Abstract
Background:
To identify whether first-degree relatives (FDRs) of gastric cancer (GC) patients have increased risk for atrophic gastritis (AG) and intestinal metaplasia (IM) in relation to other risk factors of GC.
Methods:
The study cohort consisted of 224 pairs of age-sex matched controls and FDRs. AG and IM in the gastric mucosa were scored histologically using the updated Sydney classification. Risk of having AG and IM was studied by comparing FDRs to controls. Impacts of age, H. pylori infection, smoking, dietary and socioeconomic factors on the presence of AG and IM were studied.
Results:
In multivariate regression analysis, FDRs had adjusted OR of 2.69 (95% CI 1.06–6.80, P=0.037) for antral IM in male population. Adjusted OR for antral AG and IM were 9.28 (95% CI 4.73–18.18, P<0.001) and 7.81 (95% CI 3.72–16.40, P<0.001) for the H. pylori infected subjects in total population. Getting old by 5 years increased the ORs of having AG and IM by approximately 1.25 fold (P<0.001). Spicy food increased the OR of antral IM by 2.28 fold (95% CI 1.36–3.84, P=0.002).
Conclusions:
Family history of GC was an independent risk factor for antral IM in male in our study, which could be one reason for the increase of gastric cancer in the family member of gastric cancer. It could be an evidence for the necessity of frequent endoscopy in the presence of family history of GC compared to general population in male.
Keywords: Stomach neoplasms, Helicobacter pylori, Atrophic gastritis, Metaplasia
INTRODUCTION
Classically, gastric cancer (GC) is thought to develop because of ongoing mucosal stress by dietary carcinogen. Preserved foods in ways of drying, smoking, and salting, which were all usual methods of preserving food from the ancient time, are abundant in nitrate compounds. These nitrate compounds convert to carcinogenic nitrites in the stomach by gastric bacteria such as H. pylori. Modern development of better preservation and refrigeration contributed to reduce such nitrate rich and ultimately carcinogenic diet, thereby reducing the incidence of GC. In addition, infection of H. pylori, which was classified as a carcinogen in 1994 by the International Agency for Research on Cancer,1 and shown to cause GC in 3% of infected patients compared to none of the uninfected,2 has been decreasing with the effort on eradication treatment. Thanks to fore-mentioned changes on GC dynamics, the incidence of GC is on the gradual decrease. However, despite the global trend of decreasing incidence of GC, it is still a burdensome disease, ranking third place as a cause of cancer-related mortality worldwide.3 Moreover GC is the 2nd most prevalent cancer in Korea marking as the most and the 4th most prevalent cancer in male and female respectively. GC incidence rate was 68.4 and 64.2 cases per 100,000 person-year in 1999 and 2010 respectively according to Korea national cancer information center, showing a minimal decrease during those 10 years. To prevent GC from developing and progressing, there should be multiple strategies. For that, looking into the carcinogenesis and risk factors of GC is of utmost importance.
There are two types of GC, which include diffuse type and intestinal type, classified according to Lauren’s classification system. In intestinal type, GC is thought to develop through a sequential cascade, in which mucosal inflammation develops into atrophic gastritis (AG), intestinal metaplasia (IM), dysplasia and finally into gastric cancer, postulated by Correa early in time.4 This type of GC often develops in relation to H. pylori and H. pylori infected individuals with IM has GC risk increased by 6.5 fold.2 Therefore, there have been efforts to elucidate whether H. pylori eradication can reverse or stop the cascade. Until recently, studies showed regression of atrophy but no regression of IM after H. pylori-eradication.5–10 However, H. pylori eradicated group had less progression of IM compared to H. pylori not eradicated group.11 Recently, we demonstrated that atrophy regressed in the body and even severe cases of IM showed improvement after H. pylori eradication.12 The important point is that in all of the studies mentioned, there were no regression of IM in the eradicated individuals, implicating that IM is considered to be the “point of no return”, even though H. pylori eradication can help to slow down the carcinogenic process. In secondary prevention of GC, it is important to screen individuals with high risk of developing GC and let them be checked for premalignant and malignant lesions more frequently. Risk factors of developing premalignant lesions such as AG and IM are thought to be identical to those of GC. Among the risk factors, family history of GC13 and H. pylori infection are most important. Thus, recent studies showed that the first degree relatives (FDRs) of GC and the H. pylori-infected individuals have increased risk of IM.14,15 However, most of these studies have not been stratified according to age and sex. Since age and sex are both independent risk factors of GC, it is necessary to match the population by age and sex to get a thorough comparison by reducing confounding effects of age and sex. Therefore, this study was aimed to find out how much family history of GC as well as sex and age factors contribute to the incidence of AG and IM through age-sex matched population study. Furthermore, this study looked into the relevance of H. pylori infection and environmental factors to AG and IM.
MATERIALS AND METHODS
1. Subjects
This study is a case-control study, utilizing the data which had been collected prospectively for previous studies.12,16 Collected data and medical records of healthy subjects who visited Seoul National University Bundang Hospital during the period of 2003 to 2012 were analyzed. Those who were confirmed, in endoscopic exam, not to have any evidence of GC, dysplasia, mucosa-associated lymphoid tissue lymphoma, esophageal cancer, or peptic ulcer disease at the time of visit were screened (n=564). Among them, 244 were first degree relatives (siblings, children or parents) of GC patients, and 320 were controls without such family history of GC. FDRs came to our clinic seeking counseling for their family history of GC, while controls came for routine health check-up. For the FDRs, controls were matched for age and sex. For age matching, controls within±2 years of age difference were selected. In this process, some FDRs and controls were inevitably discarded. The selection process was random. Finally, 68 male and 156 female pairs for both FDRs and controls were matched. Afterward, the selected males were matched with females by age in the same fashion making 67 male and female double pairs of subjects for FDRs and controls.
All subjects had already provided detailed information on their family history of GC and answered to a questionnaire under the supervision of a well-trained interviewer. The questionnaire included questions regarding demographic (age, sex, and residency during childhood), socioeconomic (smoking, current income and school education), and dietary (salty and spicy food diet) data. The study protocol was approved by the Ethics Committee at Seoul National University Bundang Hospital.
