Abstract
Background:
Observational epidemiological studies have shown that higher intakes of vitamins or antioxidants were inversely associated with the risk of esophageal cancer. However, randomized controlled trials (RCTs) have reported no preventive efficacy of vitamin or antioxidant supplements on esophageal cancer. This meta-analysis aimed to investigate the efficacy of vitamin and antioxidant supplements in the prevention of esophageal cancer as reported by RCTs.
Methods:
We searched PubMed, EMBASE, and the Cochrane Library in May 2013. Two authors independently reviewed and selected eligible articles based on predetermined selection criteria.
Results:
Of 171 articles searched from three databases and relevant bibliographies, 10 RCTs were included in the final analyses. In a fixed-effect meta-analysis of 10 trials, there was no efficacy of vitamin and antioxidant supplements in the prevention of esophageal cancer (relative risk [RR], 1.04; 95% confidence interval [CI], 0.86–1.25; I2=0.0%). Also, subgroup meta-analyses showed that vitamin and antioxidant supplements had no preventive efficacy on esophageal cancer both in the high risk (RR, 1.04; 95% CI, 0.85–1.28; n=4) and non-high risk (RR, 1.01; 95% CI, 0.65–1.56; n=6) groups for esophageal cancer. Further, subgroup meta-analyses revealed no preventive efficacy on esophageal cancer by type of methodological quality and type of vitamin and antioxidant supplements.
Conclusions:
Unlike observational epidemiological studies, this meta-analysis of RCTs suggests that there is no clinical evidence to support the efficacy of vitamin and antioxidant supplements in the prevention of esophageal cancer.
Keywords: Vitamin supplements, Antioxidant supplements, Esophageal cancer, Randomized controlled trials, Meta-analysis
INTRODUCTION
According to GLOBOCAN 2008 published by the International Agency for Research on Cancer, esophageal cancer is the eighth most common cancer, with 3.8% of all cancer cases, and the sixth leading cause of death from cancer, with 5.4% of all cancer deaths worldwide estimated in 2008.1 Along with genetic causes, lifestyle and environmental factors are considered to be important in the development of esophageal cancer.2 Among lifestyle factors, fruits and vegetables are rich in vitamins or antioxidants, which may have anticarcinogenic activities by various mechanisms removing free radicals and inhibiting the formation of N-nitroso compounds.2–4
Previous epidemiological studies have investigated the association between the risk of esophageal cancer and the intake of fruits and vegetables rich in vitamins and antioxidants, and those findings are mixed. In 2006, a meta-analysis of 10 epidemiological studies (1 cohort and 9 case-control studies) suggested that higher consumption of vitamin C and beta-carotene was significantly associated with a decreased risk of esophageal adenocarcinoma with odds ratios of 0.49 and 0.46, respectively.5
In the meantime, several randomized controlled trials (RCTs)6–15 have reported the association between vitamin or antioxidant supplements and the risk of esophageal cancer. However, no RCTs have suggested that there was any preventive efficacy of vitamin or antioxidant supplements on esophageal cancer. Further, there has been no quantitative meta-analysis of RCTs on this issue reported so far.
The purpose of this study was to examine the quantitative preventive efficacy of vitamin and antioxidant supplements on esophageal cancer by using a meta-analysis of RCTs by type of vitamin or antioxidant supplements, methodological quality, and high risk or non-high risk groups for esophageal cancer.
MATERIALS AND METHODS
1. Data search
We searched PubMed, EMBASE, and the Cochrane Library in May 2013, by using keywords related to vitamin and antioxidant supplements and esophageal cancer in RCTs. Also, the bibliographies of relevant articles were searched in order to locate additional studies. We used the following keywords for the literature search: “vitamin,” “antioxidant,” “beta-carotene,” or “selenium; and “esophageal cancer.”
2. Selection criteria
We included RCTs that reported the preventive efficacy of vitamin or antioxidant supplements on esophageal cancer. The main outcome measure was cancer incidence.
