulfoxide at 50 mM stock solution. The inhibitors, at 20 μM final concentration, were pre-treated to the culture medium before the treatment of H. pylori.
3. Preparation of extracts
Whole cell and nuclear extracts were prepared for respective Western blot analysis. Briefly, the harvested cells were extracted with lysis buffer (10 mM Tris-HCl, pH7.4, 10% Nonidet P-40 and protease inhibitor cocktail) and centrifuged. The supernatants were used for whole cell extracts. To prepare nuclear extracts, the cells were rinsed with ice-cold PBS, harvested by scraping into PBS, and pelleted by centrifugation at 1,500 g for 5 min. The cells were lysed in buffer containing 10 mM HEPES, 10 mM KCl, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 1.5 mM MgCl2, 0.2% Nonidet P-40, 1 mM dithiothreitol (DTT), and 0.5 mM phenylmethylsulfonylfluoride (PMSF). The nuclear pellet was resuspended on ice in nuclear extraction buffer containing 20 mM HEPES, 420 mM NaCl, 0.1 mM EDTA, 1.5 mM MgCl2, 25% glycerol, 1 mM DTT, and 0.5 mM PMSF. Protein concentrations were determined using the Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA).
4. Western blot analysis
100 μg of cellular protein was loaded per lane, separated by 10% SDS-polyacrylamide gel electrophoresis under reducing conditions, and transferred onto nitrocellulose membranes (Amersham Inc., Arlington Heights, Illinois) by electroblotting. The transfer of protein and equality of loading in all lanes was verified using reversible staining with Ponceau S. The membranes were blocked using 5% nonfat dry milk milk in TBS-T (Tris-buffered saline and 0.15% Tween 20) for 3 h at room temperature. The proteins were detected with polyclonal antibodies for IκBα, p105, p50 and p65 at 1:2000 dilution (all from Cell Signaling Technology, Inc., Bevery, Massachusetts) diluted in TBS-T containing 5% dry milk, and incubated at 4°C overnight. After washing in TBS-T, the immunoreactive proteins were visualized using goat anti-rabbit secondary antibodies conjugated to horseradish peroxidase, which was followed by enhanced chemiluminescence (Amersham). Exactly equal amount of protein, determined by Bradford method,10 was loaded in each lane. The Western result presented in each figure is the representative of five separate experiments.
RESULTS
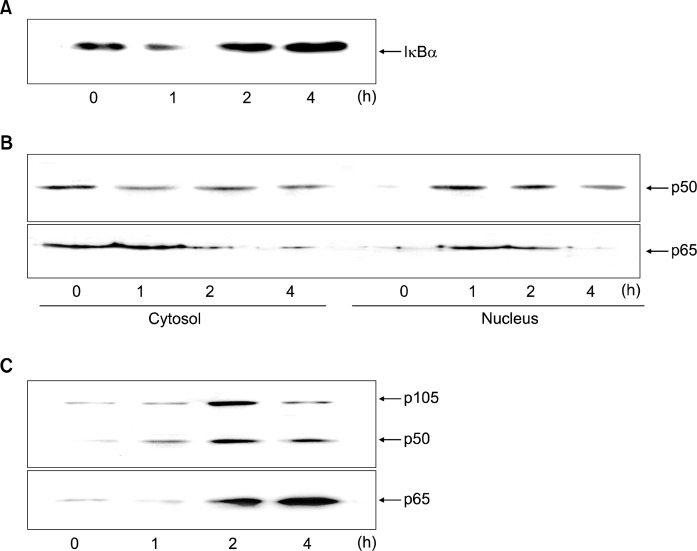
The cells with H. pylori were cultured for 4 h. Fig. 1A shows that H. pylori induced the degradation of IκBα at 1 h (Fig. 1A). To confirm that observed increase in NF-κB gene transactivation paralles increased p50 and p65 nuclear translocation, we determined cytosolic and nuclear levels of p50 and p65 (Fig. 1B). Western blot ananlyses showed significant decrease in p50 and p65 levels in cytosol 1 h and 2 h after H. pylori respectively in the gastric epithelial AGS cells. At the same time, nuclear p50 and p65 levels increased.
Fig. 1.
Protein levels of IκBα, p105, p50 and p65 in H. pylori-infected AGS cells. The cells were seeded in 12-well culture plates at 105 cells per well and cultured to reach 80% confluency. The bacterial cells were added to the cultured cells at a bacterium/cell ratio of 500:1. The cells were cultured for 4 h. The protein levels in whole cell extracts (A, C) and the protein levels in cytosolic extracts and nuclear extracts (B) were shown. Western bot result in each lane is the representative of five separate experiments.
