Abstract
Background:
Helicobacter pylori infection is associated with diverse upper gastrointestinal diseases, such as peptic and duodenal ulcers as well as gastric cancer. Longstanding period of infection impose great risk of H. pylori-related gastric disease, based on the evidence that early childhood infection is responsible for ensuing atrophic gastritis and gastric cancer related to H. pylori infection. Artemisiahas been known to be beneficial for heath for a long time. In spite of well-acknowledged cytoprotective and anti-inflammatory actions of Artemisia, the effects of the acidic polysaccharide fractions on the gastroprotection remain to be investigated.
Methods:
In the current study, we compared anti-inflammatory actions of the acidic polysaccharide fraction between Artemisia and Panax ginseng against H. pylori infection in vitro. The polysaccharide fractions were pretreated 1 h before H. pylori infection on normal gastric mucosal RGM-1 cells and gastric cancer MKN-28 cells. RT-PCR and Western blot was performed to check anti-inflammatory actions.
Results:
The expressions of inflammatory markers including COX-2, iNOS and IL-8 increased after H. pylori infection, of which levels were significantly decreased when treating with the polysaccharide fractions from Artemisia and ginseng in RGM1 and gastric cancer MKN-28 cells. In addition, the polysaccharide fractions significantly ameliorated H. pylori-induced angiogenic and invasive markers such as HIF-1α and ICAM1. Moreover, H. pylori-induced apoptosis were prevented by pretreatment with the polysaccharide fractions. The polysaccharide fraction from Artemisia showed the most protective effects among the several polysaccharide fractions used in this study.
Conclusions:
The polysaccharide fraction of Artemisia capillariscan is a candidate substance which can attenuate either H. pylori-induced gastritis or tumorigenesis based on potent anti-inflammatory action.
Keywords: Helicobacter pylori, Anti-inflammation, Artemisia capillaries, Polysaccharide, RGM-1
INTRODUCTION
Chronic Helicobacter pylori infection causes gastritis and peptic ulceration, which are based on excess oxidative stress and perpetuated inflammation.1 More than 50% of the world’s population is infected by this bacterium. Though the most are apart from risk, but a portion of patients are associated with peptic ulcer disease and its complication2 and evidence that H. pylori infection is strongly associated with the development of stomach cancer is widely accepted.3 Though the chronic infection by H. pylori generates a state of inflammation, majority of the subjects remain asymptomatic through their life.2 Nonetheless, in a subset of the H. pylori-infected population the gastric inflammation may evolve toward chronic active gastritis, and be implicated in more severe gastric diseases such as chronic atrophic gastritis and intestinal metaplasia, known as a precursor of gastric carcinogenesis, peptic ulcers, mucosa-associated lymphoid tissue lymphoma, and gastric cancer. Irrespective of outcomes, the shared features are the ability of H. pylori to infect and live persistently in the human stomach eliciting a chronic inflammatory response, which may contribute to a role in determining the varied clinical outcomes of infection. In spite of the report that prophylactic eradication of H. pylori after endoscopic resection of early gastric cancer should be used to prevent the development of metachronous gastric carcinoma4 and debates still exist, in general case of gastric cancer, the simple removal of the H. pylori etiological factor did not contribute to cancer prevention, but can attenuate the emergence of precancerous lesion. Therefore, still more information regarding the link between H. pylori infection and gastric cancer according to chronic inflammation is required for advancement of our knowledge in this field.