3. Selection of relevant trials
Two evaluators (Dr. Myung SK, Dr. Yang HJ) independently screened all the studies searched from the three databases. We tried to contact the authors of the articles with insufficient data. From the trials included in the final analysis, we extracted the following data: study name, journal, country, duration of supplement treatment and follow-up period (years), population (project name), supplement interventions, relative risk (RR) with 95% confidence intervals (CI), and number of cancer/number of participants in each intervention group.
4. Main and subgroup analyses
We examined the efficacy of vitamin and antioxidant supplements administered singly or in combination with other vitamin or antioxidant supplements on esophageal cancer, compared with placebo administration in all 10 trials. Also, we evaluated those efficacy by type of vitamin or antioxidant supplements, methodological quality, and high risk or non-high risk groups for esophageal cancer.
5. Assessment of methodological quality
We also evaluated the methodological quality of the trials by using the Jadad scale.16 This 5-point scale consists of randomization (2 points), double-blind (2 points), and follow-up (dropouts and withdrawals; 1 point) in the report of a RCT. A trial with the score of 2 or less was considered as having low-quality, and the one with the score of 3 to 5 was considered as having high-quality.
6. Statistical analyses
The pooled RR with 95% CI was calculated on the basis of both the fixed- and random-effects models; the Mantel-Haenszel method was used in the fixed-effects model, and the DerSimonian and Laird method was used in the random-effects model. We estimated heterogeneity (between-studies variability) using the Higgins I2 statistic, which measures the percentage of total variation across studies due to heterogeneity rather than chance.17,18 I2 was calculated as follows:
where Q is Cochran’s heterogeneity statistic and df is the degrees of freedom corresponding to it. Cochran’s Q statistic was calculated as follows:
where θi is the RR of each ith trial, θp is the pooled RR of all the trials, and wi is the inverse variance of each ith trial as a weight. Negative values of I2 are set at zero so that I2 ranges between 0% (no observed heterogeneity) and 100% (maximal heterogeneity). We considered an I2 value greater than 50% as indicating substantial heterogeneity. When there was no substantial heterogeneity, we reported the pooled estimate calculated based on the fixed-effects model. When there was substantial heterogeneity, we reported the pooled estimate calculated based on the random-effects model.
We estimated publication bias by using Begg’s funnel plot and Egger’s test. When the P-value was less than 0.05 by Egger’s test, the presence of a publication bias was considered. We used the Stata SE version 10.0 software package (StataCorp, College Station, Texas, USA) for statistical analysis.
RESULTS
1. Selection of trials
A total of 171 articles were retrieved after searching three databases and relevant bibliographies (Fig. 1). After excluding duplicated articles and reviewing articles based on those title and abstracts, we reviewed 24 articles with those full texts and then included 10 trials in the final analysis. We excluded 14 articles because of identical populations (n=7), insufficient data (n=4), and studies not relevant to our subject (n=3).
Fig. 1.
Flow diagram for identification of relevant studies.
2. General characteristics of trials
A total of 126,828 subjects with the 70,959 vitamin and antioxidant supplement and 55,869 placebo groups from 10 RCTs. As shown in Table 1, the selected trials were published from 1985 through 2007, spanning 22 years. The countries in which the studies were performed were as follows: US (n=3), China (n=3), Finland (n=1), Canada (n=1), UK (n=1), and France (n=1). The periods of treatment and follow-up ranges between 2 and 10.1 years. All trials used placebos as a control group. The types of vitamin and antioxidant supplements were vitamin A, vitamin B2, Vitamin C, vitamin E, beta-carotene, and selenium.
Table 1.