H. pylori-induced upregulation of p105, p50 and p65 was shown at 1 h, which was increased until 4 h (Fig. 1C). Since p105 is a precursor of p50, increased expression of p105 may induce upreguation of p50. These results suggest that NF-κB activation may be induced by upregulation of NF-κB subunits (p50, p65) as well as degradation of IκBα in the infected cells.
The cells were pretreated with the MAP kinase inhibitors, U0126 (an ERK inhibitor) and SB203580 (a p38 inhibitor) at 20 μM final concentration for 1 h, and then infected with H. pylori for 1 h (of IκBα degradation) and 2 h (expression levels of p105, p50, and p65). U0126 inhibited the degradation of IκBα (Fig. 2A) while SB203580 suppressed expression of p105, p50 and p65 in H. pylori-infected cells (Fig. 2B). These results demonstrate involvement of ERK and p38 in the activation of NF-κB differentially in H. pylori-infected AGS cells.
Fig. 2.
Protein levels of IκBα, p105, p50 and p65 in H. pylori-infected AGS cells treated with MAPK inhibitors. AGS cells were seeded in 12-well culture plates at 105 cells per well and cultured to reach 80% confluency. The MAP kinase inhibitors, U0126 (an ERK inhibitor) and SB203580 (a p38 inhibitor) (at 20 μM final concentration), were pretreated to the culture medium 1 h before the treatment of H. pylori. The bacterial cells were added to the cultured cells at a bacterium/cell ratio of 500:1 for 1 h (IκBα, A) and 2 h (p105, p50 and p65, B), respectively. None, AGS cells without treatment and cultured in the absence of H. pylori; Control, AGS cells without treatment and cultured in the presence of H. pylori; U0126, AGS cells treated with U0126 and cultured in the presence of H. pylori; SB203580, AGS cells treated with SB203580 and cultured in the presence of H. pylori.
DISCUSSION
MAPKs are known to be involved in signaling, which leads to the synthesis of pro-inflammatory cytokines and chemokines. The NF-κB element is believed to be the main regulator of inducible expression of inflammatory genes. Expression of IL-8 gene was markedly up-regulated at the levels of mRNA and protein, which was in parallel with the activation of NF-κB and increase in phospho-specific ERK1/2, JNK2/1 and p38 by H. pylori, in AGS cells. We observed that activation of NF-κB was inhibited by treatment with MAPK inhibitors (U0126 as an ERK inhibitir or SB203580 as a p38 inhibitor) in H. pylori-infected AGS cells. The results suggest that induction of cytokines by H. pylori may depend on the activation of MAPK cascade and NF-κB in gastric epithelial cells.
Keates et al.11 reported that direct contact of H. pylori with gastric epithelial cells activates NF-κB in vitro. Both the phosphorylation and proteolytic degradation of IκBα allows the release and nuclear transmigration of NF-κB, which may be induced by oxygen radicals.3,4 We previously demonstrated that lipid peroxidation, an index of oxidative membrane damage, increased by H. pylori in gastric epithelial AGS and Kato III cells, which was in parallel with a time course stimulation of IL-8 production.5,6 More recently Shimoyama et al.12 reported that oxygen radicals are important mediators for chemokine expression in human neutrophils stimulated by H. pylori. Since oxidative stress is an important regulator of chemokine gene expression13 and an inducer of the NF-κB,3,4,14 antioxidants might be beneficial for the treatment of H. pylori-induced gastric mucosal injury and inflammation caused by oxidant-mediated chemokine production.
MAPK family are composed with JNK1/2, ERK and p38 kinase. Several lines of evidence support the role of JNK pathway, which plays a major role in cellular functions, such as cell proliferation and transformation, whereas the ERK pathway was shown to suppress apoptosis and enhance cell survival or tumorigenesis.15
It has been suggested that NF-κB activation may be mediated by two distinct signaling pathways. First, the NF-κB translocation dependent IκBα phosphorylation and degradation.16 Second, phosphorylation of ERK and p38 MAPK leads to NF-κB translocation.17 Although the effect of H. pylori on NF-κB activation was well studied, the modulation of NF-κB activation through the IKK/NIK pathway between the p38 sub-group of MAP kinase has not been reported.