2. Histological evaluation
Via gastric endoscopy, 10 biopsy specimens were obtained. Two biopsy specimens were taken from the greater curvature of both the mid antrum and mid body of the stomach, and three from both the lesser curvature of the antrum and body. Among the 10 specimens, one from the antrum and one from the body were fixed in formalin, stained with hematoxylin and eosin, and used for histological evaluation. They were assessed for the degree of inflammatory cell infiltration, AG and IM. The histological features of the gastric mucosa were recorded using the updated Sydney scoring system (0=none, 1=slight, 2=moderate, and 3=marked). When the specimens were not prepared well enough to evaluate full-thickness gastric mucosa due to problems such as improper fixation, inaccurate orientation, and section inappropriateness, or whenever inflammation prevented a clear distinction between nonatrophic and atrophic phenotypes, samples were classified as “indefinite for atrophy”.17 All biopsies were examined independently by two experienced pathologists, who were unaware of the clinical history. In the event of disagreement, the biopsies were re-examined by these two pathologists until agreement was reached.
3. Helicobacter pylori testing
Among the above-mentioned 10 biopsy specimens, each from antrum and body was fixed in formalin, stained with modified Giemsa method, and assessed for the presence of H. pylori. Another set from antrum and body was examined with rapid urease testing (CLO test, Delta West, Bentley, Australia). Third set was cultured. The antral and corporal biopsy specimens were evaluated separately, and the cultured organisms were confirmed as H. pylori if they were compatible to H. pylori in Gram staining, colony morphology, and oxidase, catalase, and urease reactions.
Anti-H. pylori immunoglobulin G was determined qualitatively using an enzyme-linked immunosorbent (ELISA) assay (Genedia H. pylori ELISA; Green Cross Medical Science Corp, Seoul, Korea), when the three above-mentioned H. pylori tests were negative. If the H. pylori serology was positive but no bacteria were found on the histology, CLO test or culture, the diagnosis was past H. pylori infection without current ongoing infection, and the cases were also classified as H. pylori-positive.
4. Statistical analysis
Data were analyzed using the Pearson χ2 test, univariate and multivariate logistic regression models, and student t-test.
The occurrence of AG and IM was analyzed in several ways, using Pearson χ2 test. First, the histological grade of AG according to updated Sydney scoring system (0=none, 1=slight, 2=moderate, and 3=marked) and the histological presence of AG (absent, present) were studied. Second, histological grade of AG was studied in subgroups, in which IM was present or absent respectively. Third, the histological presence and grade of IM were studied. Above analyses compared FDRs to controls in total (224 pairs of FDR and control subjects), male (67 pairs) and female (156 pairs) populations. Analyses comparing males to females in FDRs (67 pairs of male and female subjects) and controls (67 pairs) were followed.
The effects of family history of GC, sex, H. pylori, spicy and salty diet, smoking, residency during childhood, income, education on AG and IM were analyzed with Pearson χ2 test. In analysis of aging factor, univariate logistic regression model was used. Variables with P<0.2 were subjected to multivariate logistic regression analyses. Although sex and FDR factors didn’t show P<0.2 in every analysis, they were entered to the model since they were considered to be important in this study. Model fits were assessed using Nagelkerke’s R2 test and Hosmer-Lemeshow goodness-of-fit tests. Mean age and mean values of histological grade of AG and IM in each group were analyzed using student t-test. Differences were considered significant when the P-values were <0.05. All analyses were performed using the statistical software package SPSS (version 18.0, SPSS inc., Chicago, IL, USA).
RESULTS
1. Baseline characteristics of study subjects
Table 1 shows baseline characteristics of study population. There were missing values less than 10% owing to non-answer to questionnaire. For the total 224 pairs, including both male and female subjects, mean age was about 51. The two groups did not have significant differences in residency during childhood, smoking history, final education, and H. pylori infection status. Regarding to H. pylori, almost 70% of subjects were shown to be infected. In dietary habits, the two groups had significant differences. Not surprisingly, FDRs showed preference to spicy and salty food diet. FDRs eat severely spicy food and severely salty food more by 2.6 fold and 1.5 fold, respectively, compared to controls.
Table 1.
Sequence of primers used for RT-PCR
| No. of subjects (%)
|
P-value | |||
|---|---|---|---|---|
| Control (n=224) | FDR (n=224) | |||
| Sex | Male | 68 (30.4) | 68 (30.4) | 1 |
| Female | 156 (69.6) | 156 (69.6) | ||
| Age (mean±SD) | Total | 51.67±10.39 | 51.56±10.56 | 0.91 |
| Male | 49.54±10.62 | 49.19±11.03 | 0.85 | |
| Female | 52.59±10.19 | 52.59±10.21 | 1 | |
| H. pylori | Negative | 69 (30.8) | 61 (27.2) | 0.405 |
| Positive | 155 (69.2) | 163 (72.8) | ||
| Spicy diet | Low/moderate | 131 (60.1) | 78 (36.6) | <0.001 (OR 2.61) |
| Severe | 87 (39.9) | 135 (63.4) | ||
| Salty diet | Low/moderate | 79 (36.1) | 58 (27.1) | 0.045 (OR 1.52) |
| Severe | 140 (63.9) | 156 (72.9) | ||
| Smoking | Current/ex-smoker | 58 (26.9) | 61 (28.9) | 0.635 |
| Non-smoker | 158 (73.1) | 150 (71.1) | ||
| Residency during childhood | Urban | 127 (58.8) | 110 (51.4) | 0.123 |
| Rural | 89 (41.2) | 104 (48.6) | ||
| Current income (US$/month) | <5000 | 130 (62.2) | 138 (67.6) | 0.246 |
| ≥5000 | 79 (37.8) | 66 (32.4) | ||
| Education | Elementary | 16 (7.3) | 16 (7.8) | 0.869 |
| Middle-high | 100 (45.5) | 88 (42.9) | ||
| University | 104 (47.3) | 101 (49.3) | ||
FDR, first degree relatives of gastric cancer.
P-values were calculated using χ2-test except mean age. In mean age, P-values were calculated with student t-test. Missing values were due to non-answer to questionnaire.
2. Association of atrophic gastritis and intestinal metaplasia with family history of gastric cancer and sex
After excluding cases indefinite for atrophy (70 in antrum and 61 in body), there were 32.8% (147 of 448) and 20.7% (93 of 448) of AG in antrum and body, respectively. There were 27.5% (123 of 448) and 18.5% (83 of 448) of IM in antrum and body, respectively.