General characteristics of trials included in the final analysis (n=10)
| Study name (no. of reference) | Journal | Country | Duration of supplement treatment/follow-up period (years) | Population | Supplement Interventions | RR (95% CI) | No. of esophageal cancer/no. of participants in each group | |
|---|---|---|---|---|---|---|---|---|
| 1 | 1985 Munoz et al.6 | Lancet | China | 1.1/1.1 | 610 subjects who live in the high risk of area | 15 mg of retinol (vitamin A), 200 mg of riboflavin (vitamin B2), and 50 mg of zinc vs. placebo per day | Not stated | Vitamin supplements: 4/305 Placebos: 3/305 |
| 2 | 1993 NIT7 | J Natl Cancer Inst | China | 6/6 | 3,318 persons with of esophageal dysplasia | 15 mg of beta-carotene and a combination of 10,000 IU of vitamin A, 180 mg of vitamin C, 60 IU of vitamin E, 50 μg of selenium, etc. vs. placebo per day | 0.96 (0.76–1.22) | Vitamin and antioxidant supplements: 123/1657 Placebos: 1281/1661 |
| 3 | 1996 NPC8 | JAMA | U.S. | 4.5/6.4 | 1,312 patients with a history of basal cell or squamous cell carcinomas of the skin | 200 μg of selenium vs. placebo per day | 0.34 (0.07–1.66) | Selenium supplements: 2/653 Placebos: 6/659 |
| 4 | 2002 HPS9 | Lancet | U.K. | 5/5 | 20,536 adults with coronary disease, other occlusive arterial disease, or diabetes | A combination of 600 mg of vitamin E, 250 mg vitamin C, and 20 mg beta-carotene vs. placebo per day | 1.19 (0.71–2.01) | Antioxidant supplements: 31/10269 Placebos: 26/10,267 |
| 5 | 2003 Zhu et al.10 | Chin Med J | China | 2/6 | 216 patients with atrophic gastritis | 20 mg of folate, 30 mg of natural beta-carotene, or 30 mg of synthetic beta-carotene vs. placebo per day | 0.15 (0.01–3.72) | Folate or beta-carotene supplements: 0/118 Placebos: 1/54 |
| 6 | 2004 CARET11 | J Natl Cancer Inst | U.S. | 4/4 | 18,314 participants at high risk for lung cancer because of a history of smoking or asbestos exposure | A combination of 30 mg of beta-carotene and 25,000 IU retinyl palmitate (vitamin A) vs. placebo per day | 1.43 (0.90–2.29) | Beta-carotene and vitamin A supplements: 44/9,420 Placebos: 29/8,894 |
| 7 | 2004 SUVIMAX12 | Arch Intern Med | France | 7.5/7.5 | 13,017 French adults | A combination of 120 mg of vitamin C, 30 mg of vitamin E, 6 mg of beta-carotene, 100 μg of selenium, and 20 mg of zinc vs. placebo per day | 1.01 (0.14–7.16) | Antioxidants supplements: 2/6,481 Placebos: 2/6,536 |
| 8 | 2005 Bairati et al.13 | J Natl Cancer Inst | Canada | 4.3/4.3 | 540 patients with stage or head and neck cancer treated by radiation therapy | 400 IU of vitamin E and 30 mg of beta-carotene vs. placebo per day | 0.98 (0.06–15.56) | Antioxidants supplements: 1/273 Placebos: 1/267 |
| 9 | 2005 WHS14 | JAMA | U.S. | 10.1/10.1 | 39,876 apparently healthy US women | 600 IU of natural-source vitamin E or aspirin vs. placebo on alternate days | 1.33 (0.30–5.96) | Vitamin E supplements: 47/19,937 Placebos: 3/19,939 |
| 10 | 2007 ATBC15 | Cancer | Finland | 6.1/6.1 | 29,133 male smokers | 50 mg of alpha-tocopherol or 20 mg of beta-carotene vs. placebo per day | 0.81 (0.34–1.95) | Antioxidant supplements: 17/21,846 Placebo: 7/7,287 |
RR, relative risk; CI, confidence interval; NIT, the nutrition intervention trial; NPC, the nutritional prevention of cancer study; HPS, the heart protection study; CARET, the beta-carotene and retinol efficacy trial; SUVIMAX, the supplemenatation en vitamines et mineraux antioxydants; WHS, the women’s health study; ATBC, the alpha-tocopherol beta-carotene cancer prevention study.