Furthermore, our data suggest that a reduction in ERK activation by U0126 may inhibit IκBα degradation. Although, previous reports indicate that MAP kinase activation may be involved in the activation of NF-κB,18 very little is known about interaction of MEK/ERK pathway with IκBα/NF-κB. Previous study along with the present data support the idea of the potential crosstalk between the ERK and the IκBα/NF-κB signal transduction pathways following H. pylori infection.
In concusion. MAP kinases, such as ERK and p38, may control the activation of NF-κB in H. pylori-infected AGS cells in a different way. Activated ERK induces the dissociaton of IκBα from NF-κB, therefore allowing nuclear translocation and DNA-binding of NF-κB. p38 induces the expression of p65 and p50. Therefore, H. pylori activates transcription factor NF-κB, which is mediated by ERK and p38 in gastric epithelial cells.
Acknowledgments
This study was supported by a 2012 grant from NRF Korea (NRF-2012R1A1A2043423).
REFERENCES
- 1.Dixon MF. Histological responses to Helicobacter pylori infection: gastritis, atropy and preneoplasia. Bailliere Clin Gastroenterol. 1995;9:467–86. doi: 10.1016/0950-3528(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 2.Grisham DY. Pathogenic mechanisms loading to Helicobacter pylori-induced inflammation. Eur J Gastroenterol Hepatol. 1992;4:S9–16. [Google Scholar]
- 3.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–55. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 4.Thanos D, Maniatis T. NF-kappa B: a lesion in family values. Cell. 1995;80:529–32. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Seo JY, Kim KH. Effects of mannitol and dimethylthiourea on Helicobacter pylori-induced IL-8 production in gastric epithelial cells. Pharmacology. 1999;59:201–11. doi: 10.1159/000028321. [DOI] [PubMed] [Google Scholar]
- 6.Kim H, Seo JY, Kim KH. Inhibition of lipid peroxidation, NF-κB activation and IL-8 production by rebamipide in Helicobacter pylori-stimulated gastric epithelial cells. Dig Dis Sci. 2000;45:621–8. doi: 10.1023/a:1005474013988. [DOI] [PubMed] [Google Scholar]
- 7.Lim JW, Kim H, Kim KH. NF-κB, inducible nitric oxide snthase and apoptosis by Helicobacter pylori infection. Free Rad Biol Med. 2001;31:355–66. doi: 10.1016/s0891-5849(01)00592-5. [DOI] [PubMed] [Google Scholar]
- 8.Palombella VJ, Rando OJ, Goldberg AL. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78:773–85. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 9.Seo JH, Lim JW, Kim H, Kim KH. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest. 2004;84:49–62. doi: 10.1038/sj.labinvest.3700010. [DOI] [PubMed] [Google Scholar]
- 10.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Keates S, Hitti YS, Upton M, Kelly CP. Helicobacter pylori infection activates NF-κB in gastric epithelial cells. Gastroenterology. 1997;113:1099–109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- 12.Shimoyama T, Fukuda S, Liu Q, Nakaji S, Fukuda Y, Sugawara K. Production of chemokines and reactive oxygen species by human neutrophils stimulated by Helicobacter pylori. Helicobacter. 2002;7:170–4. doi: 10.1046/j.1523-5378.2002.00077.x. [DOI] [PubMed] [Google Scholar]
- 13.DeForge LE, Preston AM, Takeuchi E, Kenney J, Boxer LA, Remick DG. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993;268:25568–76. [PubMed] [Google Scholar]
- 14.Hsu TC, Young MR, Cmarik J, Colburn NH. Activator protein 1 and nuclear factor κB-depemndent transcription events in carcinogenesis. Free Rad Biol Me. 2000;28:1338–48. doi: 10.1016/s0891-5849(00)00220-3. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, O’Toole PW, Doig P, Trust TJ. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–8. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awasthi YC, Sharma R, Cheng JZ, Yang Y, Sharma A, Singhal SS, et al. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Mol Aspects Med. 2003;24:219–30. doi: 10.1016/s0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- 17.Craig R, Larkin A, Mingo AM, Thuerauf DJ, Andrews C, McDonough PM, et al. p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J Biol Chem. 2000;275:23814–24. doi: 10.1074/jbc.M909695199. [DOI] [PubMed] [Google Scholar]
- 18.Bhat NR, Feinstein DL, Shen Q, Bhat AN. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. J Biol Chem. 2002;277:29584–92. doi: 10.1074/jbc.M204994200. [DOI] [PubMed] [Google Scholar]