Extracts of the whole herb of Artemisia had been used in traditional oriental medicine to treat inflammation and to accelerate regeneration as well as food component based on its good flavor. Since an ethanol extract of Artemisia was reported to possess anti-oxidative and anti-inflammatory effects in various experiments and to exhibit cytoprotective effects against experimentally induced gastrointestinal, hepatic and pancreatic damage, their formulated pills come to clinic for the treatment of inflammation based diseases such as gastritis and colitis.5,6 The preclinical facts that the ethanol extracts of Artemisia very effectively ameliorated the severity of trinitrozobenzoic acid (TNBS)-induced colitis through either inhibition of reactive oxygen species generation or down-regulation of pro-inflammatory signaling as well as significant protection from reflux esophagitis6 and various irritants-induced gastric damages.7 The discovery of an acidic polysaccharide fraction from the root of Panax ginseng C.A. Meyer (Araliaceae) which inhibited H. pylori adherence tohost cells was based on hemagglutinating activities.8 Woo and colleagues9 have reported the acidic polysaccharides from Artemisia capillaris inhibited the adhesion of H. pylori to host cells via development of the method that quantitated the inhibition of H. pylori binding to carbohydrate epitopes present on the glycoprotein via conjugating with peroxidase. Since several phytochemicals or drugs have been used in attempts to decrease H. pylori-associated gastric inflammation,10 the acidic polysaccharides from Artemisia capillaris could be one of the candidates to attenuate H. pylori-induced gastric epithelial injury. Therefore, in the present study,we examined the preotective effects of the acidic polysaccharide from A. capillaris by measuring H. pylori-induced pro-inflammatory signaling molecules on rat gastric mucosa RGM-1 cells under the comparison between the polysaccharide from Panax ginseng and green tea.
MATERIALS AND METHODS
1. Reagents
All chemical reagents were obtained from Sigma (St. Louis, MO, USA). The polysaccharide fractions from Artemisia capillaris (MP), Panax ginseng (GP), green tea (GT) and the mixture of GP and MP (MPG6) were provided by S&D Co., Ltd. (Yeongi, Korea). Western blotting detection reagents were obtained from Amersham Biotechnology (Bucks, UK). Primers for RT-PCR were synthesized by Bioneer (Daejeon, Korea). Reverse transcriptase was from Promega (Madison, WI, USA). Antibodies cyclooxygenase-2 (COX-2), cleaved caspase-3, Bcl-2, and PARP were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2. Bacteria culture
H. pylori strain ATCC 43504 (American Type Culture Collection, a cagA+ and vacA s1-m1 type’s strain) for in vitro model and Sydney Strain (SS1, a cagA+, vacA s2-m2 strain) for in vivo model were used in this study. H. pylori were cultured at 37°C in BBL Trypticase Soy (TS) Agar plate with 5% sheep blood (TSA II; BD Biosciences, Franklin Lakes, NJ, USA) under microaerophilic condition (BD GasPaK EZ Gas Generating Systems, BD Biosciences) for 3 days. The bacteria were harvested in clean TS broth, centrifuged at 3000×g for 5 min, and resuspended in broth at a final concentration of 109 colony-forming units (CFUs)/ml. In all experiments, cultures grown for 48 h on TS agar plates were used.
3. Cell culture and cytotoxicity assay
The rat gastric mucosal cells, RGM1, were kindly given by Prof. Hirofumi Matsui (University of Tsukuba, Japan) and human gastric cancer cells, MKN-28 cells were purchased from ATCC (Manassas, VA, USA). Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2 and cultured in Dulbecco’s modified Eagle’s medium containing 10% (v/v) fetal bovine serum and 100 U/ml penicillin. Cell cytotoxicity was measured by MTT [3-(4, 5-dimethy-lthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay.