3. Efficacy of vitamin or antioxidant supplements in all 10 trials
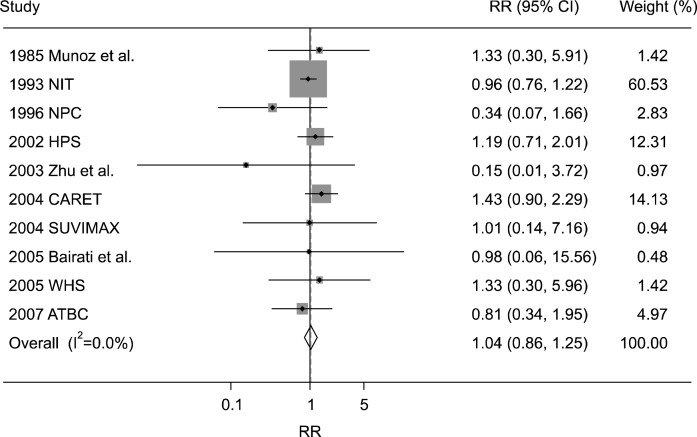
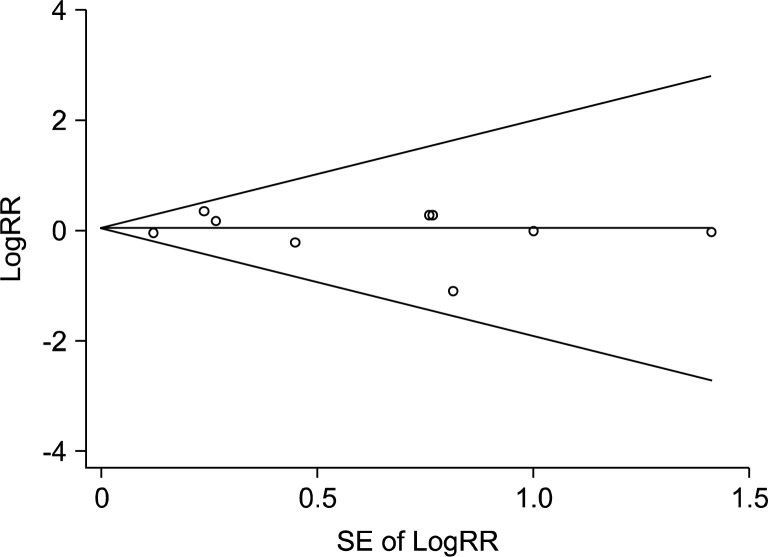
In a fixed-effects model meta-analysis of all 22 trials, there was no efficacy of vitamin and antioxidant supplements in the prevention of esophageal cancer (RR, 1.04; 95% CI, 0.86–1.25; I2=0.0%) (Fig. 2). There was no evidence of publication bias in the selected studies (Egger’s test, P for bias=0.913; The Begg’s funnel plot was symmetrical) (Fig. 3).
Fig. 2.
Efficacy of vitamin and antioxidant supplements in prevention of esophageal cancer by a fixed-effect model meta-analysis of randomized controlled trials. RR, relative risk; CI, confidence interval; NIT, the nutrition intervention trial; NPC, the nutritional prevention of cancer study; HPS, the heart protection study; CARET, the beta-carotene and retinol efficacy trial; SUVIMAX, the supplemenatation en vitamines et mine-raux antioxydants; WHS, the women’s health study; ATBC, the alpha-tocopherol beta-carotene cancer prevention study.
Fig. 3.
Funnel plots and egger’s test for identifying publication bias (P=.913) in a meta-analysis of trials (n=9). RR, relative risk; SE, standard error.
4. Methodological quality of trials
Table 2 shows the methodological quality of trials based on the Jadad scale. Eight trials received 4 or higher points and were classified as having a high quality, whereas two trials received 3 points and were classified as having a low quality.
Table 2.