In antrum, there was a tendency that FDRs have AG and IM more frequently (Table 2). In male group analysis, FDRs had more cases of AG by 2.4 fold (P=0.034) and IM by 2.71 fold (P=0.012) compared to controls. In total population, FDRs had more cases of IM by 1.68 fold compared to controls (P=0.015). In terms of severity, it was not evident whether FDRs had severer cases of AG and IM owing to small number of severe cases observed. Some of the results, however, reached statistical significance. In male group analysis, FDRs seemed to have severer cases of both AG and IM with P-value of 0.009 and 0.043 respectively. In total population, FDRs had severer cases of IM with P-value of 0.032. Comparison was done between sex in FDR and control populations respectively (data not shown). In controls, males had fewer cases of AG and IM. In FDRs, males had more cases of AG and IM. In terms of severity, males were shown to have severer cases of metaplastic AG and IM with P-value of 0.066 and 0.055, respectively.
Table 2.
Atrophic gastritis and intestinal metaplasia in the antrum
| No. of subjects (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Total population | Male | Female | |||||||||
|
| |||||||||||
| Control (n=224) | FDR (n=224) | P-value (OR) | Control (n=68) | FDR (n=68) | P-value (OR) | Control (n=156) | FDR (n=156) | P-value (OR) | |||
| Atrophic gastritis | IAa | 34 (15.2) | 36 (16.1) | 15 (22.1) | 11 (16.2) | 19 (12.2) | 25 (16.0) | ||||
| Absent | 123 (54.9) | 108 (48.2) | 0.50* | 37 (54.4) | 28 (41.2) | 0.009* | 86 (55.1) | 80 (51.3) | 0.87* | ||
| Present | 67 (29.9) | 80 (35.7) | 0.15** (1.36) | 16 (23.5) | 29 (42.6) | 0.034** (2.40) | 51 (32.7) | 51 (32.7) | 0.77** (1.08) | ||
| Metaplasticb | None | 13 (5.8) | 16 (7.1) | 0.82† | 3 (4.4) | 6 (8.8) | 0.039† | 10 (6.4) | 10 (6.4) | 0.97† | |
| Slight | 19 (8.5) | 31 (13.8) | 3 (4.4) | 15 (22.1) | 16 (10.3) | 16 (10.3) | |||||
| Moderate | 8 (3.6) | 8 (3.6) | 2 (2.9) | 0 (0) | 6 (3.8) | 8 (5.1) | |||||
| Marked | 2 (0.9) | 2 (0.9) | 0 (0) | 0 (0) | 2 (1.3) | 2 (1.3) | |||||
| Non-Metaplasticc | None | 110 (49.1) | 92 (41.1) | 0.75†† | 34 (50.0) | 22 (32.4) | 0.26†† | 76 (48.7) | 70 (44.9) | 0.80†† | |
| Slight | 35 (15.6) | 36 (16.1) | 9 (13.2) | 13 (19.1) | 26 (16.7) | 23 (14.7) | |||||
| Moderate | 3 (1.3) | 3 (1.3) | 2 (2.9) | 1 (1.5) | 1 (0.6) | 2 (1.3) | |||||
| Marked | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Intestinal metaplasia | Absent | 174 (77.7) | 151 (67.4) | 0.032‡ | 56 (82.4) | 43 (63.2) | 0.043‡ | 118 (75.6) | 108 (69.2) | 0.22‡ | |
| Present | Total | 50 (22.3) | 73 (32.6) | 0.015‡‡ (1.68) | 12 (17.6) | 25 (36.8) | 0.012‡‡ (2.71) | 38 (24.4) | 48 (30.8) | 0.21‡‡ (1.38) | |
| Slight | 22 (9.8) | 43 (19.2) | 6 (8.8) | 14 (20.6) | 16 (10.3) | 29 (18.6) | |||||
| Moderate | 25 (11.1) | 28 (12.5) | 5 (7.4) | 11 (16.2) | 20 (12.8) | 17 (10.9) | |||||
| Marked | 3 (1.33) | 2 (0.9) | 1 (1.5) | 0 (0) | 2 (1.28) | 2 (1.3) | |||||
FDR, first degree relatives of gastric cancer.
Indefinite for atrophy.
Atrophic gastritis with intestinal metaplasia present.
Atrophic gastritis without intestinal metaplasia present P-values were calculated using χ2-test.
Histological grade is according to updated Sydney scoring system (0=none, 1=slight, 2=moderate, and 3=marked).
Compared histological grade of atrophic gastritis regardless of presence of intestinal metaplasia;
compared histological presence of atrophic gastritis regardless of presence of intestinal metaplasia;
compared histological grade of metaplastic atrophic gastritis;
compared histological grade of non-metaplastic atrophic gastritis;
compared histological grade of intestinal metaplasia;
compared histological presence of intestinal metaplasia.
Analysis of body was done in the same manner (Table 3). However, the results were neither consistent nor statistically significant.
Table 3.