4. RT-PCR
This assay was performed as previously described. After incubation, media was removed by suction and cells were washed with PBS twice. Trizol (Invitrogen) was added to plates, which were then incubated for 10 min at 4°C. Trizol was harvested and placed in a 1.5 ml tube, and 100 μl chloroform (Merck) was added and gently mixed. After incubation for 10 min in ice, samples were centrifuged at 10,000 g for 30 min. Supernatants were extracted and mixed with 200 μl isopropanol (Merck), and mixtures were incubated at 4°C for 1 h. After centrifuging at 13,000 g for 30 min, pellets were washed with 70% (v/v) ethanol. After allowing the ethanol to evaporate completely, pellets were dissolved in 40μl of DEPC-treated water (Invitrogen). cDNA was prepared using reverse transcriptase originating from Murine-Moloney leukemia virus (Promega), according to the manufacturer’s instructions. PCR was performed over 25 cycles of: 94°C for 20 s, 55°C for 30 s, and 72°C for 45 s. Oligonucleotide primers designed by authors using NCBI/primer-blast. Oligonucleotide primers were purchased from Bioneer (Daejeon, Korea). Oligonu-cleotide primers were as follows; for COX-2, sense 5′-GAA ATG GCT GCA GAG TTG AA-3′, antisense 5′-TCA TCT AGT CTG GAG TGG GA-3′, for iNOS, sense 5′-TTT TCC CAG GCA ACC AGA CG-3′, antisense 5′-GTA GCG GGG CTT CAG AAT GG-3′, for IL-8, sense 5′-CTC AAG ACC TTC AGC TCC AA-3′, antisense 5′-TTC TCA TAG GAG TCC AGG TG-3′and for VEGF, sense 5′-AAG AGA CTT CCA GCC AGT TG-3′, antisense 5′-TGG ATG GTC TTG GTC CTT AG-3′, and for GAPDH.
5. Western blot analysis
This assay was performed as previously described. Briefly, treated cells were washed twice with PBS and then lysed in ice-cold cell lysis buffer (Cell Signaling Technology, MA, USA) containing 1 mM PMSF. After 1 h of incubation, samples were centrifuged at 12,000 g for15 min. Supernatants were then collected. Proteins were separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes, which were incubated with appropriate antibodies and visualized using an enhanced chemiluminescence (ECL) system (GE Healthcare, Bucking-hamshire, UK).
6. Statistical analysis
Results are expressed as the mean±SD. The data were analyzed by one-way ANOVA, and the statistical significance between groups was determined by Duncan’s multiple range test. Statistical significance was accepted at P<0.05.
RESULTS
1. The acidic polysaccharide fractions of Artemisia capillaris, Panax ginseng and green tea attenuated the inflammatory signaling induced by H. pylori infection
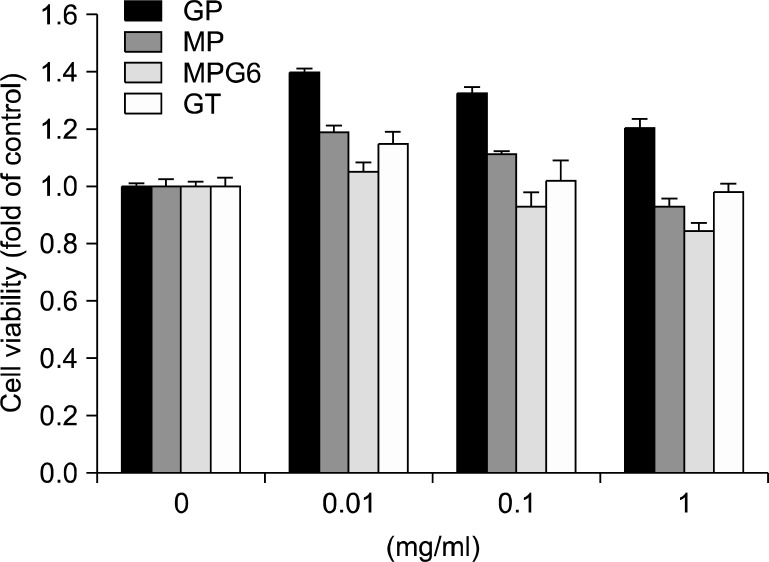
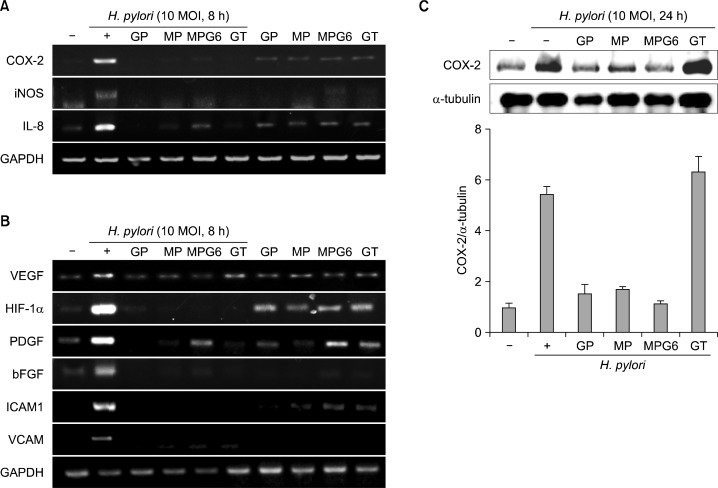
RGM-1 cells cultured with the acidic polysaccharides at the concentrations (0, 0.01, 0.1, 1 mg/ml) for 24 h. As seen in Fig. 1, there was no change in cell viability up to 1 mg/ml concentration, suggesting the polysaccharides from Artemisia capillaris, Panax ginseng and green tea have no cytotoxicity in the gastric mucosal cells. To compare the anti-inflammatory effects of the polysaccharides on H. pylori-induced inflammation in RGM-1 cells, the inflammatory mediators