Methodological quality of trials based on the jadad scale (n=10)
| Source (project name) | Randomization | Description of randomization methods | Double-blind | Using identical placebo | Follow-up reporting | Total score | |
|---|---|---|---|---|---|---|---|
| 1 | 1985 Munoz et al. | 1 | 0 | 1 | 1 | 1 | 4 |
| 2 | 1993 NIT | 1 | 0 | 1 | 1 | 1 | 4 |
| 3 | 1996 NPC | 1 | 0 | 1 | 1 | 1 | 4 |
| 4 | 2002 HPS | 1 | 1 | 1 | 1 | 1 | 5 |
| 5 | 2003 Zhu et al. | 1 | 0 | 1 | 1 | 0 | 3 |
| 6 | 2004 CARET | 1 | 0 | 1 | 1 | 1 | 4 |
| 7 | 2004 SUVIMAX | 1 | 0 | 1 | 1 | 1 | 4 |
| 8 | 2005 Bairati et al. | 1 | 1 | 1 | 1 | 1 | 5 |
| 9 | 2005 WHS | 1 | 0 | 1 | 1 | 1 | 4 |
| 10 | 2007 ATBC | 1 | 0 | 1 | 1 | 0 | 3 |
NIT, the nutrition intervention trial; NPC, the nutritional prevention of cancer study; HPS, the heart protection study; CARET, the beta-carotene and retinol efficacy trial; SUVIMAX, the supplemenatation en vitamines et mineraux antioxydants; WHS, the women’s health study; ATBC, the alpha-tocopherol beta-carotene cancer prevention study.
5. Subgroup meta-analyses by type of supplements
In the subgroup meta-analyses by type of supplements based on the fixed-effect model, none of the antioxidant supplements had any significant preventive efficacy in the prevention of esophageal cancer: vitamin A (RR, 1.06; 95% CI, 0.86–1.30; n=3), vitamin B2 (RR, 1.33; 95% CI, 0.30–5.91; n=1), vitamin C (RR, 1.00; RR, 0.81–1.24: n=3), vitamin E (RR, 1.00; 95% CI, 0.81–1.22; n=6), folate (RR, 0.41; 95% CI, 0.02–9.76), beta-carotene (RR, 1.05; 95% CI, 0.87–1.27; n=7), and selenium (RR, 0.94; 95% CI, 0.74–1.18; n=3) (Table 3).
Table 3.
Efficacy of vitamin and antioxidant supplements in prevention of esophageal cancer in subgroup meta-analyses
| Variable | No. of trials | Summary RR (95% CI) | Heterogeneity, I2 | Model used |
|---|---|---|---|---|
| All | 10 | 1.04 (0.86–1.25) | 0.0% | Fixed-effect |
| Type of supplements | ||||
| Vitamin A | 3 | 1.06 (0.86–1.30) | 13.1% | Fixed-effect |
| Vitamin B2 | 1 | 1.33 (0.30–5.91) | NA | NA |
| Vitamin C | 3 | 1.00 (0.81–1.24) | 0.0% | Fixed-effect |
| Vitamin E | 6 | 1.00 (0.81–1.22) | 0.0% | Fixed-effect |
| Folate | 1 | 0.41 (0.02–9.76) | NA | NA |
| Beta-carotene | 7 | 1.05 (0.87–1.27) | 0.0% | Fixed-effect |
| Methodological quality | ||||
| High quality (score>3) | 8 | 1.06 (0.87–1.28) | 0.0% | Fixed-effect |
| Low quality (score≤3) | 2 | 0.70 (0.31–1.61) | 0.0% | Fixed-effect |
| Risk group for esophageal cancer | ||||
| High risk group | 4 | 1.04 (0.85–1.28) | 0.0% | Fixed-effect |
| Non-high risk group | 6 | 1.01 (0.65–1.56) | 0.0% | Fixed-effect |
RR, relative risk; CI, confidence interval; NA, not applicable.