Atrophic gastritis and intestinal metaplasia in the body
| No. of subjects (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Total population | Male | Female | |||||||||
|
| |||||||||||
| Control (n=224) | FDR (n=224) | P-value (OR) | Control (n=68) | FDR (n=68) | P-value (OR) | Control (n=156) | FDR (n=156) | P-value (OR) | |||
| Atrophic gastritis | IAa | 27 (12.1) | 34 (15.2) | 6 (8.8) | 14 (20.6) | 21 (13.5) | 20 (12.8) | ||||
| Absent | 148 (66.1) | 146 (65.2) | 0.61* | 51 (75) | 43 (63.2) | 0.94* | 97 (62.2) | 103 (66.0) | 0.49* | ||
| Present | 49 (21.9) | 44 (19.6) | 0.69** (0.91) | 11 (16.2) | 11 (16.2) | 0.47** (1.19) | 38 (24.4) | 33 (21.2) | 0.47** (0.82) | ||
| Metaplasticb | None | 11 (4.9) | 8 (3.6) | 0.40† | 4 (5.9) | 2 (2.9) | 0.54† | 7 (4.5) | 6 (3.85) | 0.57† | |
| Slight | 13 (5.8) | 18 (8.0) | 4 (5.9) | 6 (8.8) | 9 (5.8) | 12 (7.7) | |||||
| Moderate | 7 (3.1) | 11 (4.9) | 2 (2.9) | 3 (4.4) | 5 (3.2) | 8 (5.1) | |||||
| Marked | 3 (1.3) | 1 (0.45) | 0 (0) | 0 (0) | 3 (1.9) | 1 (0.6) | |||||
| Non-Metaplasticc | None | 137 (61.2) | 138 (61.6) | 0.19†† | 47 (69.1) | 41 (60.3) | 0.54†† | 90 (57.7) | 97 (62.2) | 0.22†† | |
| Slight | 23 (10.3) | 12 (5.4) | 4 (5.9) | 2 (2.9) | 19 (12.2) | 10 (6.4) | |||||
| Moderate | 3 (1.3) | 2 (0.9) | 1 (1.5) | 0 (0) | 2 (1.3) | 2 (1.3) | |||||
| Marked | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Intestinal metaplasia | Absent | 186 (83.0) | 179 (79.9) | 0.36‡ | 57 (83.8) | 54 (79.4) | 0.66‡ | 129 (82.7) | 125 (80.1) | 0.14‡ | |
| Present | Total | 38 (17.0) | 45 (20.1) | 0.40‡‡ (1.23) | 11 (16.2) | 14 (20.6) | 0.51‡‡ (1.34) | 27 (17.3) | 31 (19.9) | 0.56‡‡ (1.19) | |
| Slight | 22 (9.8) | 29 (12.9) | 8 (11.8) | 8 (11.8) | 14 (9.0) | 21 (13.5) | |||||
| Moderate | 12 (5.3) | 15 (6.7) | 3 (4.4) | 5 (7.4) | 9 (5.8) | 10 (6.4) | |||||
| Marked | 4 (1.8) | 1 (0.4) | 0 (0) | 1 (1.5) | 4 (2.6) | 0 (0) | |||||
P-values were calculated using χ2-test.
Histological grade is according to updated Sydney scoring system (0=none, 1=slight, 2=moderate, and 3=marked).
FDR, first degree relatives of gastric cancer.
Indefinite for atrophy.
Atrophic gastritis with intestinal metaplasia present.
Atrophic gastritis without intestinal metaplasia present
Compared histological grade of atrophic gastritis regardless of presence of intestinal metaplasia;
compared histological presence of atrophic gastritis regardless of presence of intestinal metaplasia;
compared histological grade of metaplastic atrophic gastritis;
compared histological grade of non-metaplastic atrophic gastritis;
compared histological grade of intestinal metaplasia;
compared histological presence of intestinal metaplasia.
3. Association of H. pylori infection with the presence of atrophic gastritis and intestinal metaplasia
To evaluate the impact of H. pylori infection on development of AG and IM, the total population is sorted into H. pylori negative and positive groups irrespective of age and sex. Since there was no significant difference in H. pylori infection between FDRs and controls in the initial analysis of demographics, the population can be conceived to be virtually H. pylori and FDR matched. The presence of AG and IM was counted in these sets of new groups (Table 4). In this analysis, the results were prominent to show that AG and IM happen more in H. pylori infected subjects. Odds ratios of developing AG and IM in antrum were 8.74 and 5.96 (P<0.001), and in body 3.75 and 2.86 (P<0.001), respectively.
Table 4.
H. pylori, smoking, dietary and socioeconomic factors in relation to the presence of atrophic gastritis and intestinal metaplasia
| No. of patients (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| H. pylori | Smoking | Spicy diet | Salty diet | |||||||||||
|
| ||||||||||||||
| Negative (n=131) | Positive (n=317) | P-value (OR) | Non-smoker (n=308) | Current/ex-smoker (n=119) | P-value (OR) | Low/moderate (n=209) | Severe (n=222) | P-value (OR) | Low/moderate (n=137) | Severe (n=296) | P-value (OR) | |||
| Antrum | Atrophic gastritis | Absent | 101 (89.4) | 130 (49.1) | <0.001 | 149 (58.0) | 64 (63.4) | 0.35 | 111 (66.5) | 108 (55.1) | 0.027 | 76 (67.9) | 144 (57.1) | 0.054 |
| Present | 12 (10.6) | 135 (50.9) | (8.74) | 108 (42.0) | 37 (36.6) | (0.80) | 56 (33.5) | 88 (44.9) | (1.62) | 36 (32.1) | 108 (42.9) | (1.58) | ||
| Intestinal metaplasia | Absent | 119 (90.8) | 246 (77.6) | <0.001 | 225 (73.1) | 85 (71.4) | 0.74 | 168 (80.4) | 145 (65.3) | < 0.001 | 107 (78.1) | 206 (69.6) | 0.066 | |
| Present | 12 (9.2) | 71 (22.4) | (5.96) | 83 (26.9) | 34 (28.6) | (1.08) | 41 (19.6) | 77 (34.7) | (2.18) | 30 (21.9) | 90 (30.4) | (1.56) | ||
| Body | Atrophic gastritis | Absent | 105 (89.7) | 189 (70.0) | <0.001 | 193 (72.3) | 87 (85.3) | 0.009 | 135 (77.6) | 147 (73.9) | 0.40 | 94 (78.3) | 189 (74.1) | 0.38 |
| Present | 12 (10.3) | 81 (30.0) | (3.75) | 74 (27.7) | 15 (14.7) | (0.45) | 39 (22.4) | 52 (26.1) | (1.22) | 26 (21.7) | 66 (25.9) | (1.26) | ||
| Intestinal metaplasia | Absent | 120 (91.6) | 205 (64.7) | <0.001 | 251 (81.5) | 97 (81.5) | 1.00 | 174 (83.3) | 175 (78.8) | 0.24 | 110 (80.3) | 240 (81.1) | 0.85 | |
| Present | 11 (8.4) | 112 (35.3) | (2.86) | 57 (18.5) | 22 (18.5) | (1) | 35 (16.7) | 47 (21.2) | (1.34) | 27 (19.7) | 56 (18.9) | (0.95) | ||
| No. of patients (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Residency during childhood | Current income (US$/month) | Education | ||||||||||
|
| ||||||||||||
| Urban (n=237) | Rural (n=193) | P-value (OR) | ≥ 5000 (n=145) | <5000 (n=268) | P-value (OR) | Elementary (n=32) | Middle-High (n=188) | University (n=205) | P-value | |||
| Antrum | Atrophic gastritis | Absent | 127 (65.1) | 90 (53.9) | 0.030 (1.60) | 79 (65.8) | 137 (59.6) | 0.25 (1.31) | 17 (58.6) | 92 (56.1) | 108 (65.5) | 0.216 |
| Present | 68 (34.9) | 77 (46.1) | 41 (34.2) | 93 (40.4) | 12 (41.4) | 72 (43.9) | 57 (34.5) | |||||
| Intestinal metaplasia | Absent | 176 (74.3) | 136 (70.5) | 0.38 (1.21) | 107 (73.8) | 194 (72.4) | 0.76 (1.07) | 19 (59.4) | 134 (71.3) | 154 (75.1) | 0.167 | |
| Present | 61 (25.7) | 57 (29.5) | 38 (26.2) | 74 (27.6) | 13 (40.6) | 54 (28.7) | 51 (24.9) | |||||
| Body | Atrophic gastritis | Absent | 157 (78.5) | 125 (72.7) | 0.19 (1.37) | 100 (80.6) | 173 (73.3) | 0.122 (1.52) | 19 (67.9) | 119 (71.3) | 139 (79.4) | 0.148 |
| Present | 43 (21.5) | 47 (27.3) | 24 (19.4) | 63 (26.7) | 9 (32.1) | 48 (28.7) | 36 (20.6) | |||||
| Intestinal metaplasia | Absent | 199 (84.0) | 150 (77.7) | 0.099 (1.50) | 113 (77.9) | 222 (82.8) | 0.22 (0.73) | 26 (81.3) | 145 (77.1) | 172 (83.9) | 0.235 | |
| Present | 38 (16.0) | 43 (22.3) | 32 (22.1) | 46 (17.2) | 6 (18.8) | 43 (22.9) | 33 (16.1) | |||||
P-values were calculated using χ2-test. Missing values were due to cases “indefinite for atrophy” and non-answer to questionnaire.