were investigated. H. pylori infection is associated with robust induction of inflammatory mediators including COX-2 and iNOS.1 COX-2 is one of core mediator involved in either H. pylori-associated gastritis or carcinogenesis, by which several drugs or strategy had been tried to prevent various gastrointestinal cancers including H. pylori-associated gastric tumorigenesis using COX inhibitors. iNOS also has been reported to be engaged in either H. pylori-associated gastritis and carcinogensis as evidence that iNOS knock-out mice was resistant to these pathologies of H. pylori infection. IL-8 is another important chemokines strongly associated with pathogenesis of H. pylori infection. Among the inflammatory mediators, IL-8 plays a crucial role in initiating inflammatory response by chemoattracting and activating neutrophils to the H. pylori-infected gastric mucosa.11 As expected, 10 MOI of H. pylori-stimulated RGM-1 cells increased the expression of the inflammation-associated enzymes, iNOS and COX-2, and the representative cytokines IL-8, as determined by RT-PCR and these increases were significantly inhibited by treatment with the GP, MP, MPG6 and GT (Fig. 2A). Since enhanced angiogenic activations in H. pylori infection had been highly implicated in either inflammation perpetuation or gastric carcinogenesis,12 we have extended the elucidation of the changes of the expressions of angiogenic markers. As seen in Fig. 2B, H. pylori infection highly induced the mRNA expressions of angiogenic markers including VEGF, HIF-1α, platelet-derived growth factor (PDGF), one of the growth factors that plays key role in blood vessel formation, basic fibroblast growth factor (bFGF), one of the growth factors implicated in either cell growth or angiogenesis, inter-cellular adhesion molecule-1 (ICAM-1, well-known as CD54) and vascular cell adhesion protein 1 (VCAM1). However, these increases associated with H. pylori infection were all significantly decreased with polysaccharides GP, MP, MPG6 and GT (Fig. 2B). To compare the anti-inflammatory effects of the four polysaccharides, the protein levels of COX-2 were investigated. As shown in Fig. 2C, the polysaccharides from A. capillaries (MP and MPG6) and Panax ginseng (GP) significantly inhibited the expression of COX-2 induced by H. pylori. However, the polysaccharides from green tea had no inhibitory effect on the protein level of COX-2 induced by H. pylori. The reduction of these angiogenic factors with the acidic polysaccharides administration suggested that H. pylori- associated chronic inflammation were associated with increased angiogenesis but the polysaccharides, especially from A. capillaris significantly attenuated these inevitable gastric cell damage provoked by H. pylori infection.
Fig. 1.
The polysaccharides from Artemisia, Panax ginseng and green tea had no cytotoxicity. GP, MP, MPG6 and GT (0.01, 0.1 or 1 mg/ml) were tested for their cytotoxic activity using the MTT colorimetric assay. The data were presented as mean±SD for three different experiments performed in triplicate.
Fig. 2.