6. Subgroup meta-analyses by type of quality and risk group
The subgroup meta-analyses by type of quality showed no preventive efficacy of vitamin and antioxidant supplements on esophageal cancer for both high quality (RR, 1.06; 95% CI, 0.87–1.28; n=8) and low quality (RR, 0.70; 95% CI, 0.31–1.61; n=2) trials. Similarly, no preventive efficacy was found in both high risk (RR, 1.04; 95% CI, 0.85–1.28; n=4) and non-high risk (RR, 1.01; 95% CI, 0.65–1.568; n=6) groups for esophageal cancer.
DISCUSSION
The current meta-analysis of RCTs found that there was no efficacy of vitamin and antioxidant supplements in the prevention of esophageal cancer. Further, subgroup analyses by type of supplements, methodological quality, and risk group for esophageal cancer revealed no preventive efficacy of those supplements.
Our findings were inconsistent with those of previously published epidemiological studies and a meta-analysis of epidemiological studies, which had reported that people with the highest levels of antioxidant intake such as vitamin C or beta-carotene had an about 50% lower risk compared to those with lower levels of intake.5
There are several possible explanations for the inconsistent findings between observational epidemiological studies and RCTs. First, retrospective case-control studies are susceptible to recall and selection biases.18 Esophageal cancer patients might recall wrongly their consumption of fruit and vegetables rich in vitamin and antioxidants. Even though they had have adequate intakes of fruit and vegetables long before their diagnosis of esophageal cancer, they might think incorrectly that they had consumed less foods because they had dyspepsia and loss of appetite due to cancer right before that diagnosis. Also, selection bias might affect the results because cases or controls are not representative of the population. Second, there are differences in functions and components between natural vitamin or antioxidants and synthetic ones. For example, synthetic alpha-tocopherol (all-rac-alpha-tocopherol), which is composed of equal amounts of the 8 different stereoisomers of alpha-tocopherol, is different from its natural form (RRR-alpha-tocopherol).19 Also, the human body absorbs and excretes natural and synthetic vitamin E differently, and those biological activities are different.19,20 As for beta-carotene, experimental studies reported that beta-carotene might play a potential protective role against cancer initiation.21 However, it may act as a prooxidant in the presence of chronic oxidative stress such as smoking; this may induce the oxidation of beta-carotene and DNA oxidative damage and finally lead to lung cancer.18,22
Third, eliminating reactive oxygen species by antioxidant supplementation might interfere with several essential defensive mechanisms like apoptosis and detoxification and unexpectedly increase mortality.23 Last, antioxidant supplements might have interdependency and show those efficacy only when given in combination.24
There are several limitations in our study. First, we were unable to include several recent RCTs25,26 because data for esophageal cancer were not reported. Second, the statistical power of the current meta-analysis is very low because the incidence of esophageal cancer is very low, compared with other types of common cancers. Further lager RCTs are needed to confirm our findings. Last, we were unable to apply our findings to healthy populations. Of 10 trials, only two trials involved general healthy populations, while the remaining trials involved a history of certain diseases or high risk populations for esophageal cancer.
In summary, unlike observational epidemiological studies, the current meta-analysis of RCTs found that there is no clinical evidence to support the efficacy of vitamin and antioxidant supplements in the prevention of esophageal cancer.
Acknowledgments
Seung-Kwon Myung is responsible for the initial plan, study design, data screening, data selection, data interpretation, statistical analysis, manuscript drafting, and for conducting the study.