4. Association of other environmental factors with the presence of atrophic gastritis and intestinal metaplasia
To evaluate the association of other environmental factors with the presence of AG and IM, the total population is sorted in the same manner used in the analysis of H. pylori infection with the presence of AG and IM (Table 4). Smoking, which is a known risk factor of gastric cancer,18 presented rather a contradicting result. History of smoking reduced the OR of having corporal AG by 0.45 fold (P=0.009). Severely spicy food diet showed significant association with antral AG and IM with OR (P-value) of 1.62 (0.027) and 2.18 (<0.001) respectively. Severely salty food diet failed to reach statistical significance. Childhood residency in rural area is positively associated with presence of antral AG with statistical significance OR of 1.6 (P=0.03). There were no statistically significant association of current income and education with AG and IM.
5. Presence of atrophic gastritis and intestinal metaplasia according to age
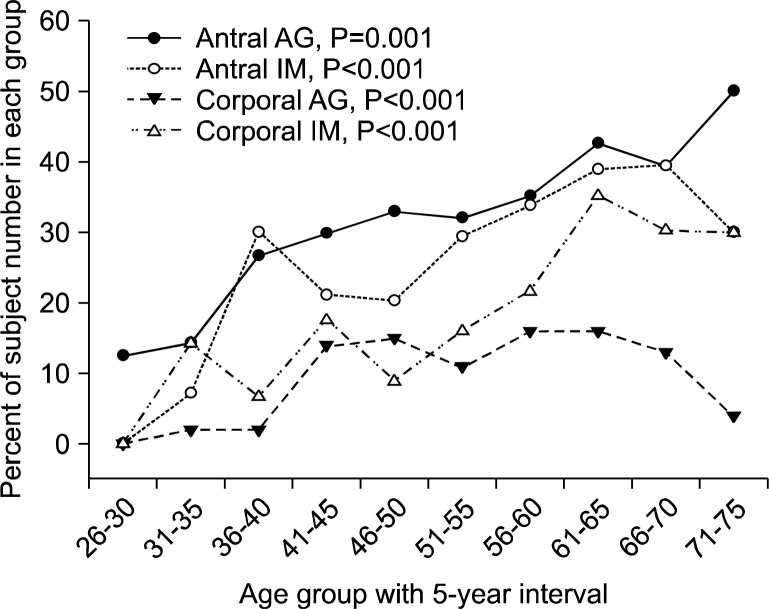
The total population is put into age groups of 5-year interval from 26–30 to 71–75. The presence of AG and IM is counted in each group. Univariate logistic regression analysis was done according to the age groups with group 26–30 set as the reference. The distribution of AG and IM increases with age (Table 5, Fig. 1). The odds ratio was about 1.2 per 5-year (P≤0.001). Moreover, AG and IM showed more than 30% prevalence in ages above 61 years.
Table 5.
Age distribution of atrophic gastritis and intestinal metaplasia in the total population
| Age | n | No. of subjects (%) | |||
|---|---|---|---|---|---|
|
| |||||
| Antrum | Body | ||||
|
| |||||
| Atrophic gastritis | Intestinal metaplasia | Atrophic gastritis | Intestinal metaplasia | ||
| 26–30 | 8 | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 31–35 | 28 | 4 (14.3) | 2 (7.1) | 2 (7.1) | 4 (14.3) |
| 36–40 | 30 | 8 (26.7) | 9 (30.0) | 2 (6.7) | 2 (6.7) |
| 41–45 | 57 | 17 (29.8) | 12 (21.1) | 14 (24.6) | 10 (17.5) |
| 46–50 | 79 | 26 (32.9) | 16 (20.3) | 15 (19.0) | 7 (8.9) |
| 51–55 | 75 | 24 (32.0) | 22 (29.3) | 11 (14.7) | 12 (16.0) |
| 56–60 | 74 | 26 (35.1) | 25 (33.8) | 16 (21.6) | 16 (21.6) |
| 61–65 | 54 | 23 (42.6) | 21 (38.9) | 16 (29.6) | 19 (35.2) |
| 66–70 | 33 | 13 (39.4) | 13 (39.4) | 13 (39.4) | 10 (30.3) |
| 71–75 | 10 | 5 (50.0) | 3 (30.0) | 4 (40.0) | 3 (30.0) |
| Total | 448 | 147 | 93 | 123 | 83 |
| P-value | 0.001 | <0.001 | <0.001 | <0.001 | |
| OR (95% CI) | 1.19 (1.07–1.31) | 1.22 (1.10–1.36) | 1.28 (1.13–1.44) | 1.28 (1.13–1.45) | |
P-values were calculated using univariate logistic regression analysis.
Fig. 1.
Age distribution of atrophic gastritis and intestinal metaplasia; AG, atrophic gastritis; IM, intestinal metaplasia.