Inhibitory effects of the polysaccharides on the expression of inflammatory mediators in H. pylori-infected gastric epithelial cells. RGM-1 cells were pretreated with polysaccharides (0.1 mg/ml) for 1 h and stimulated with H. pylori (10 MOI) for 8 h. Expressions of inflammatory mediator such as COX-2, iNOS and IL-8 (A) and angiogenic markers such as VEGF, HIF-1α, PDGF, bFGF, ICAM1 and VCAM (B) were analyzed by RT-PCR. (C) RGM-1 cells were pretreated the polysaccharides GP, MP, MPG6 and GT (0.1 mg/ml) for 1 hour before H. pylori stimulation (10 MOI, 24 h) then checked the protein levels of COX-2.
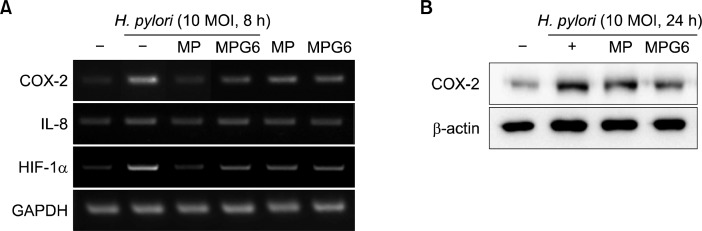
To confirm the findings from H. pylori-treated RGM-1 cells, the gastric cancer cell line MKN-28 was used. As expected, the polysaccharides from A. capillaries, MP and MPG6 significantly inhibited the expression of COX-2, IL-8 and iNOS induced by H. pylori in MKN-28 cells (Fig. 3A and B). The treatment with MPG6, the mixture of MP and GP showed much more inhibitory effect on the COX-2 expression than the treatment with MP only in H. pylori-infected gastric cancer cells.
Fig. 3.
Inhibitory effects of the polysaccharides including A. capillaris on the expression of inflammatory mediatorsin H. pylori-infected gastric cancer cells. MKN-28 cells were pretreated with polysaccharides (0.1 mg/ml) for 1 h and stimulated with H. pylori (10 MOI) for 8 h or 24 h for RT-PCR or Western blotting, respectively. The mRNA expressions of COX-2, IL-8 and HIF-1α (A) and the protein levels of COX-2 (B) were measured.
2. The acidic polysaccharide fractions of Artemisia capillaris restored the apoptosis induced by H. pylori infection
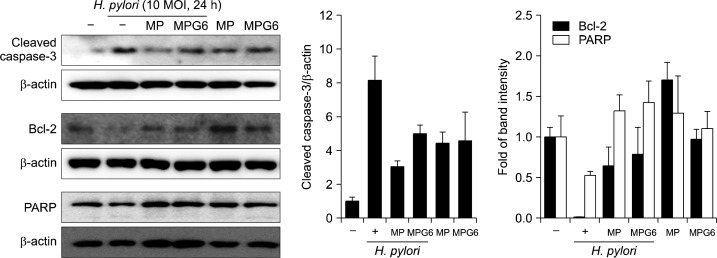
As H. pylori can cause very diverse clinical outcomes, including neoplasms in some individuals, in others atrophy, and in most an unaltered tissue mass, attention has recently been paid to examining the effect of H. pylori on the balance between gastric epithelial cell apoptosis and proliferation. Numerous evidences for the induction of apoptosis by H. pylori has been obtained in various models including cultured gastric epithelial cells in vitro.13 It hasreported the role of the Bcl-2 family in the decision step of H. pylori induced apoptosis as Bcl-2 and its related family members control a key downstream common cell cycle checkpoint, beyond which apoptosis is inevitable.14 Western blot analysis revealed that cleaved caspase-3 was significantly increased andthe expressions of Bcl-2 and PARP cleavage were down-regulated in the H. pylori-infected RGM-1 cells (Fig. 4). However, the expressions of these proteins were restored with the pretreatment of the polysaccharides from Artemisia, MP and MPG6. These results suggest that the polysaccharides from Artemisia imposed the cytoprotective effects against apoptotic insults induced by H. pylori.