Hyo Jin Yang is responsible for data screening and data selection. SK Myung is the guarantor for this paper and has full responsibility for this study.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Kubo A, Corley DA, Jensen CD, Kaur R. Dietary factors and the risks of oesophageal adenocarcinoma and Barrett’s oesophagus. Nutr Res Rev. 2010;23:230–46. doi: 10.1017/S0954422410000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes Control. 1991;2:427–42. doi: 10.1007/BF00054304. [DOI] [PubMed] [Google Scholar]
- 4.Mirvish SS, Wallcave L, Eagen M, Shubik P. Ascorbate-nitrite reaction: possible means of blocking the formation of carcinogenic N-nitroso compounds. Science. 1972;177:65–8. doi: 10.1126/science.177.4043.65. [DOI] [PubMed] [Google Scholar]
- 5.Kubo A, Corley DA. Meta-analysis of antioxidant intake and the risk of esophageal and gastric cardia adenocarcinoma. Am J Gastroenterol. 2007;102:2323–30. doi: 10.1111/j.1572-0241.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- 6.Munoz N, Wahrendorf J, Bang LJ, Crespi M, Thurnham DI, Day NE, et al. No effect of riboflavine, retinol, and zinc on prevalence of precancerous lesions of oesophagus. Randomised double-blind intervention study in high-risk population of China. Lancet. 1985;2:111–4. doi: 10.1016/s0140-6736(85)90223-5. [DOI] [PubMed] [Google Scholar]
- 7.Li JY, Taylor PR, Li B, Dawsey S, Wang GQ, Ershow AG, et al. Nutrition intervention trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia. J Natl Cancer Inst. 1993;85:1492–8. doi: 10.1093/jnci/85.18.1492. [DOI] [PubMed] [Google Scholar]
- 8.Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–63. [PubMed] [Google Scholar]
- 9.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 10.Zhu S, Mason J, Shi Y, Hu Y, Li R, Wahg M, et al. The effect of folic acid on the development of stomach and other gastrointestinal cancers. Chin Med J (Engl) 2003;116:15–9. [PubMed] [Google Scholar]
- 11.Goodman GE, Thornquist MD, Balmes J, Cullen MR, Meyskens FL, Jr, Omenn GS, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96:1743–50. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 12.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335–42. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 13.Bairati I, Meyer F, Gelinas M, Fortin A, Nabid A, Brochet F, et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J Natl Cancer Inst. 2005;97:481–8. doi: 10.1093/jnci/dji095. [DOI] [PubMed] [Google Scholar]
- 14.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 15.Wright ME, Virtamo J, Hartman AM, Pietinen P, Edwards BK, Taylor PR, et al. Effects of alpha-tocopherol and beta-carotene supplementation on upper aerodigestive tract cancers in a large, randomized controlled trial. Cancer. 2007;109:891–8. doi: 10.1002/cncr.22482. [DOI] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Myung SK, Kim Y, Ju W, Choi HJ, Bae WK. Effects of antioxidant supplements on cancer prevention: meta-analysis of randomized controlled trials. Ann Oncol. 2010;21:166–79. doi: 10.1093/annonc/mdp286. [DOI] [PubMed] [Google Scholar]
- 19.Kiyose C, Muramatsu R, Kameyama Y, Ueda T, Igarashi O. Biodiscrimination of alpha-tocopherol stereoisomers in humans after oral administration. Am J Clin Nutr. 1997;65:785–9. doi: 10.1093/ajcn/65.3.785. [DOI] [PubMed] [Google Scholar]
- 20.Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, et al. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998;67:669–84. doi: 10.1093/ajcn/67.4.669. [DOI] [PubMed] [Google Scholar]
- 21.De Flora S, Bagnasco M, Vainio H. Modulation of genotoxic and related effects by carotenoids and vitamin A in experimental models: mechanistic issues. Mutagenesis. 1999;14:153–72. doi: 10.1093/mutage/14.2.153. [DOI] [PubMed] [Google Scholar]
- 22.Mayne ST, Handelman GJ, Beecher G. Beta-Carotene and lung cancer promotion in heavy smokers--a plausible relationship? J Natl Cancer Inst. 1996;88:1513–5. doi: 10.1093/jnci/88.21.1516-a. [DOI] [PubMed] [Google Scholar]
- 23.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 24.Hercberg S, Galan P, Preziosi P, Alfarez MJ, Vazquez C. The potential role of antioxidant vitamins in preventing cardiovascular diseases and cancers. Nutrition. 1998;14:513–20. doi: 10.1016/s0899-9007(98)00040-9. [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14–23. doi: 10.1093/jnci/djn438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]