6. Multivariate logistic regression analysis of risk factors of atrophic gastiritis and intestinal metaplasia
Although FDR and sex didn’t show P<0.2 in every analysis, they were entered into the model since they were considered to be important in this study. In both AG and IM, age and H. pylori infection had considerable impacts. In antral IM, spicy food diet showed significant association. FDR did not show significant association with AG and IM (Table 6). However, considering that FDRs showed stronger association with both antral AG and IM in male population, multivariate regression analysis in male sub-group was executed (Table 7). There was a significant association with OR of 2.69 (P=0.037) between FDRs with antral IM.
Table 6.
Multivariate logistic regression analysis of risk factors of atrophic gastiritis and intestinal metaplasia in total population
| Variable | Antral AG | Antral IM | Corporal AG | Corporal IM | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Adjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
| Aging by 5-year | 1.25 (1.11–1.42) | <0.001 | 1.25 (1.10–1.42) | 0.001 | 1.25 (1.09–1.44) | 0.002 | 1.26(1.11–1.44) | 0.001 |
| FDR | 1.22 (0.75–2.00) | 0.42 | 1.50 (0.93–2.42) | 0.097 | 1.01 (0.60–1.71) | 0.96 | 1.31 (0.79–2.16) | 0.30 |
| H. pylori infected | 9.28 (4.73–18.18) | <0.001 | 7.81 (3.72–16.40) | <0.001 | 4.46 (2.16–9.22) | <0.001 | 3.00 (1.49–5.89) | 0.002 |
| Male | 1.22 (0.72–2.07) | 0.47 | 1.04 (0.62–1.74) | 0.87 | 1.31 (0.59–2.91) | 0.51 | 1.07 (0.62–1.86) | 0.80 |
| Severely spicy diet | 1.54 (0.92–2.58) | 0.10 | 2.28 (1.36–3.84) | 0.002 | ||||
| Severely salty diet | 1.48 (0.85–2.58) | 0.17 | 1.14 (0.66–1.97) | 0.64 | ||||
| Current or ex-smoker | 0.43 (0.18–1.01) | 0.051 | ||||||
| Rural raised | 1.40 (0.86–2.26) | 0.17 | 1.36 (0.83–2.25) | 0.226 | ||||
| Monthly income<US $5000 | 1.20 (0.66–2.15) | 0.55 | ||||||
| Final education compared to university education | ||||||||
| Elementary | 0.99 (0.60–1.63) | 0.95 | 1.39 (0.79–2.46) | 0.29 | ||||
| Middle-high | 1.23 (0.50–3.03) | 0.66 | 0.93 (0.33–2.63) | 0.88 | ||||
| Nagelkerke’s R2 | 0.286 | 0.236 | 0.167 | 0.104 | ||||
| Hosmer-Lemeshow Goodness-of-fit test, P-value | 0.764 | 0.487 | 0.228 | 0.199 | ||||
FDR, first degree relatives of gastric cancer; AG, atrophic gastritis; IM, intestinal metaplasia. For each column of data, sex, FDR and variables with P<0.2 were entered into the model.
Table 7.
Multivariate logistic regression analysis of risk factors of antral atrophic gastiritis and intestinal metaplasia in male population
| Variable | Male | |||
|---|---|---|---|---|
|
| ||||
| Antral AG | Antral IM | |||
|
| ||||
| Adjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
| Aging by 5-year | 1.41 (1.12-1.79) | 0.004 | 1.46 (1.15-1.85) | 0.002 |
| FDR | 2.38 (0.89-6.37) | 0.085 | 2.69 (1.06-6.80) | 0.037 |
| H. pylori infected | 15.5 (3.15-75.9) | 0.001 | 19.6 (2.37-161.7) | 0.006 |
| Severely spicy diet | 1.74 (0.59-5.16) | 0.32 | 3.07 (1.19-7.91) | 0.02 |
| Severely saltydiet | 1.18 (0.37-3.72) | 0.78 | ||
| Rural raised | 1.27 (0.49-3.26) | 0.62 | ||
| Nagelkerke’s R2 | 0.410 | 0.367 | ||
| Hosmer-Lemeshow Goodness-of-fit test, P-value | 0.843 | 0.57 | ||
FDR, first degree relatives of gastric cancer; AG, atrophic gastritis; IM, intestinal metaplasia. For each column of data, FDR and variables with P<0.2 were entered into the model.
DISCUSSION
The latest meta-analysis of the same topic which included only age-sex matched population studies showed that FDRs had increased risk of having AG and IM with OR of 2.2 and 1.98 respectively.14 This study reviewed literatures up until 2009, including six studies for analysis of AG and eight studies for analysis of IM. After that, there was one more such study from Iran in 2012, in which only atrophy, not IM, was shown to be of significant risk in FDRs compared to controls.19 The reason why family history is an independent risk factor of premalignant lesions of GC is because GC has been attributed to shared socioeconomic environments and genetic traits. Regarding the latter, we have shown that TGFB1-509 TT genotype might be one of the culprits.20
As FDR is considered to be a risk factor of premalignant lesions of GC and GC itself from above studies, we anticipated to get similar results from our study. However, in our study, significant result was found only in male sub- population analysis that FDRs had increased risk of antral IM by 2.69 fold. Overall small numbers of subjects can be one of the reasons why the results were not as anticipated. To prove the significance of family history of GC as a risk factor for premalignant lesions of GC in female further investigation is needed in larger population. Another point to note is that male sub-population study has unraveling effect even with small numbers of subjects since male sex is an independent risk factor of GC. Furthermore the fact that the ORs for the H. pylori infected subjects to have premalignant lesions increased significantly in males (Antral AG 15.2, antral IM 19.6) compared to total population (Antral AG 9.28, antral IM 7.81) points to the possibility that male sex might have a synergistic interaction with H. pylori infection. So far, why male sex acts as a risk factor for GC is unknown, but there are hypotheses that androgen receptor might take a role in carcinogenesis.21
From the results of the present study, H. pylori infection, once again, proved itself to be the strongest risk factor of premalignant lesions. Regarding to the age, its relevance was consistently significant. Even though the ORs of having AG or IM either in antrum or body by aging 5 years were approximately 1.25, estimated ORs of aging 40 years (which means aging from age of 26–30 to age of 66–70) are 5.96 (calculated from 1.258). Thus, age is just as powerful to indicate premalignant lesions. Among other environmental factors, severely spicy diet is significantly associated with antral IM (OR 2.28, P=0.002). Moreover, FDRs’ predilection for severely spicy and salty diet (OR 2.61, P<0.001 and OR 1.52, P=0.045 respectively) from the baseline characteristics seems to be traits shared in families with history of GC. Socioeconomic indices seem to represent unhealthy diet; however, they didn’t reach statistical significance.