Fig. 4.
Protective effects of the polysaccharides including A. capillaris on the apoptosisin H. pylori-infected gastric epithelial cells. The expression of apoptotic markers such as cleaved caspase-3, Bcl-2 and PARP were analyzed by Western blotting. Representative band were shown. Three independent experiments were performed.
DISCUSSION
This study was designed to determine whether dietary consumption of the polysaccharide fractions from Artemisia capillaris, Panax ginseng and green tea can inhibit H. pylori-induced active inflammation and angiogenesis. We found that the acidic polysaccharides from Artemisia significantly attenuated H. pylori-induced gastric inflammation as well as apoptosis based on its potential pharmacological actions of anti-inflammation and cytoprotection. Since the ethanol extracts of Artemisia are available in clinic for the treatment of gastritis and gastric ulcer, we expect the acidic polysaccharide fraction of Artemisiacan also impose the clinical efficacy supported with the anti-inflammatory mechanisms. Before our study, Artemisia extracts had been widely used for the treatment of gynecological disorders, including infertility and dysmenorrhea, which can be commonly caused by endometriosis,15 antimicrobial purpose,16 increasing anti-nociceptive and antipyretic activities,17 improving penile erection,18 and treatment of gastritis, gastric ulcer, pancreatitis, and hepatic fibrosis in either western clinic or oriental clinic as well as folk medicine.
In this study, weput hypothesis that the polysaccharides from Artemisia can be used for ameliorating gastric inflammation caused by H. pylori infection as it efficiently attenuated inflammatory mediator such as COX-2 and iNOS expression. Since effective modulation of inflammation can confer the possibility of cancer prevention in diverse kinds of GI cancers associated with inflammation on their pathogenesis such as chronic atrophic gastritis, chronic reflux esophagitis, cholangitis, pancreatitis, inflammatory bowel disease,19 we extended our hypothesis that long-term administration of the polysaccharides from Artemisia can provide the hope of chemoprevention of H. pylori-associated chronic gastritis. Considering the link between inflammation and carcinogenesis, for instances, the pro-inflammatory enzymes including COX-2 and iNOS have been implicated in carcinogenesis,20 continuous administration of the polysaccharides from Artemisia lead to safe and efficient achievement of prevention of H. pylori-associated gastric tumorigenesis through ameliorating these inflammatory mediators including COX-2. In addition to the anti-inflammatory action of the polysaccharides from Artemisia against H. pylori infection, we have found additional chemopreventive actions of the polysaccharides from Artemisia such as inhibition of angiogenesis with accentuated reduction of VEGF expression since angiogenic growth factors induced by H. pylori may play a critical role in the development and progression of gastric cancer21 and gastric adenocarcinomas frequently showed high levels of VEGF expression.22 According to our previous investigations using cytokine array, basic Fibroblast growth factor (bFGF), intercellular adhesion molecule-1 (ICAM-1, well-known as CD54), Lungkine (CXC-chemokine), Thymus-CK1 (Chemokine ligand 7), TNF-related activation induced cytokine (TRNACE), and TNF-receptor super family cytokine (TROY) levels were all significantly increased with H. pylori infection. The genes including bFGF, CD32, CD54, CXC-chemokine, Chemokine ligand 7, TRNACE, and TROY identified with H. pylori challenge were all the genes reported to be implicated in stomach carcinogenesis and tumor angiogenesis beyond imflammation.23 Real mucosal levels of IL-8, TNF-α, IL-1β, IL-6, and IL-12 expression, all principally implicated genes in either severe gastritis as well as stomach carcinogenesis.24