Regarding to smoking history, the results were rather contradictory to the results of previous studies where smoking acted as a risk factor of GC.18 In the present study, however, smoking acted as if it is a protective factor of corporal AG. This suggests that smoking might have been a confounding factor in previous studies since previous studies were not age and sex matched and males usually smoke.
After H. pylori infection, gastric atrophy and intestinal metaplasia usually start from antrum, and they progress to the body as time goes by. Based on the results of the present study, there is a possibility that FDR has more susceptibility to initial development of intestinal metaplasia in the antrum after H. pylori infection. However, during long-time chronic H. pylori infection, since many environmental factors other than family history of gastric cancer could affect progression of intestinal metaplasia from antrum to body, they might be confounding factors on the final pathology of gastric body. Therefore, to elucidate the relationship between FDR and corporal intestinal metaplasia, further studies are needed.
With above findings in mind, there can be multiple approaches to prevent GC. As a primary prevention, diet habit should be changed so as to eat fresh food more, salted, pickled, smoked, and spicy food less.22 In the population less than 40-years of age, most importantly in 20–30’s,16 H. pylori eradication gets utmost importance since it is the best way to prevent IM from developing and progressing further. Once IM is developed, early and regular screening with esophagogastroduodenoscopy (EGD) is important since it raises the chance to detect the malignant lesions better than any other screening methods,23 thereby making it possible to treat the malignant lesions in early stages by endoscopic resection.
When it comes to screening method and interval, there are on-going disputes and international variations due to the matter of cost-effectiveness, since the long-term risks of GC from AG and IM are relatively low. For example, the life time risk of GC was 0.3% for AG and 1.0% for IM in American cohort study24 and 10-year risk of GC was 0.8% for AG and 1.8% for IM in Netherland cohort study. Furthermore, the fact that the incidence rate varies widely in different countries makes it difficult to reach a global agreement. Recent European guideline suggested operative link for gastritis assessment (OLGA) and operative link for gastric intestinal metaplasia (OLGIM), serum pepsinogen level, H. pylori serologic testing, family history of GC to be useful into detecting and differentiating the high risk group, but it states that neither age, gender, H. pylori virulence factors nor host genetic variations change the clinical recommendations. In addition, the guideline recommended EGD every 3 years for the individuals with extensive AG and IM but not for the individuals with mild to moderate lesions.25 However, in high risk regions like Korea, China, and Japan, it’s long been suggested that annual EGD screening is cost-effective.23,26–29 Recent study in Korea revealed that the proportions of early gastric cancer (EGC) in the annual and biennial screening groups were 98.6% and 80.7% respectively. Furthermore 56.9% and 33.3% of them, respectively, were able to treat the cancer via endoscopic modality. However, in the group who were with more than 2-year intervals of screening, only 54.6% were diagnosed as EGC. The overall 5-year survival rate for the annual, biennial and more than 2-year interval group were 98.5%, 92%, and 86.1%, respectively.30 Clearly it is beneficial to screen gastric cancer frequently since it enables to detect GC in early stage when they are still treatable with endoscopic modality, reducing the overall medical cost and increasing survival and quality of life. Currently in Korea, nationwide screening with either UGIS (upper gastrointestinal series) or EGD is provided biennially by National Cancer Screening Program. However, those with risk factors studied in this article are reasonably recommended to go under more frequent screening.
In summary, this study suggests that H. pylori infection, age, and spicy food are strong markers to predict the occurrence of AG and IM. However it failed to prove that family history is an independent risk factor of AG or IM. The results only hinted that family history and male sex might be risk factors and there could be interaction in between both factors. To prove that, there should be more studies in larger scale with age-sex matched population. Since premalignant lesions are definite risk factors, it is important to let the people with premalignant lesions and risk factors of GC know that they have benefit with frequent EGD screening.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant for the Global Core Research Center (GCRC) funded by the Korea government (MSIP) (No. 2011-0030001).
REFERENCES
- 1.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohkusa T, Fujiki K, Takashimizu I, Kumagai J, Tanizawa T, Eishi Y, et al. Improvement in atrophic gastritis and intestinal metaplasia in patients in whom Helicobacter pylori was eradicated. Ann Intern Med. 2001;134:380–6. doi: 10.7326/0003-4819-134-5-200103060-00010. [DOI] [PubMed] [Google Scholar]
- 6.Ito M, Haruma K, Kamada T, Mihara M, Kim S, Kitadai Y, et al. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther. 2002;16:1449–56. doi: 10.1046/j.1365-2036.2002.01311.x. [DOI] [PubMed] [Google Scholar]
- 7.Sung JJ, Lin SR, Ching JY, Zhou LY, To KF, Wang RT, et al. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterology. 2000;119:7–14. doi: 10.1053/gast.2000.8550. [DOI] [PubMed] [Google Scholar]
- 8.Forbes GM, Warren JR, Glaser ME, Cullen DJ, Marshall BJ, Collins BJ. Long-term follow-up of gastric histology after Helicobacter pylori eradication. J Gastroenterol Hepatol. 1996;11:670–3. doi: 10.1111/j.1440-1746.1996.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 9.Van der Hulst R, van der Ende A, Dekker F, Ten Kate F, Weel J, Keller J, et al. Effect of Helicobacter pylori eradication on gastritis in relation to caga: a prospective 1-year follow-up study. Gastroenterology. 1997;113:25–30. doi: 10.1016/s0016-5085(97)70076-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Xu L, Shi R, Huang X, Li SWH, Huang Z, et al. Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: a meta-analysis. Digestion. 2011;83:253–60. doi: 10.1159/000280318. [DOI] [PubMed] [Google Scholar]