Furthermore, the treatment with polysaccharides from Artemisia inhibited H. pylori-induced apoptosis, in which another pivotal cancer preventive action of the polysaccharides from Artemisia was through the inhibition of apoptotic signaling in the gastric mucosal cellsvia the inhibition of caspase-3 and PARP cleavage as well as the recovery of anti-apoptotic molecule Bcl-2. In the development of gastric cancer, H. pylori infection induces apoptosis in the mucosa, which further aggravated gastric mucosal damage and perpetuated inflammation.1,2 In this unpleasant environment relevant to H. pylori infection, sodium chloride further cooperates as cancer promoters by enhancing chronic gastric mucosal membrane inflammation and cellular proliferations.25 Apoptosis in response to H. pylori factors may play an important role in the process by which gastric cancer develops in H. pylori-infected humans. The exact mechanisms by which H. pylori-associated apoptosis may predispose to gastric cancer are not yet entirely clear, but enhanced rates of cell loss could potentially accelerate the development of gastric atrophy or intestinal metaplasia.26
H. pylori show a wide spectrum of different specificities in adhesion to host cells, suggesting a multifactorial adherence. The polysaccharide fraction from Artemisia (MP)had reported to show the inhibitory activity with the content of uronic acids, particularly galacturonic acid, reaching approximately 22% in total carbohydrate.9 Large amounts of uronic acids were also detected in the acidic polysaccharide fraction isolated from P. ginseng (GP).27 Thus, we compared the anti-inflammatory activities of MP, GP and the mixture of MP and GP (MPG6) on the damage induced by H. pylori. We observed MPG6 have the strong anti-inflammatory effect on the H. pylori infection. The carbohydrate components of Artemisia and P. ginseng may exhibit the synergistic inhibitory effect on the inflammation, adhesion and angiogenesis induced by H. pylori in host-bacterial interactions. Therefore, the acidic polysaccharides from Artemisia or P. ginseng may be useful dietary substances to control H. pylori-induced gastric disorders.
Acknowledgments
This research was supported by High Value-added Food Technology Development Program, iPET (Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries; 111121-3), Republic of Korea.
REFERENCES
- 1.Hahm KB, Song YJ, Oh TY, Lee JS, Surh YJ, Kim YB, et al. Chemoprevention of Helicobacter pylori-associated gastric carcinogenesis in a mouse model: is it possible? J Biochem Mol Biol. 2003;36:82–94. doi: 10.5483/bmbrep.2003.36.1.082. [DOI] [PubMed] [Google Scholar]
- 2.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 3.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 4.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–7. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 5.Seo HJ, Park KK, Han SS, Chung WY, Son MW, Kim WB, et al. Inhibitory effects of the standardized extract (DA-9601) of Artemisia asiatica Nakai on phorbol ester-induced ornithine decarboxylase activity, papilloma formation, cyclooxygenase-2 expression, inducible nitric oxide synthase expression and nuclear transcription factor kappa B activation in mouse skin. Int J Cancer. 2002;100:456–62. doi: 10.1002/ijc.10489. [DOI] [PubMed] [Google Scholar]
- 6.Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, et al. Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 2001;49:364–71. doi: 10.1136/gut.49.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baek YH, Lee KN, Jun DW, Yoon BC, Kim JM, Oh TY, et al. Augmenting Effect of DA-9601 on Ghrelin in an Acute Gastric Injury Model. Gut Liver. 2011;5:52–6. doi: 10.5009/gnl.2011.5.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belogortseva NI, Yoon JY, Kim KH. Inhibition of Helicobacter pylori hemagglutination by polysaccharide fractions from roots of Panax ginseng. Planta Med. 2000;66:217–20. doi: 10.1055/s-2000-8658. [DOI] [PubMed] [Google Scholar]
- 9.Woo JS H, Kim TG, Lim Y, Kim KH. Inhibition of Helicobacter pylori adhesion by acidic polysaccharide isolated from Artemisia capillaris. J Miocrobiol Biotechnol. 2003;13:853–8. [Google Scholar]
- 10.Qasim A, O’Morain CA. Review article: treatment of Helicobacter pylori infection and factors influencing eradication. Aliment Pharmacol Ther. 2002;16(Suppl 1):24–30. doi: 10.1046/j.1365-2036.2002.0160s1024.x. [DOI] [PubMed] [Google Scholar]
- 11.Crabtree JE. Gastric mucosal inflammatory responses to Helicobacter pylori. Aliment Pharmacol Ther. 1996;10(Suppl 1):29–37. doi: 10.1046/j.1365-2036.1996.22164003.x. [DOI] [PubMed] [Google Scholar]
- 12.Strowski MZ, Cramer T, Schafer G, Juttner S, Walduck A, Schipani E, et al. Helicobacter pylori stimulates host vascular endothelial growth factor-A (vegf-A) gene expression via MEK/ERK-dependent activation of Sp1 and Sp3. FASEB J. 2004;18:218–20. doi: 10.1096/fj.03-0055fje. [DOI] [PubMed] [Google Scholar]
- 13.Shirin H, Moss SF. Helicobacter pylori induced apoptosis. Gut. 1998;43:592–4. doi: 10.1136/gut.43.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–6. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Jung SH, Yang YI, Ahn JH, Cho JG, Lee KT, et al. Artemisia leaf extract induces apoptosis in human endometriotic cells through regulation of the p38 and NFkappaB pathways. J Ethnopharmacol. 2013;145:767–75. doi: 10.1016/j.jep.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Ahameethunisa AR, Hopper W. In vitro antimicrobial activity on clinical microbial strains and antioxidant properties of Artemisia parviflora. Ann Clin Microbiol Antimicrob. 2012;11:30. doi: 10.1186/1476-0711-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habib M, Waheed I. Evaluation of anti-nociceptive, anti-inflammatory and antipyretic activities of Artemisia scoparia hydromethanolic extract. J Ethnopharmacol. 2013;145:18–24. doi: 10.1016/j.jep.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Kim HK, Choi BR, Bak YO, Zhao C, Lee SW, Jeon JH, et al. The role of capillarisin from Artemisia capillaris on penile erection. Phytother Res. 2012;26:800–5. doi: 10.1002/ptr.3635. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Cho JY, Song H, Kim EH, Hahm KB. Revaprazan, a novel acid pump antagonist, exerts anti-inflammatory action against Helicobacter pylori-induced COX-2 expression by inactivating Akt signaling. J Clin Biochem Nutr. 2012;51:77–83. doi: 10.3164/jcbn.11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan S, Epstein JB. Carcinogenesis and cyclooxygenase: the potential role of COX-2 inhibition in upper aerodigestive tract cancer. Oral Oncol. 2003;39:537–46. doi: 10.1016/s1368-8375(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 21.Kitadai Y, Sasaki A, Ito M, Tanaka S, Oue N, Yasui W, et al. Helicobacter pylori infection influences expression of genes related to angiogenesis and invasion in human gastric carcinoma cells. Biochem Biophys Res Commun. 2003;311:809–14. doi: 10.1016/j.bbrc.2003.10.077. [DOI] [PubMed] [Google Scholar]
- 22.Maeda K, Kang SM, Onoda N, Ogawa M, Kato Y, Sawada T, et al. Vascular endothelial growth factor expression in preoperative biopsy specimens correlates with disease recurrence in patients with early gastric carcinoma. Cancer. 1999;86:566–71. doi: 10.1002/(sici)1097-0142(19990815)86:4<566::aid-cncr4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Patel MK, Trombly MI, Kurt-Jones EA. Innate immune responses to Helicobacter pylori infection: an overview. Methods Mol Biol. 2012;921:205–7. doi: 10.1007/978-1-62703-005-2_23. [DOI] [PubMed] [Google Scholar]
- 24.Kundu JK, Surh YJ. Emerging avenues linking inflammation and cancer. Free Radic Biol Med. 2012;52:2013–37. doi: 10.1016/j.freeradbiomed.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 25.Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59:4823–8. [PubMed] [Google Scholar]
- 26.Cover TL, Krishna US, Israel DA, Peek RM., Jr Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63:951–7. [PubMed] [Google Scholar]
- 27.Lee JH, Shim JS, Lee JS, Kim MK, Chung MS, Kim KH. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr Res. 2006;341:1154–63. doi: 10.1016/j.carres.2006.03.032. [DOI] [PubMed] [Google Scholar]