- 11.Massarrat S, Haj-Sheykholeslami A, Mohamadkhani A, Zendehdel N, Rakhshani N, Stolte M, et al. Precancerous conditions after H. pylori eradication: a randomized double blind study in first degree relatives of gastric cancer patients. Arch Iran Med. 2012;15:664–9. [PubMed] [Google Scholar]
- 12.Kang JM, Kim N, Shin CM, Lee HS, Lee DH, Jung HC, et al. Predictive factors for improvement of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication: a three-year follow-up study in Korea. Helicobacter. 2012;17:86–95. doi: 10.1111/j.1523-5378.2011.00918.x. [DOI] [PubMed] [Google Scholar]
- 13.Shin CM, Kim N, Yang HJ, Cho S-I, Lee HS, Kim JS, et al. Stomach cancer risk in gastric cancer relatives: interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J Clin Gastroenterol. 2010;44:e34–9. doi: 10.1097/MCG.0b013e3181a159c4. [DOI] [PubMed] [Google Scholar]
- 14.Rokkas T, Sechopoulos P, Pistiolas D, Margantinis G, Koukoulis G. Helicobacter pylori infection and gastric histology in first-degree relatives of gastric cancer patients: a meta-analysis. Eur J Gastroenterol Hepatol. 2010;22:1128–33. doi: 10.1097/MEG.0b013e3283398d37. [DOI] [PubMed] [Google Scholar]
- 15.Marcos-Pinto R, Carneiro F, Dinis-Ribeiro M, Wen X, Lopes C, Figueiredo C, et al. First-degree relatives of patients with early-onset gastric carcinoma show even at young ages a high prevalence of advanced OLGA/OLGIM stages and dysplasia. Aliment Pharmacol Ther. 2012;35:1451–9. doi: 10.1111/j.1365-2036.2012.05111.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim N, Park YS, Cho SI, Lee HS, Choe G, Kim IW, et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia in a korean population without significant gastroduodenal disease. Helicobacter. 2008;13:245–55. doi: 10.1111/j.1523-5378.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 17.Rugge M, Correa P, Dixon M, Fiocca R, Hattori T, Lechago J, et al. Gastric mucosal atrophy: Interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther. 2002;16:1249–59. doi: 10.1046/j.1365-2036.2002.01301.x. [DOI] [PubMed] [Google Scholar]
- 18.La Torre G, Chiaradia G, Gianfagna F, De Lauretis A, Boccia S, Mannocci A, et al. Smoking status and gastric cancer risk: an updated meta-analysis of case-control studies published in the past ten years. Tumori. 2009;95:13–22. doi: 10.1177/030089160909500103. [DOI] [PubMed] [Google Scholar]
- 19.Mansour-Ghanaei F, Joukar F, Baghaei SM, Yousefi-Mashhoor M, Naghipour MR, Sanaei O, et al. Gastric precancerous lesions in first degree relatives of patients with known gastric cancer: a cross-sectional prospective study in Guilan Province, north of Iran. Asian Pac J Cancer Prev. 2012;13:1779–82. doi: 10.7314/apjcp.2012.13.5.1779. [DOI] [PubMed] [Google Scholar]
- 20.Shin CM, Kim N, Lee HS, Lee DH, Kim JS, Jung HC, et al. Intrafamilial aggregation of gastric cancer: a comprehensive approach including environmental factors, Helicobacter pylori virulence, and genetic susceptibility. Eur J Gastroenterol Hepatol. 2011;23:411–7. doi: 10.1097/MEG.0b013e328343b7f5. [DOI] [PubMed] [Google Scholar]
- 21.Tian Y, Wan H, Lin Y, Xie X, Li Z, Tan G. Androgen receptor may be responsible for gender disparity in gastric cancer. Med Hypotheses. 2013;80:672–4. doi: 10.1016/j.mehy.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Compare D, Rocco A, Nardone G. Risk factors in gastric cancer. Eur Rev Med Pharmacol Sci. 2010;14:302–8. [PubMed] [Google Scholar]
- 23.Tashiro A, Sano M, Kinameri K, Fujita K, Takeuchi Y. Comparing mass screening techniques for gastric cancer in japan. World J Gastroenterol. 2006;12:4873–4. doi: 10.3748/wjg.v12.i30.4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh JM, Hur C, Kuntz KM, Ezzati M, Goldie SJ. Cost-effectiveness of treatment and endoscopic surveillance of precancerous lesions to prevent gastric cancer. Cancer. 2010;116:2941–53. doi: 10.1002/cncr.25030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinis-Ribeiro M, Areia M, de Vries A, Marcos-Pinto R, Monteiro-Soares M, O’Connor A, et al. Management of precancerous conditions and lesions in the stomach (MAPS): Guideline from the European society of gastrointestinal endoscopy (ESGE), European Helicobacter study group (EHSG), European society of pathology (ESP), and the sociedade Portuguesa de endoscopia digestiva (SPED) Virchows Arch. 2012;460:19–46. doi: 10.1007/s00428-011-1177-8. [DOI] [PubMed] [Google Scholar]
- 26.Dan YY, So J, Yeoh KG. Endoscopic screening for gastric cancer. Clin Gastroenterol Hepatol. 2006;4:709–16. doi: 10.1016/j.cgh.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Morii Y, Arita T, Shimoda K, Yasuda K, Yoshida T, Kitano S. Effect of periodic endoscopy for gastric cancer on early detection and improvement of survival. Gastric Cancer. 2001;4:132–6. doi: 10.1007/pl00011735. [DOI] [PubMed] [Google Scholar]
- 28.Aida K, Yoshikawa H, Mochizuki C, Mori A, Muto S, Fukuda T, et al. Clinicopathological features of gastric cancer detected by endoscopy as part of annual health checkup. J Gastroenterol Hepatol. 2008;23:632–7. doi: 10.1111/j.1440-1746.2008.05346.x. [DOI] [PubMed] [Google Scholar]
- 29.Nam SY, Choi IJ, Park KW, Kim CG, Lee JY, Kook MC, et al. Effect of repeated endoscopic screening on the incidence and treatment of gastric cancer in health screenees. Eur J Gastroenterol Hepatol. 2009;21:855–60. doi: 10.1097/MEG.0b013e328318ed42. [DOI] [PubMed] [Google Scholar]
- 30.Chung SJ, Park MJ, Kang SJ, Kang HY, Chung GE, Kim SG, et al. Effect of annual endoscopic screening on clinicopathologic characteristics and treatment modality of gastric cancer in a high-incidence region of Korea. Int J Cancer. 2012;131:2376–84. doi: 10.1002/ijc.27501. [DOI] [PubMed] [Google Scholar]